Abstract
Zeolite and limestone were tested for their capability of removing As and Fe from acidic water in batch and column experiments. Synthetic acidic water with 3 mg/L As and 50 or 100 mg/L Fe at pH = 2 was used in the column experiments. In the batch experiments, the As concentration, the mass of media, and the contact time were varied between 0.2 and 5 mg As/L, 0.5 and 50 g, and 0.25 and 42 h, respectively. Maximum As sorption capacity as indicated by the Langmuir model was 0.17 mg/g for zeolite and 1.3 mg/g for limestone, at 18-h contact time and 6.3 g/L medium concentration. Energy dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy analyses revealed that As and Fe were retained in zeolite at the end of the batch experiments. The main factors affecting As and Fe removal efficiency and pH raising capacity were the contact time and the media concentration. This was confirmed in the column experiments, since zeolite and limestone columns presented 99% As removal, under a hydraulic loading rate of 21.8 mm/day. However, limestone columns presented a higher Fe removal: 99 versus 73% for zeolite. The results indicate that limestone could be more appropriate than zeolite when As and Fe are present under acidic conditions, given its higher capacity to remove both As and Fe and to raise pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) is well known for being carcinogenic, causing bladder, lung, and non-melanoma skin cancer when ingested via drinking water (Marshall et al. 2007). About 100 million people in the world are at risk because of exposure to As concentrations over 10 μg/L, the WHO drinking water guideline value (Singh et al. 2015). The presence of As in natural waters is a worldwide problem, with Bangladesh, China, India, the USA, Chile, and many other countries affected (Jain and Singh 2012).
In northern Chile, in the Lluta River basin, the Azufre River presents high levels of As and also iron (Fe) at acidic pH, due to the influence of the Tacora volcano and the legacy of sulfur mining. In fact, acid mine drainage (AMD) is observed at the foot of mine tailings located on the Tacora crater and its western flank (Leiva et al. 2014).
Many efforts have been made to develop various arsenic remediation techniques (Sarkar and Paul 2016). However, arsenic removal from AMD has received little attention because manganese (Mn) and iron often are considered the main pollutants (Wallace and Knight 2006). In mine waters, As can be present in varying concentrations, fluctuating between 0.005 and 72 mg/L for pH values between 0.52 and 10 (Williams 2001). Because arsenic is soluble across a wide pH range—a major contrast from most heavy metals (ibid)—other environmental factors must be taken into account to elucidate As speciation, especially the presence of Fe (Lizama A. et al. 2011a).
AMD is treated conventionally by adding finely powdered limestone (CaCO3) or lime (Ca(OH)2) to raise its pH, so the dissolved metals precipitate out as basic metal carbonates or oxyhydroxides (Wang et al. 2003). Limestone also has been employed as a fixed medium in passive and semi-passive AMD treatment systems, which include various limestone-based methods such as limestone drains and wetlands (Cravotta III 2010).
Constructed wetlands are a “green” technology able to remove a variety of pollutants, including metals and metalloids such as As (Marchand et al. 2010). The main application of constructed wetlands for heavy metals removal has been in fact the treatment of AMD, for which they have been employed around the world (Zipper and Skousen 2010). Surface flow (SF) wetlands have been the predominant treatment of choice (Kadlec and Wallace 2009); therefore, less is known about the performance of subsurface flow (SSF) constructed wetlands for this purpose. Because many of the heavy metal removal mechanisms in wetlands treating AMD occur in the substrate (Sheoran and Sheoran 2006), suitable substrates that enhance the performance of SSF should be further investigated.
Lately, SSF constructed wetlands have been further investigated for their capability of removing As (Rahman et al. 2011; Olmos-Márquez et al. 2012; Schwindaman et al. 2014). In addition, it has been shown that the supporting medium (substrate) has a key role in this capability (Zurita et al. 2012). The use of alternative media instead of conventional gravel media has been recommended but still not thoroughly investigated. Limestone and zeolite have been very effective in vertical subsurface flow (VSSF) and horizontal subsurface flow (HSSF) constructed wetlands (Lizama Allende et al. 2011b, 2012, 2014). The use of alternative media has raised other questions that must be tackled; for example, the removal capacity of the media itself and the changes on relevant water quality parameters related to the characteristics of the media, particularly pH. This study will address these questions regarding the use of limestone and zeolite.
Zeolite, as opposed to limestone, has been investigated mainly in laboratory experiments for AMD treatment. Despite the fact that Motsi et al. (2009) concluded that natural zeolite (clinoptilolite) has a great potential for this purpose, it appears that zeolite has not yet been employed in AMD treatment systems. As mentioned previously, the target pollutants in these systems are Fe and Mn rather than As; therefore, little is known about the potential of zeolite and limestone for AMD treatment with elevated As concentrations. The capability of limestone for As removal has been documented (Davis et al. 2007; Armienta et al. 2009, 2012; Romero et al. 2011). However, few studies have focused on the capability of limestone to remove As from acidic water in batch reactors (e.g., Labastida et al. 2013) or as a filter medium (e.g., Pagnanelli et al. 2011).
Different types of zeolites—natural, synthetic, modified—have been investigated for their As removal capacity (Elizalde-González et al. 2001; Chutia et al. 2009; Jeon et al. 2009; Šiljeg et al. 2012; Baskan and Pala 2014; Bakatula et al. 2017). The typical approach has been to vary key water quality parameters (pH, As concentration) so as to obtain sorption capacities under different conditions. Few studies have focused on a particular kind of water and very few on acidic waters (e.g., Li et al. 2011). It appears that no study has investigated As removal by natural zeolite under highly acidic conditions. The present study will contribute to fill this gap.
To probe As speciation and association and removal mechanisms, different techniques exist. X-ray photoelectron spectroscopy (XPS) has been widely used to characterize sorbent materials aiming As removal (e.g., Qin et al. 2016; Ocinski et al. 2016). However, it rarely has been used to identify possible changes in As oxidation state when As is sorbed onto zeolite. Up to now, apparently only Šiljeg et al. (2009) and Simsek and Beker (2014) have done so. Elucidating the fate of As in complex water treatment systems such as constructed wetlands has been suggested but still barely done, probably because As speciation analysis in the solid phase remains a challenge (Liu and Cai 2007).
The objective of this study is therefore to quantify the As and Fe removal capacity of zeolite and limestone from highly acidic water. This capacity was studied in batch and column experiments. Other relevant water quality parameters (pH, temperature, electrical conductivity, and dissolved oxygen) were monitored, alongside Al, Si, Ca, and Mg concentrations, so as to evaluate the effects on water quality when using these media under different environmental conditions. This is a new approach compared to previous work on As removal using both media in batch and column experiments. To the authors’ knowledge, the performance of zeolite for As removal under highly acidic conditions has not been investigated, and despite the wide use of limestone in AMD treatment, As removal from AMD has been barely investigated. We considered the case study of the Azufre River in northern Chile, because it represents a typical acid drainage with elevated As concentrations. Findings from this study can be used to guide the design of water treatment systems using limestone and zeolite, giving further information on their role as main supporting media in constructed wetlands for the removal of As and Fe from acidic water. This study is part of a broader study that will consider the use of these media in constructed wetlands systems treating highly acidic contaminated water to enhance performance. In addition, this study will give a first attempt to identify any possible changes in As and Fe oxidation states in zeolite, aiming to compare with future analyses to be performed in constructed wetlands.
2 Materials and Characterization
2.1 Media
Zeolite and limestone obtained from local suppliers were employed. Their main characteristics are presented in Table 1. Main chemical composition was provided by the products datasheet, whereas the physical properties were obtained in the Department of Civil Engineering, Universidad de Chile. The porosity of zeolite and limestone was calculated using the data from Table 1 and it corresponds to 0.58 and 0.43, respectively.
To identify the presence and speciation of As and Fe before and after exposure, the elemental chemical information of natural zeolite and zeolite exposed to contaminated water in a batch reactor was obtained by means of energy dispersive X-ray spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). The exposed zeolite sample was selected given the high removal of As obtained in the corresponding reactor. For the EDS analyses, a FEI Quanta 250 scanning electron microscope (SEM) was employed. The XPS analyses were obtained in a Physical Electronics System equipment model 1257 using Al Kα emission (1486.6 eV).
2.2 Specific Surface Area and Mineralogy
Brunauer, Emmett, and Teller (BET) specific surface area (Brunauer et al. 1938) was determined by N2 sorptometry on a Micromeritics ASAP 2010 equipment after degassing the samples at 200 °C. Zeolite-specific surface area was 49.8 m2/g, pore diameter was 5.55 nm, and pore volume was 0.07 cm3/g. For limestone the specific surface was 0.38 m2/g—within the range of reported values; e.g., Canga et al. (2016)—and no data regarding pore diameter or pore volume was obtained.
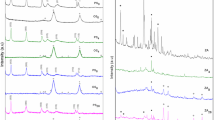
To verify the composition of the media, the crystalline minerals in zeolite and limestone were identified using X-ray diffraction (XRD). A Bruker D8 Advance diffractometer with a Cu anode (λ = 1.54 Å) was used. The scans were performed every 0.1 s with a scan step size of 0.02° at 24 °C.
2.3 Synthetic Water
The case study of the Azufre River, northern Chile, was selected for consistency with previous studies (Lizama Allende et al. 2012; Lizama Allende et al. 2014) as it is affected by acid drainage (Guerra et al. 2016). Synthetic acidic water simulating the water quality of this river was prepared using distilled water. The following reagents were added per liter of water: 3 mL 1000 mg/L arsenic standard solution (arsenic acid As2O5 in H2O), 3 mL 10,000 mg/L boron standard solution (boric acid H3BO3 in H2O), 0.5 g FeSO4·7H2O, and 0.425 mL H2SO4 (95–97% Merck ISO grade). In most of the experiments, the target concentrations were As = 3 mg/L, B = 30 mg/L, and Fe = 100 mg/L at pH = 2. In the sorption isotherm experiment, the amount of the corresponding reagent was modified according to the required target concentrations: arsenic standard solution and FeSO4·7H2O. In the column experiment, the Fe concentration was 50 mg/L for the 60 first days of the experimental period, and then it was increased to 100 mg/L. The synthetic water was freshly prepared every 10 days using the procedure explained above, and it was stored in a 6-L feeding tank for continuous dosing.
2.4 Experimental Setup
2.4.1 Batch Experiments
In all batch experiments, the following factors remained fixed: volume of solution (V) = 240 mL, pH = 2, boron [B] = 30 mg/L. Experiments were performed in triplicate. Reactors consisted of 250-mL high-density polyethylene (HDPE) containers that were continuously stirred in a shaker at the laboratory temperature (22 ± 1 °C).
Three types of experiments were performed in order to investigate the effect of one key factor at a time: As concentration (C 0), contact time (θ), and mass of media (M).
Effect of As concentration: the initial As concentrations C 0 were 0.2, 0.5, 1, 2, 3, 4, and 5 mg/L, for θ = 18 h, M = 1.5 g, and [Fe] = 50 and 100 mg/L.
Effect of contact time: reactors were agitated for θ = 0.25, 1, 18, and 42 h, for [Fe] = 50 mg/L; and for θ = 0.5, 3, and 18 h, for [Fe] = 100 mg/L. In both cases, [As] = 3 mg/L and M = 1.5 g.
Effect of media concentration: The mass of solid (M) used in each reactor was 0.5, 1.5, 2.5, 5, 10, and 50 g, for θ = 18 h, [As] = 3 mg/L, and [Fe] = 50 mg/L.
The sorbed amount q (mg/g) was calculated by using the difference between the initial concentration C 0 and the equilibrium concentration C e, as q = V(C 0 − C e)/M. Removal efficiencies (%) were calculated as (C 0 − C e)/C 0 × 100.
2.4.2 Column Experiments
The column system consisted of six columns built using acrylic, 30-cm long and 5.8-cm diameter. Three columns were filled with zeolite and three with limestone. In each column, the medium was compacted in 5-cm layers up to a total depth of 22 cm, achieving an average density of 0.86 g/cm3 in zeolite columns and 1.57 g/cm3 in limestone columns. Because the volume of the columns was fixed and the media have different specific weights (Table 1), it was preferred that the columns had a similar compaction rather than a similar mass (500 g of zeolite were used in each zeolite column and 915 g of limestone were used in each limestone column). The columns were washed with distilled water prior to the experiment. A Masterflex peristaltic pump was used to fix the outflow from each column to 0.04 mL/min, corresponding to a hydraulic loading rate (HLR) of 21.8 mm/day. This rate was chosen, because it is similar to those used in constructed wetlands for As removal (Kröpfelová et al. 2009; Rahman et al. 2011). Nominal hydraulic retention time in zeolite and limestone columns was 9.5 and 8 days, respectively. A picture of the experimental setup is presented in Figure S1.
2.4.3 Sampling and Analysis
At the end of each batch experiment, water samples from the supernatant in each reactor were filtered through 0.45-um cellulose acetate filters and acidified with nitric acid immediately after sampling. They were stored at 4 °C prior to heavy metals analyses.
The column system was continuously dosed with the acidic wastewater for 90 days (HLR = 21.8 mm/day), and the effluent from each column was continuously stored in a collection container. Every 10 days, samples were collected from each of the six containers and from the feed tank following the procedure explained above.
The concentrations of As, Fe, Ca, Si, Mg, and Al in the samples were analyzed in an INN (Instituto Nacional de Normalización, Chile, www.inn.cl) accredited laboratory by ICP-OES or MS depending on the detection limits. ICP-MS detection limits (mg/L) were 0.001 for As, 0.02 for Fe, 1.78 for Ca, 0.06 for Si, 0.35 for Mg, and 0.0145 for Al. For samples reported below the detection limit, half of the corresponding value was considered as the actual concentration, a common practice when dealing with this type of data for statistical analyses (Croghan and Egeghy 2003). Water quality parameters also were measured in situ for inflow and outflow samples: pH and temperature were monitored in column experiments, whereas in batch experiments ORP, electrical conductivity, and dissolved oxygen also were monitored. Temperature, pH, and ORP were measured with a Kedida E201 probe and a WD-35805-15 Oakton probe, respectively, connected to a Hanna meter, model HI2550; electrical conductivity was monitored with a Corning Check Mate 90 conductivimeter, probe number 473621. Dissolved oxygen was measured with a YSI model 57 oxygen meter, 5421 series.
2.5 Statistical Analyses
2.5.1 Performance of the Column System
To analyze the performance of the column system, statistical tests were performed using IBM SPSS Statistics 22 (IBM Corporation 2013) and a significance level of α = 0.05. Among these tests, the Kolmogorov-Smirnov test was performed first to check data normality.
The effect of the column media type was statistically assessed by one-way ANOVA. Rather than a simple comparison between the two media types, we compared the inflow concentration and the outflow concentrations of As, Fe, Ca, Mg, and Al from all columns, so as to detect a significant difference (i.e., to know if the concentrations in the outflow are lower or higher than in the inflow). The same analysis was undertaken for the pH values. When a significant difference was found (ANOVA p < 0.05), multiple comparison post hoc tests were performed to distinguish any significant difference between the two media types: Tukey’s test was applied when the assumption of homogeneity of variances was satisfied (as determined by Levene’s test), or Games-Howell’s test when this assumption was not satisfied.
If the assumption of normality was not achieved, a Kruskal-Wallis analysis was performed instead of ANOVA. In these cases, when a significant difference was found between inflow and outflow concentration (Kruskal-Wallis p < 0.05), the Mann-Whitney post hoc test was performed to identify any significant difference between the two media types (Mann-Whitney p < 0.05).
2.5.2 Correlation Between Pollutant Levels
The Spearman rank ρ correlation coefficient was obtained to determine possible relationships between the concentration of As, metals, and pH. Spearman ρ was used instead of Pearson’s correlation due to the non-normal distribution of most of the data as shown by Kolmogorov-Smirnov test.
3 Results and Discussion
3.1 Media Characterization
Figure 1 presents the diffractograms for (a) zeolite and (b) limestone. These analyses confirmed the crystalline phases present in each medium type.
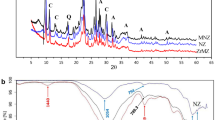
In addition, SEM-EDS analyses confirmed the elemental chemical composition of pure zeolite and also corroborated the presence of Fe as indicated in the datasheet (Table 1). The spectra and the images of the corresponding sample are presented in Fig. 2 for (a) pure zeolite and (b) exposed zeolite from a batch reactor. According to this, As is only present in the zeolite after being in contact with the contaminated water. There also was Fe retention, because its average atomic percentage increased from 1.67 (pure zeolite) up to 2.41 (exposed zeolite). The estimated elemental composition of the samples is presented in Table S1.
3.2 Batch Studies
3.2.1 Sorption Isotherms
The Langmuir isotherm sorption model (Eq. (1)) was employed to find the maximum sorption capacity q max for each media type. Model parameters q max and K were estimated by using the absolute least squares method (Sáez and Rittmann 1992).
In Eq. (1), C e corresponds to the equilibrium As concentration (analyzed in the supernatant at the end of the experiment, refer to Sect. 2.5).
According to the model, limestone has maximum sorption capacities that are an order of magnitude greater than those of zeolite, and this capacity is greater when the Fe concentration is greater (Fig. 3b). This effect was not observed in zeolite, because the value of q max is the same for both Fe concentrations (Fig. 3a). This indicates that Fe concentration does not affect As removal in zeolite. Because both As sorption (Armienta et al. 2012) and coprecipitation with Fe (Nilsson et al. 1994; Labastida et al. 2013) have been reported when removing As using limestone, a sorption isotherm might not be appropriate to describe the overall As removal process; however, it can be used to quantify and compare removal efficiencies of both media.
Arsenic sorption isotherm for zeolite (a) and limestone (b). (a) Langmuir model parameters q max = 0.17 and K = 1.76 for [Fe] = 50 mg/L; q max = 0.17 and K = 2.05 for [Fe] = 100 mg/L. (b) q max = 1.13 and K = 0.006 for [Fe] = 50 mg/L; q max = 1.3 and K = 0.007 for [Fe] = 100 mg/L. Each point represents an independent batch reactor using 1.5 g media, contact time = 18 h and T = 22 ± 1 °C
The main changes in water quality parameters monitored during the As sorption isotherm experiment are presented in Table S2. As expected, the pH increased markedly in the limestone reactors, but very slightly in the zeolite reactors. The pH also was monitored in the other batch experiments and a similar effect was detected (data not shown). Because zeolite increases pH due to H+ adsorption (Motsi et al. 2009), the results indicate that the contact time was not sufficient for this reaction to occur, and/or the mass of zeolite was low given the elevated H+ concentration. The electrical conductivity decreased in both reactor types, consistent with the decrease of the target pollutants. The increase in temperature can be explained by the agitation in the orbital shaker after the 18 h of duration of the experiment. The DO decreased in all reactors, while the ORP increased in zeolite reactors and decreased in limestone reactors. The oxidation of iron and the associated oxygen consumption can explain the change in DO levels. There is no clear effect of the initial As concentration on any parameter.
3.2.2 Effect of Contact Time
Results show an important effect of contact time on the removal efficiency in limestone reactors, with 100% As removal within 18 h for [Fe] = 50 mg/L (Fig. 4a), whereas for [Fe] = 100 mg/L, this time was within 3 h (Fig. 4b). These results confirm that the concentration of Fe plays a key role when removing As using limestone. Arsenic removal efficiencies up to 90 and 98% for 48 h contact time have been previously reported for limestone in batch experiments by Davis et al. (2007) and Romero et al. (2004), respectively.
Zeolite reactors only reached a maximum of 40% As removal for [Fe] = 50 mg/L after 42 h of contact time, with a modest removal increase through time for both Fe concentrations. Because the adsorption of As onto zeolite is highly dependent on contact time (Chutia et al. 2009; Li et al. 2011), these results indicate that the contact time might have not been sufficient to reach As equilibrium concentrations.
Similar results were found for Fe removal, as limestone could remove up to almost 100% Fe for both Fe concentrations (Fig. 5a, b), within a shorter time (18 versus 42 h) for the highest Fe concentration. This is in agreement with the Fe oxidation kinetics for pH >5.5, which is first order with respect to Fe concentration and oxygen partial pressure, and second order with respect to OH− (Snoeyink and Jenkins 1980). In fact, for the data presented in Fig. 5b, the difference between the actual data and the predicted by the model is negligible (considering pH = 6.2 and oxygen partial pressure = 0.21). Zeolite, however, presented much lower Fe removal efficiencies than limestone (10–20% with certain data dispersion, even presenting negative values for [Fe] = 50 mg/L), and they decreased instead of increasing through time, as opposed to the case of As. In this case, it is likely that the competition between H+ and Fe ions for sorption sites affected Fe sorption. Motsi (2010) reported that Fe removal using zeolite increased as contact time increased, but the pH was 3.5 and no other cations were in the solution. Thus, the presence and concentration of competing ions appear to be major factors affecting Fe sorption onto zeolite.
The final concentrations of Al, Si, and Mg were greater in zeolite reactors than in limestone reactors, and they increased as contact time increased (Fig. S2). This was expected, given the composition of zeolite and limestone (Sect. 2.1). Consistent with this, the concentration of Ca was greater in limestone reactors than in zeolite reactors, and it also increased as contact time increased. The implications of the release of these elements will depend on the target water quality: the Chilean guideline for drinking water only includes Mg (125 mg/L), whereas the irrigation guideline only includes Al (5 mg/L). No guideline value for hardness has been proposed by the WHO, because it is not a health concern. In fact, Ca levels between 100 and 300 mg/L could affect water taste (World Health Organization 2011). The main purpose of monitoring the concentration of Ca was as an indicator of calcite dissolution and thus the buffering capacity. The dissolution rate is a key factor that has been studied by some authors (e.g., Cravotta III and Trahan 1999; Santomartino and Webb 2007) and should be considered in estimating the lifespan of limestone-based treatment systems.
3.2.3 Effect of Media Concentration
The removal of As and Fe in batch experiments increased as the media concentration increased. In the case of limestone, only a mass concentration of 2 g/L was required to achieve removal efficiencies of 100% for As and 90% for Fe; whereas for zeolite, a mass concentration of around 20 times that of limestone was required to achieve similar removal efficiencies (Fig. S3).
This fact confirms that the main removal mechanism in zeolite is sorption, which is highly dependent on the available surface sites and thus the mass available. Similarly, Motsi (2010) reported that Fe removal increased up to around 100% as the zeolite concentration increased, for [Fe] = 400 mg/L. For As, Jeon et al. (2009) reported that the removal increased up to around 100% when increasing the zeolite concentration to 100 g/L, for [As] = 20.1 mg/L. Romero et al. (2004) reported that As removal increased as the limestone concentration increased, attributing sorption as the main removal mechanism.
3.2.4 Arsenic and Iron in the Solid Phase
According to XPS analyses (Figs. 6 and 7), As was only present in zeolite after being in contact with the contaminated water in the batch reactor, whereas Fe was present before and after. These analyses are consistent with the EDS analyses (Sect. 3.1) and the available information regarding the chemical composition of zeolite. Therefore, the presence of As in zeolite from the reactor can be attributed to sorption, whereas the presence of Fe can be attributed to its natural content and also to sorption.
In the exposed zeolite sample, the deconvolution of the centered peak from the As 3d signal revealed As was present as As(0) and As(III) only (Fig. 7b), whereas for the Fe 2p3/2, it was revealed that Fe was present as Fe(0), Fe(II), and Fe(III) (Fig. 7c). This indicates that Fe(II) could have reduced As(V), as Fe(II) was the species that was originally added to the acidic water (Sect. 2.3). This is supported by Šiljeg et al. (2009), who proposed that As(V) is reduced to As(III) while Fe(II) is oxidized to Fe(III), based on XPS analyses when investigating As(V) removal by iron-modified zeolite.
Even though many studies have investigated As removal by natural zeolite (Sect. 1), very few have probed the As speciation in the solid phase after the removal process, despite the fact that sorption of aqueous As(III) and As(V) onto zeolite has been reported. Because As(V) reduction to As(III) commonly occurs in microaerobic to anoxic environments (Inskeep et al. 2002), the presence of As(0) and As(III) as indicated by the XPS analyses seems unlikely given the environmental conditions of the experiment (Table S2). However, this could be possible, given the non-equilibrium behavior of the As(V)/As(III) couple (ibid).
The Fe oxidation states Fe(0), Fe(II), and Fe(III) coincide with those detected in the pure zeolite sample (Fig. 6), as reported by Wichterlová et al. (1982) within the structure of natural zeolite. To verify Fe retention and to discover any possible changes in Fe speciation caused by this retention, the relative content of Fe and the relative concentrations of each Fe species in both zeolites samples were obtained. These values were estimated by using the areas below the curves after the deconvolution of the Fe 2p3/2 photoelectron signal, and by using the intensities ratio between the Fe 2p3/2 and O 1s photoelectron signals from the corresponding XPS spectra, respectively (Table 2). Results indicate that the relative content of total Fe increased considerably after exposure to the contaminated water, and that Fe(III) followed Fe(II) in relative content after exposure, thus supporting the hypothesis of the As(V) reduction by the Fe(II) oxidation.
3.3 Column Studies
3.3.1 Overall Performance
Table 3 presents the overall performance of the column system. The concentrations of As and Fe in the outflow from both column types were lower than those in the inflow. Both had similar As removal rates (≅99%), but the Fe removal rate was greater in limestone columns than in zeolite columns (99 and 73%, respectively). As expected, the concentrations of Si, Mg, Ca, and Al in the outflow were greater than in the inflow, particularly Si concentration in the outflow from zeolite columns, and Ca concentration in the outflow from limestone columns. As mentioned in Sect. 3.2.2, this increase is due to the dissolution of limestone and zeolite, and also to the ion exchange capacity of zeolite. In addition, pH increased markedly in both column types, and was higher in the outflow from limestone cells than from zeolite cells. The fact that the pH values in the outflow from zeolite columns were higher than those in the zeolite batch reactors confirms the strong effect of contact time on zeolite reactivity, not only on As and Fe sorption but also on H+ sorption.
3.3.2 Arsenic and Iron Removal
Significant differences were found between As and Fe inflow and outflow concentrations (pKw < 0.001 in both cases). Both column types significantly decreased the concentrations of As and Fe (pMW < 0.001 for both cases), although limestone columns showed higher removal efficiencies than the zeolite columns (Table 2). Arsenic concentrations decreased and Fe concentrations remained steady over time in limestone columns; in zeolite columns, both As and Fe tended to increase through time (Fig. 8). The similarity of the As removal efficiencies between the two column types indicates that the q max obtained for zeolite in the sorption isotherm experiment was underestimated, because the nominal retention time was similar for all columns (9.5 and 8 days for zeolite and limestone columns, respectively). However, given the difference between inflow and outflow As concentrations (two orders of magnitude), the removal percentages do not represent performance of each column type properly. In fact, the As concentrations in the outflow from limestone columns were significantly less than those from zeolite columns (pMW = 0.029). As such, limestone has a greater As removal capacity than zeolite in both batch and column experiments. Because Fe concentrations in the outflow from limestone columns were significantly less than those from zeolite columns (pMW < 0.001), and Fe concentrations in batch limestone reactors were markedly less than those in zeolite reactors (Fig. 5), limestone also had a greater Fe removal capacity than zeolite in both batch and column experiments. Arsenic coprecipitation with iron oxyhydroxides has been proposed as the main As removal mechanism in limestone (Labastida et al. 2013), which explains the greater capacity of limestone to remove both As and Fe.
3.3.3 Al, Ca, Mg, and Si Release
Al, Ca, Mg, and Si concentrations in the outflow were greater than those in the inflow (pKw < 0.001 for Si, Ca, and Mg, pKw = 0.005 for Al). The concentrations of Ca, Mg, and Si increased significantly in zeolite (pMW < 0.001 for all cases) and limestone columns (pMW < 0.001 for all cases). Furthermore, these concentrations were significantly different between both column types (pMW = 0.003 for Al, and pMW < 0.001 for Si, Ca, and Mg). In batch zeolite reactors, the concentrations of Si and Mg were greater than those in limestone reactors, and the concentrations of Ca were smaller (refer to Sect. 3.2 and Fig. S2).
The concentration of Al increased significantly in the outflow from zeolite columns (pMW = 0.002) but did not increase in the outflow from limestone columns (pMW = 0.221). In the batch experiments, Al concentrations also were greater in zeolite reactors than in the limestone ones (Sect. 3.2). The concentrations of Al and Si increased markedly through time in the outflow from zeolite columns, whereas the concentrations of Ca and Mg did not. In limestone columns, the concentration of Al tended to increase through time, but the concentration of Si did not (Fig. S4).
These results confirm that the dissolution rates are higher for those elements which are major components of the corresponding media, i.e., Si, Al, and Mg for zeolite, and Ca for limestone. They also indicate that limestone could meet the Chilean irrigation guidelines for all elements but zeolite exceeded the maximum Fe concentration of 5 mg/L.
3.3.4 Changes in pH
The column experiments significantly affected the pH (ANOVA p < 0.001). The pH values in the inflow were significantly more acidic than those in the outflow from both column types (Games-Howell p < 0.001). In addition, the pH values in the outflow from limestone columns were significantly less acidic than those from zeolite columns (Games-Howell p < 0.001). In limestone columns, the outflow pH values remained fairly constant during the experimental period, whereas in zeolite columns, those values decreased through time. Temperature was not significantly affected by the column system.
The contact time appears as a key factor in the capacity of zeolite to raise pH, because it barely increased in the batch experiments (around 0.2–0.3 pH units, Table S2), but it increased significantly in the column experiments (between 3 and 4.5 pH units, Fig. S5).
3.3.5 Correlation Between Pollutant Concentrations
A positive correlation was detected between As and Fe levels in zeolite columns, while this correlation was negative in limestone columns. Spearman ρ correlation coefficients for significant correlations between the monitored parameters in both column types are presented in Table S3. It was expected that this correlation would be positive in the limestone columns because Fe precipitation causes As coprecipitation (Lizama Allende et al. 2011b). This inconsistency can be explained by the fact that most of the As and Fe measurements were below the detection limit, so only a few of them could be used for the statistical analyses. In fact, Fe oxides were observed throughout the limestone columns; thus, it is very likely that As was coprecipitated with Fe hydroxides (Labastida et al. 2013). In the case of zeolite, both positive and negative correlations have been reported when used as supporting media in VSSF and HSSF constructed wetlands (Lizama Allende et al. 2012; Lizama Allende et al. 2014). Different As removal mechanisms can occur: for example, As sorption onto Fe cations (Payne and Abdel-Fattah 2005) and/or onto Fe hydroxides (Jeon et al. 2009), which implies that both are removed together and thus giving a positive correlation between As and Fe levels; and the As exchange by aluminol or silanol hydroxyl groups (Chutia et al. 2009), which is not related to Fe levels but to pH levels. In fact, As and pH levels were negatively correlated in zeolite columns. This could indicate that the dominant mechanism is As sorption onto Fe cations rather than As exchange by hydroxyl groups, because higher hydroxyl concentrations imply more competition between arsenate and hydroxyl groups. In limestone columns, the same negative correlation between As and pH levels was expected, given that As is likely being removed when Fe is being removed; however, this correlation was not significant. Given the nature of the As and Fe data, results of the statistical analyses should be considered carefully.
A negative correlation was detected between pH and Fe levels in zeolite columns, consistent with the capacity of zeolite to sorb/exchange cations. The fact that both Fe and H+ concentrations increased through time could mean that they were removed by the same mechanism, and given the elevated concentrations of both in the inflow, the available sorption/exchange sites were decreasing. The solubility of Fe decreases as pH increases (Stumm and Morgan 1996); in fact, precipitation of Fe by zeolite has been reported by Motsi et al. (2009). This phenomenon probably occurred, although it could not be observed in the zeolite columns in the way it was observed in the limestone columns.
Given the effect of pH on Fe solubility, a negative correlation also was expected in limestone columns, but this correlation was not significant. This also can be attributed to the nature of the Fe measurements. In addition, the fact that neither pH values nor Fe concentrations presented a particular trend over time indicates that the duration of the experiment was not enough to detect the effect of limestone dissolution. Long-term studies are required to quantify the life span of the media; the capacity of limestone to provide alkalinity and thus to raise pH must be careful evaluated, since it has been reported that this capacity can decrease through time due to the deposition of iron hydroxides (Groudev et al. 2008), in addition to the dissolution of calcite. Because coating of limestone surface with iron hydroxides decreases its reactivity and thus its As removal capacity (Labastida et al. 2013), breakthrough experiments should also be performed to estimate the total arsenic removal capacity.
4 Conclusions
This study investigated the As and Fe removal capacity of zeolite and limestone in batch and column experiments. In the batch experiments, the As sorption capacities were adequately represented by the Langmuir isotherm and were 0.17 mg/g for zeolite and 1.3 mg/g for limestone at contact time of 18 h and media concentration of 6.3 g/L. However, given the higher removal efficiency of limestone, this was observed only for low As concentrations. In the column experiments, both media performed similarly, since the removal efficiencies for As were 99.5 and 99.8% in zeolite and limestone columns, respectively, whereas for Fe they were 73 and 99% respectively, for a hydraulic loading rate of 21.8 mm/day.
Results provide valuable information regarding the capacity of these media under highly acidic conditions (pH = 2) and elevated As concentrations (3 mg/L). Limestone appears to be the most effective medium, because it showed a greater As and Fe removal capacity than zeolite. It also showed a greater pH-raising capacity than zeolite in both batch and column experiments. In batch experiments, zeolite increased the pH between 0 and 0.3 pH units; whereas limestone increased the pH between 2.5 and 4.7 pH units (initial pH between 1.5 and 2, Table S2). In column experiments, zeolite increased the pH between 2.5 and 4.7 pH units; whereas limestone increased the pH between 5 and 6 pH units (initial pH ≅1.8, Fig. S5). This indicates that As coprecipitation with Fe oxides is more effective than sorption/ion exchange under the same experimental conditions.
This study showed the capacity of natural zeolite to remove As and Fe from highly acidic water in column experiments, which had not been reported previously, and also confirmed the capacity of limestone. As such, both media can be used as filter media and/or wetland media for this purpose. Limestone also could be used to remove As and metals from other types of contaminated water, because it has been found to be effective as a filter medium in storm water treatment (Cederkvist et al. 2010; Wium-Andersen et al. 2012). This indicates that processes other than coprecipitation with Fe can remove As. The key role of the supporting media in enhancing constructed wetlands performance has been recognized by different authors, not only for the removal of As and metals (e.g., Zurita et al. 2012; Lizama Allende et al. 2014) but also for the removal of other pollutants such as nitrogen (Saeed and Sun 2011) and phosphorus (Sakadevan and Bavor 1998). Therefore, this is an aspect that should be addressed to strengthen their application. Results indicate that limestone is more suitable, given its effectiveness; however, the final choice of the media will depend on several factors such as availability, costs, and capability of plants to adapt to the media. In addition, the changes in the As and Fe content and speciation in zeolite that could be identified in this study are the very first step to probe the removal mechanisms associated with the application of zeolite for removing As and Fe from highly acidic water, with the final aim of guiding the design of water treatment systems for this purpose.
References
Armienta, M. A., Micete, S., & Flores-Valverde, E. (2009). Feasibility of arsenic removal from contaminated water using indigenous limestone. In J. Bundschuh, M. A. Armienta, P. Birkle, P. Bhattacharya, J. Matschullat, & A. B. Mukherjee (Eds.), Natural arsenic in groundwaters of Latin America (pp. 505–510, Interdisciplinary Books Arsenic in the Environment, Vol. 1). Lisse, The Netherlands: CRC Press/Balkema Publishers.
Armienta, M. A., Villaseñor, G., Cruz, O., Ceniceros, N., Aguayo, A., & Morton, O. (2012). Geochemical processes and mobilization of toxic metals and metalloids in an As-rich base metal waste pile in Zimapán, Central Mexico. Applied Geochemistry, 27(11), 2225–2237. doi:10.1016/j.apgeochem.2012.01.015.
Baskan, M. B., & Pala, A. (2014). Batch and fixed-bed column studies of arsenic adsorption on the natural and modified clinoptilolite. Water, Air, and Soil Pollution, 225(1). doi:10.1007/s11270-013-1798-4.
Bakatula, E. N., Molaudzi, R., Nekhunguni, P., & Tutu, H. (2017). The removal of arsenic and uranium from aqueous solutions by sorption onto iron oxide-coated zeolite (IOCZ). Water, Air, and Soil Pollution, 228.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60(2), 309–319.
Canga, E., Kjaergaard, C., Iversen, B. V., & Heckrath, G. J. (2016). Agricultural drainage filters. I. Filter hydro-physical properties and tracer transport. Water, Air, and Soil Pollution, 227.
Cederkvist, K., Holm, P. E., & Jensen, M. B. (2010). Full-scale removal of arsenate and chromate from water using a limestone and ochreous sludge mixture as a low-cost sorbent material. Water Environment Research, 82(5), 401–408. doi:10.2175/106143009X12487095237314.
Cravotta III, C. A. (2010). Abandoned mine drainage in the Swatara Creek Basin, Southern Anthracite Coalfield, Pennsylvania, USA: 2. Performance of treatment systems. Mine Water and the Environment, 29(3), 200–216. doi:10.1007/s10230-010-0113-5.
Cravotta III, C. A., & Trahan, M. K. (1999). Limestone drains to increase pH and remove dissolved metals from acidic mine drainage. Applied Geochemistry, 14(5), 581–606.
Croghan, C. W., & Egeghy, P. P. (2003). Methods of dealing with values below the limit of detection using SAS. Presented at Southeastern SAS User Group, St. Petersburg, FL, September 22–24, 2003.
Chutia, P., Kato, S., Kojima, T., & Satokawa, S. (2009). Arsenic adsorption from aqueous solution on synthetic zeolites. Journal of Hazardous Materials, 162(1), 440–447.
Davis, A., Webb, C., Dixon, D., Sorensen, J., & Dawadi, S. (2007). Arsenic removal from drinking water by limestone-based material. Mining Engineering, 59(2), 71–74.
Elizalde-González, M. P., Mattusch, J., Einicke, W. D., & Wennrich, R. (2001). Sorption on natural solids for arsenic removal. Chemical Engineering Journal, 81(1–3), 187–195.
Groudev, S., Georgiev, P., Spasova, I., & Nicolova, M. (2008). Bioremediation of acid mine drainage in a uranium deposit. Hydrometallurgy, 94(1–4), 93–99.
Guerra, P., Gonzalez, C., Escauriaza, C., Pizarro, G., & Pasten, P. (2016). Incomplete mixing in the fate and transport of arsenic at a river affected by acid drainage. Water, Air, & Soil Pollution, 227, (3). doi:10.1007/s11270-016-2767-5.
IBM Corporation. (2013). SPSS statistics (p. 22).
Inskeep, W. P., McDermott, T. R., & Fendorf, S. (2002). Arsenic (V)/(III) cycling in soils and natural waters: Chemical and microbiological processes. In W. T. Frankenberg Jr. (Ed.), Environmental chemistry of arsenic (pp. 183–215). New York: Marcel Dekker, Inc..
Jain, C. K., & Singh, R. D. (2012). Technological options for the removal of arsenic with special reference to South East Asia. Journal of Environmental Management, 107, 1–18. doi:10.1016/j.jenvman.2012.04.016.
Jeon, C. S., Baek, K., Park, J. K., Oh, Y. K., & Lee, S. D. (2009). Adsorption characteristics of As(V) on iron-coated zeolite. Journal of Hazardous Materials, 163(2–3), 804–808. doi:10.1016/j.jhazmat.2008.07.052.
Kadlec, R. H., & Wallace, S. D. (2009). Treatment wetlands (second ed.). Boca Raton, FL: CRC Press.
Kröpfelová, L., Vymazal, J., Švehla, J., & Štíchová, J. (2009). Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech Republic. Environmental Pollution, 157(4), 1186–1194. doi:10.1016/j.envpol.2008.12.003.
Labastida, I., Armienta, M. A., Lara-Castro, R. H., Aguayo, A., Cruz, O., & Ceniceros, N. (2013). Treatment of mining acidic leachates with indigenous limestone, Zimapan Mexico. Journal of Hazardous Materials, 262, 1187–1195.
Leiva, E. D., Rámila, C. D., Vargas, I. T., Escauriaza, C. R., Bonilla, C. A., Pizarro, G. E., et al. (2014). Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed. Science of the Total Environment, 466-467, 490–502. doi:10.1016/j.scitotenv.2013.07.009.
Li, Z., Jean, J. S., Jiang, W. T., Chang, P. H., Chen, C. J., & Liao, L. (2011). Removal of arsenic from water using Fe-exchanged natural zeolite. Journal of Hazardous Materials, 187(1–3), 318–323. doi:10.1016/j.jhazmat.2011.01.030.
Liu, G., & Cai, Y. (2007). Chapter 31 Arsenic speciation in soils: an analytical challenge for understanding arsenic biogeochemistry. In R. D. Dibyendu Sarkar, & H. Robyn (Eds.), Developments in environmental science (pp. 685–708): Elsevier.
Lizama, A. K., Fletcher, T. D., & Sun, G. (2011a). Removal processes for arsenic in constructed wetlands. Chemosphere, 84(8), 1032–1043. doi:10.1016/j.chemosphere.2011.04.022.
Lizama, A. K., Fletcher, T. D., & Sun, G. (2011b). Enhancing the removal of arsenic, boron and heavy metals in subsurface flow constructed wetlands using different supporting media. Water Science and Technology, 63(11), 2612–2618. doi:10.2166/wst.2011.533.
Lizama, A. K., Fletcher, T. D., & Sun, G. (2012). The effect of substrate media on the removal of arsenic, boron and iron from an acidic wastewater in planted column reactors. Chemical Engineering Journal, 179, 119–130. doi:10.1016/j.cej.2011.10.069.
Lizama, A. K., McCarthy, D. T., & Fletcher, T. D. (2014). The influence of media type on removal of arsenic, iron and boron from acidic wastewater in horizontal flow wetland microcosms planted with Phragmites australis. Chemical Engineering Journal, 246(0), 217–228. doi:10.1016/j.cej.2014.02.035.
Marchand, L., Mench, M., Jacob, D. L., & Otte, M. L. (2010). Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: a review. Environmental Pollution, 158(12), 3447–3461.
Marshall, G., Ferreccio, C., Yuan, Y., Bates, M. N., Steinmaus, C., Selvin, S., et al. (2007). Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. Journal of the National Cancer Institute, 99(12), 920–928. doi:10.1093/jnci/djm004.
Motsi, T. (2010). Remediation of acid mine drainage using natural zeolite. University of Birmingham.
Motsi, T., Rowson, N. A., & Simmons, M. J. H. (2009). Adsorption of heavy metals from acid mine drainage by natural zeolite. International Journal of Mineral Processing, 92(1–2), 42–48. doi:10.1016/j.minpro.2009.02.005.
Nilsson, L., Pettersson, S., & Sandstrom, A. (1994). Iron and arsenic removal from bacterial leaching effluents by precipitation with limestone. Scandinavian Journal of Metallurgy, 23(4), 184–189.
Ocinski, D., Jacukowicz-Sobala, I., Mazur, P., Raczyk, J., & Kociolek-Balawejder, E. (2016). Water treatment residuals containing iron and manganese oxides for arsenic removal from water—characterization of physicochemical properties and adsorption studies. Chemical Engineering Journal, 294, 210–221. doi:10.1016/j.cej.2016.02.111.
Olmos-Márquez, M. A., Alarcón-Herrera, M. T., & Martín-Domínguez, I. R. (2012). Performance of Eleocharis macrostachya and its importance for arsenic retention in constructed wetlands. Environmental Science and Pollution Research, 19(3), 763–771. doi:10.1007/s11356-011-0598-x.
Pagnanelli, F., De Michelis, I., Di Tommaso, M., Ferella, F., Toro, L., & Vegliò, F. (2011). Treatment of acid mine drainage by a combined chemical/biological column apparatus: mechanisms of heavy metal removal. In Mine drainage and related problems (pp. 239-263): Nova science publishers, Inc.
Payne, K. B., & Abdel-Fattah, T. M. (2005). Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: effects of pH, temperature, and ionic strength. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering, 40(4), 723–749.
Qin, C., Liu, L., Han, Y., Chen, C., & Lan, Y. (2016). Mesoporous magnetic ferrum-yttrium binary oxide: a novel adsorbent for efficient arsenic removal from aqueous solution. Water, Air, and Soil Pollution, 227(9). doi:10.1007/s11270-016-3032-7.
Rahman, K. Z., Wiessner, A., Kuschk, P., van Afferden, M., Mattusch, J., & Müller, R. A. (2011). Fate and distribution of arsenic in laboratory-scale subsurface horizontal-flow constructed wetlands treating an artificial wastewater. Ecological Engineering, 37(8), 1214–1224.
Romero, F. M., Armienta, M. A., & Carrillo-Chavez, A. (2004). Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapán, México. Archives of Environmental Contamination and Toxicology, 47(1), 1–13.
Romero, F. M., Núñez, L., Gutiérrez, M. E., Armienta, M. A., & Ceniceros-Gómez, A. E. (2011). Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area, Mexico. Archives of Environmental Contamination and Toxicology, 60(2), 191–203. doi:10.1007/s00244-010-9544-z.
Saeed, T., & Sun, G. (2011). Enhanced denitrification and organics removal in hybrid wetland columns: Comparative experiments. Bioresource Technology, 102(2), 967–974. doi:10.1016/j.biortech.2010.09.056.
Sáez, P. B., & Rittmann, B. E. (1992). Model-parameter estimation using least squares. Water Research, 26(6), 789–796. doi:10.1016/0043-1354(92)90010-2.
Sakadevan, K., & Bavor, H. J. (1998). Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Water Research, 32(2), 393–399.
Santomartino, S., & Webb, J. A. (2007). Estimating the longevity of limestone drains in treating acid mine drainage containing high concentrations of iron. Applied Geochemistry, 22(11), 2344–2361.
Sarkar, A., & Paul, B. (2016). The global menace of arsenic and its conventional remediation—a critical review. Chemosphere, 158, 37–49.
Schwindaman, J. P., Castle, J. W., & Rodgers, J. H. (2014). Biogeochemical process-based design and performance of a pilot-scale constructed wetland for arsenic removal from simulated Bangladesh groundwater. Water, Air, & Soil Pollution, 225(6), 2009. doi:10.1007/s11270-014-2009-7.
Sheoran, A. S., & Sheoran, V. (2006). Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Minerals Engineering, 19(2), 105–116.
Šiljeg, M., Foglar, L., & Gudelj, I. (2012). The removal of arsenic from water with natural and modified clinoptilolite. Chemistry and Ecology, 28(1), 75–87. doi:10.1080/02757540.2011.619531.
Šiljeg, M., Stefanovic, S. C., Mazaj, M., Tušar, N. N., Arcon, I., Kovac, J., et al. (2009). Structure investigation of As(III)- and As(V)-species bound to Fe-modified clinoptilolite tuffs. Microporous and Mesoporous Materials, 118(1–3), 408–415. doi:10.1016/j.micromeso.2008.09.009.
Simsek, E. B., & Beker, U. (2014). Equilibrium arsenic adsorption onto metallic oxides : isotherm models, error analysis and removal mechanism. Korean Journal of Chemical Engineering, 31(11), 2057–2069. doi:10.1007/s11814-014-0176-2.
Singh, R., Singh, S., Parihar, P., Singh, V. P., & Prasad, S. M. (2015). Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicology and Environmental Safety, 112, 247–270. doi:10.1016/j.ecoenv.2014.10.009.
Snoeyink, V. L., & Jenkins, D. (1980). Water chemistry. New York: John Wiley & Sons.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry: chemical equilibria and rates in natural waters (3ed ed.). John Wiley & Sons, INC.
Wallace, S. D., & Knight, R. (2006). Small-scale constructed wetland treatment systems: feasibility, design criteria, and O&M requirements. Final report (p. 350). Alexandria, VA: Water Environment Research Foundation (WERF) and IWA Publishing.
Wang, J. W., Bejan, D., & Bunce, N. J. (2003). Removal of arsenic from synthetic acid mine drainage by electrochemical pH adjustment and coprecipitation with iron hydroxide. Environmental Science and Technology, 37(19), 4500–4506.
Wichterlová, B., Kubelková, L., Nováková, J., & Jíru, P. (1982). Behaviour of Fe species in zeolite structure. Studies in Surface Science and Catalysis, 12, 143–150.
Williams, M. (2001). Arsenic in mine waters: international study. Environmental Geology, 40(3), 267–278.
Wium-Andersen, T., Nielsen, A. H., Hvitved-Jacobsen, T., Kristensen, N. K., Brix, H., Arias, C., et al. (2012). Sorption media for stormwater treatment—a laboratory evaluation of five low-cost media for their ability to remove metals and phosphorus from artificial stormwater. Water Environment Research, 84(7), 605–616. doi:10.2175/106143012X13373550426832.
World Health Organization (2011). Guidelines for drinking-water quality, fourth edition. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/ . Accessed 22 September 2016.
Zipper, C. E., & Skousen, J. G. (2010). Influent water quality affects performance of passive treatment systems for acid mine drainage. Mine Water and the Environment, 29(2), 135–143. doi:10.1007/s10230-010-0101-9.
Zurita, F., Del Toro-Sánchez, C. L., Gutierrez-Lomelí, M., Rodriguez-Sahagún, A., Castellanos-Hernandez, O. A., Ramírez-Martínez, G., et al. (2012). Preliminary study on the potential of arsenic removal by subsurface flow constructed mesocosms. Ecological Engineering, 47, 101–104. doi:10.1016/j.ecoleng.2012.06.018.
Acknowledgements
We thank Prof. Francisco Gracia from the Department of Chemical Engineering and Biotechnology, Universidad de Chile, for the BET analyses; Prof. César Pastén from the Department of Civil Engineering for the media characterization; Andrés Ibáñez from the Physics Department for the XRD analyses; Fernando Guzmán and Prof. Víctor Fuenzalida from the Physics Department for the XPS analyses; and Christian Nievas from the Andean Geothermal Centre of Excellence (CEGA) for the EDS analyses. We also thank Viviana Lorca and Natalia Fuentes for their support in the experiments, Felipe Proschle and José Ayala for their cooperation in some sections of the manuscript, and Prof. Marcelo Olivares for his comments on an early version of this manuscript.
Funding
This work was supported by the Department of Civil Engineering, the Concurso U-Inicia VID 2014, Universidad de Chile, and by CONICYT FONDECYT initiation into research grant 11150401.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
ESM 1
(PDF 423 kb)
Rights and permissions
About this article
Cite this article
Lizama-Allende, K., Henry-Pinilla, D. & Diaz-Droguett, D.E. Removal of Arsenic and Iron from Acidic Water Using Zeolite and Limestone: Batch and Column Studies. Water Air Soil Pollut 228, 275 (2017). https://doi.org/10.1007/s11270-017-3466-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3466-6