Abstract
In the Taxco mining area, sulfide mineral oxidation from inactive tailings impoundments and abandoned underground mines has produced acid mine drainage (AMD; pH 2.2–2.9) enriched in dissolved concentrations (mg l−1) sulfate, heavy metals, and arsenic (As): SO4 2− (pH 1470–5454), zinc (Zn; 3.0–859), iron (Fe; pH 5.5–504), copper (Cu; pH 0.7–16.3), cadmium (Cd; pH 0.3–6.7), lead (Pb; pH < 0.05–1.8), and As (pH < 0.002–0.6). Passive-treatment systems using limestone have been widely used to remediate AMD in many parts of the world. In limestone-treatment systems, calcite simultaneously plays the role of neutralizing and precipitating agent. However, the acid-neutralizing potential of limestone decreases when surfaces of the calcite particles become less reactive as they are progressively coated by metal precipitates. This study constitutes first-stage development of passive-treatment systems for treating AMD in the Taxco mine area using indigenous calcareous shale. This geologic material consists of a mixture of calcite, quartz, muscovite, albite, and montmorillonite. Results of batch leaching test indicate that calcareous shale significantly increased the pH (to values of 6.6–7.4) and decreased heavy metal and As concentrations in treated mine leachates. Calcareous shale had maximum removal efficiency (100%) for As, Pb, Cu, and Fe. The most mobile metals ions were Cd and Zn, and their average percentage removal was 87% and 89%, respectively. In this natural system (calcareous shale), calcite provides a source of alkalinity, whereas the surfaces of quartz and aluminosilicate minerals possibly serve as a preferred locus of deposition for metals, resulting in the neutralizing agent (calcite) beings less rapidly coated with the precipitating metals and therefore able to continue its neutralizing function for a longer time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intense mining operations of zinc (Zn), lead (Pb), and silver ore deposits have existed in the Taxco mining area, in southern Mexico, for several centuries. These operations have generated approximately 30 millions of tons of sulfide-rich mine waste (Talavera et al. 2005).
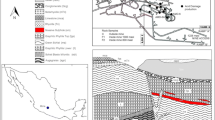
The Taxco mining area has been one of the main important base- and precious-metal producers since pre-Hispanic times (Talavera et al. 2005). The mineralization mainly appears in hydrothermal veins, replacement ores, and stockworks hosted in limestone, calcareous shale, and schist (Fig. 1). The principal sulfide minerals in the area are pyrite (10% to 15%), sphalerite (11%), and galena (4%). Among the main gangue minerals are quartz, calcite, and feldspar. Other primary sulphide minerals in veins throughout the Taxco area are chalcopyrite, argentite, pirargite, proustite, and arsenopyrite. The ore also contains minor quantities of goethite, hematite, cerusite, anglesite, melanterite, and barite. The Taxco area is located at an altitude of 1700 m. It has a warm and subhumid climate that averages 1000 mm of precipitation annually and a mean annual temperature of 28°C.
According to Armienta et al. (2003), Talavera Mendoza et al. (2006), and Romero et al. (2007), the oxidation and dissolution of sulfide minerals within mine waste have produced “acid mine drainage” (AMD). This AMD is characterized by low pH values (pH < 3) and high levels of dissolved arsenic (As) and heavy metals, which are considered of environmental and toxicologic concern. AMD emanating from abandoned underground mines has also been identified in this area (Nuñez Alvarez 2008).
AMD has been recognized as the main environmental problem derived from mining activities (Dold and Fontbote 2001; Holmstrom et al. 2001; Costello 2003). The released potentially toxic elements (PTEs) may be transported to the surrounding environment and contaminate soils, sediments, ground, and surface waters (Bain et al. 2000; Armienta et al. 2001; Jung 2001). It is important to emphasize that in many places around the world it has been reported that in addition to AMD resulting from mining wastes, these acid solutions are also produced naturally in mineralized zones. Among these zones can be listed Iron Mountain, California (Nordstrom and Alpers 1999); Sangre de Cristo Mountains, New Mexico (Shaw et al. 2003); Macmillan Pass, Yukon, Canada (Kwong and Whiteley 1992); and Rio Tinto, Spain (Galan et al. 1999).
Considering that AMD is a significant and costly environmental concern in the mining industry, increasing attention has been paid to studying the generation and control of AMD (1) to develop an effective treatment to prevent damage to wildlife or their habitats and to other natural resources as well as (2) to protect public health and safety.
A variety of active and passive technologies for treating AMD have been widely reported. However, active treatment of AMD to remove PTEs and acidity is often an expensive, long-term liability (Younger 2000; López-Pamo et al. 2002). In recent years, a variety of passive-treatment systems have been developed, and they do not require continuous chemical inputs and have the advantage of requiring relatively little maintenance compared with active systems (Johnson and Hallberg 2005). The primary passive technologies include constructed wetlands, anoxic limestone drains, successive alkalinity-producing systems, limestone ponds, and open limestone channels.
The main objective of these passive technologies is the neutralization of AMD to favour the formation of insoluble species and retention of metallic cations (López-Pamo et al. 2002; Simon et al. 2005). Other passive technologies include treatment system using pelletized hydrous ferric oxide as a sorbent for removing Zn from circumneutral mine waters (Mayes et al. 2009). In addition, the use of caustic magnesia (low-cost reagent) has been reported as an alternative material for passive-remediation systems to remove divalent metals from acid solutions (Rötting et al. 2006).
Many investigators have reported the successful application of passive-treatment systems. Ziemkiewicz et al. (2003) reported the long-term performance of AMD-treatment systems in many places from the eastern United States. At the Nickel Rim site (Canada), Benner et al. (1999) reported that use of permeable reactive barriers have been effective for the treatment of AMD.
Passive-treatment systems provide an ideal approach for treating AMD because they have the advantage of relatively low cost (López-Pamo et al. 2002; Johnson and Hallberg 2005). Most of the reported systems address the removal of common heavy metals; however, due to the high potential ecologic risk caused by metalloids, such as As, there is a need to develop practical and affordable passive AMD-treatment procedures for As removal from these effluents. Limestone is the geologic material commonly used in both active and passive AMD treatment. Treatment of AMD with limestone allows increasing the pH of drainage and decreases the mobility of heavy metals (Cravotta and Trahan 1999; Simon et al. 2005; Santomartino and Webb 2007; Cravotta 2008). However, some researchers have postulated that the acid-neutralizing potential of limestone decreases with time due to the inevitable metallic precipitation on limestone surface and its tendency to develop an external coating, or armor, of ferric hydroxide when added to AMD (Johnson and Hallberg 2005; Santomartino and Webb 2007; Cravotta 2008). The local geology of the Taxco mining area is dominated by calcareous shale (Fig. 1).
This investigation was carried out to study the capacity of this indigenous geologic material, using batch-leaching tests, to neutralize the acidity and activate the precipitation and sorption of As and heavy metals. This research will provide useful information for the experimental design of long-term laboratory tests to estimate the longevity of calcareous shale in treating AMD in the Taxco mining area, which is essential for implementing this passive-treatment technology at the field scale.
Materials and Methods
Sampling
Calcareous shale from outcrops distributed in two different zones of the Taxco area was selected for sampling. Five outcrops were sampled: A1, A2, A3, A4, and A5 (Fig. 1). At each site, four subsamples, 1.0 kg each, were taken within a 10-m circular area radius. In the laboratory, the rock samples were dried and crushed to 10 mesh. Five composite samples were prepared by mixing and quartering the four subsamples taken at each outcrop for chemical and mineralogical analyses. Two batch reactor systems (BR1 and BR2) were built by mixing composite rock samples.
Two kind of acid mine leachates were used for the batch experiments: (1) natural mine leachate discharges from oxidized tailings (SL1 in Fig. 1) and underground mine (SL2 in Fig. 1); and (2) synthetic mine leachates from composite tailing samples (T1–T4 in Fig. 1).
Synthetic mine leachates from tailing samples were prepared to assess the chemical composition of leachates that could be generated over the whole tailings dam because natural leachate discharge only was observed at the extreme northern part of the dam. These synthetic mine leachates were prepared from superficial oxidized tailing samples (0- to 20-cm deep) using water in equilibrium with the atmosphere and according to the procedure described by American Society for Testing and Materials D3987-85 method (ASTM 1985). Four mine leachates were prepared from composites of four tailings subsamples collected from traverses across the oxidized dam (T1–T4 in Fig. 1).
Methods
Batch leaching experiments (BR1 and BR2) were performed to determine the potential of indigenous calcareous shale to increase pH and remove As and heavy metals of acid mine leached from the Taxco mining area. Batch tests were carried out using the following ratio of solution to solid: 20:1 (140 ml acid mine leachates and 7 g crushed calcareous shale). Sample suspensions were equilibrated for 18 ± 0.25 h in the batch reactors and continuously shaken to maintain a constant solution-to-solid ratio. After the equilibration time, the suspension was centrifuged for 15 min at 3000 rpm. The supernatant was filtered through a 0.45-μm membrane, transferred to a vial, and stored at 4°C until chemical analyses were performed. Duplicates were run throughout these experiments. This method has been successfully used to study the capacity of As removal by natural geologic materials in mining zones (Carrillo and Drever 1998; Romero et al. 2007; Armienta et al. 2008).
Chemical and Mineralogical Analyses
The PTEs of solid samples were analyzed with inductively coupled plasma–atomic emission spectroscopy (ICP-AES) according to United States Environmental Protection Agency (EPA) 6010 A method (USEPA 1996). The analyses of solid samples were performed after microwave-assisted acid digestion according to USEPA 3051 procedure (USEPA 1994).
Before batch experiments, acid mine leachates and aqueous extracts were analyzed for pH, electrical conductivity, PTEs, and sulfates. In addition, aqueous extracts before batch experiments were analyzed for calcium (Ca) and bicarbonates. The pH was measured in an Accumet pH meter (Fisher Scientific), and electric conductivity was measured using a conductivity meter (model 441; Corning, NY). Potentially toxic elements were analyzed using ICP-AES. Sulfate was determined by turbidimetric method through the formation of BaSO4 by adding BaCl2, and bicarbonates were measured by acid titration.
A quality control was implemented and included blanks, duplicate samples, certified reference materials, and certified standards. One blank was analyzed for every three samples, and the concentrations of analyzed elements were lower than detection limits. Analyses of duplicates on five samples showed precision error rates between 3% and 7%. The recoveries of PTEs in the standard reference materials (sulfide ore mill tailing RTS-3 Canadian standard) were in the range of 93% to 112%.
In addition, solid samples were analyzed by X-ray diffraction (XRD) and scanning electron microscopy with energy dispersive X-ray spectrometry (SEM–EDS). XRD was performed with a Siemens D5000 with Cu Kα radiation (λ = 1.5406 A), and SEM–EDS analyses were performed using a JEOL JXA-8900R superprobe in SEM mode to obtain backscattered images.
Geochemical Modeling
Geochemical modeling of the aqueous extracts was performed using the MINTEQA2 (Allison et al. 1991) computer code. Concentrations determined in leachates were used to calculate ionic strength by the model. Activity coefficients were calculated with the Davies equation. MINTEQA2 terminates the calculations if the charge balance exceeds 10%. Saturation indexes were computed with the model considering a temperature of 25°C. Ksp values were taken from the database of the geochemical program.
Results and Discussion
Mineralogical and Chemical Characterization of Calcareous Shale and Tailings
Based on XRD patterns, calcareous shale is composed of quartz, calcite, and aluminosilicate minerals as muscovite, albite, and montmorillonite (Table 1). Chemically, calcareous shale is a material rich in silicon (Si; 69.5–77.3 wt%), Ca (14.4–23.5 wt%), and aluminum (Al; 2.2–2.4 wt%) and has a lower content of other aluminosilicate-bound elements, such as magnesium (Mg; 0.4–0.9 wt%), sodium (Na; 0.02–1.10 wt%), potassium (K; 0.06–0.78 wt%), and manganese (Mn; 0.03–0.07 wt%). It is important to indicate that this indigenous geologic material has low concentrations of sulfide-bound elements: As (2–12 mg kg−1), Cd; <1.0 mg kg−1), copper (Cu; 20–42 mg kg−1), iron (Fe; 2.3–3.6 wt%), Pb (20–47 mg kg−1), and Zn (46–89 mg kg−1) (Table 1).
These results indicate that calcareous shale of the studied area is characterized by the presence of minerals with high and moderate acid-neutralization potential. Calcite dissolves rapidly, and its dissolution can maintain circumneutral pH conditions. Relative to Ca carbonate minerals, aluminosilicate minerals dissolve slowly and maintain neutral pH conditions when carbonate minerals have been depleted (Lin 1997). In contrast, montmorillonite presence is important for treatment of AMD due to its great capacity for heavy-metal retention (Takahasi and Imai 1983; Abollino et al. 2003).
From Table 1,it is evident that oxidized mine tailings of the Taxco area are characterized by high total concentrations of sulfide-bound elements, with average values of 2383 mg kg−1 As, 9.5 wt% Fe, and 3695 mg kg−1 Pb. Total concentrations of other heavy metals are relatively low (49 mg kg−1 Cd, 162 mg kg−1 Cu, and 814 mg kg−1 Zn). Alkali elements, such as Ca (4.6% wt), Mg (336 mg kg−1), Mn (197 mg kg−1), Na (98 mg kg−1), and K (920 mg kg−1), show relatively low concentrations due to losses of carbonate and aluminosilicate minerals during weathering of mine tailings.
XRD studies of oxidized tailings showed that the primary minerals are dominated only by quartz and microcline. Sulfide and carbonate minerals were not observed. Oxidation and acid-neutralization reactions have depleted the content of other primary sulfides and carbonates in these oxidized tailings, thus allowing the formation of secondary minerals. XRD analysis showed that the secondary mineralogy of oxidized tailings is dominated by gypsum and jarosite. Other secondary minerals detected were goethite and lepidocrocite.
SEM–EDS analysis showed small (<10 μm) dense particles scattered throughout the mine waste that contain sulfide-bound elements. Three distinct types of particles containing these elements were identified: (a) Fe–S-As, (b) Fe–S-Pb-Cu, and (c) Fe–S-Pb-Cu-As (Fig. 2). The presence of Si, Al, Ca, and K detected in these particles is due to the occurrence of bulk quartz and aluminosilicate in the matrix samples. Particles (a) and (b) can be associated with the secondary Fe minerals and traces of As, Pb, and Cu, whereas, particles (c) can correspond to beundantite, which has been previously identified in these studied tailings (Romero et al. 2007).
Potentially Toxic Elements in Mine Leachates
Table 2 lists the pH and redox potential values (Eh), conductivity, and concentrations of dissolved Ca and PTEs in mine leachates from the Taxco area. The waters discharged from underground mine have the lowest pH (2.5), the highest conductivity values (6.0 mS/cm), and the highest dissolved concentrations of sulfates (5454 mg l−1 mg l−1) and PTEs: 858.9 mg l−1 Zn, 504 mg l−1 Fe, 16.3 mg l−1 Cu, 6.7 mg l−1 Cd, 1.8 mg l−1 Pb, and 0.65 mg l−1 As.
A general decrease in the dissolved concentration, in mine leachates generated by oxidized tailings, was observed across all elements considered (Table 2). Natural leachate discharges from tailings are acidic (pH 2.7) and contain 2469 mg l−1 SO4 2−, 313.5 mg l−1 Zn, 39.7 mg l−1 Fe, 7.4 mg l−1 Cu, 3.6 mg l−1 Cd, 0.01 mg l−1, Pb, and <0.0002 mg l−1 As. Nevertheless, in acidic synthetic leachates (pH 2.8), the lowest concentrations of the studied elements were achieved and reached averages values of 1550 mg l−1 SO 24 , 8.8 mg l−1 Zn, 20.5 mg l−1 Fe, 1.8 mg l−1 Cu, 0.5 mg l−1 Cd, <0.005 mg l−1 Pb, and 0.08 mg l−1 As.
These results indicate that dissolved concentrations of Zn, Cd, and Cu in natural leachates, are higher than those determined in synthetic leachates by factor of 103, 11, and 10, respectively. This discrepancy suggests that the standard test method used for extraction of solid waste with water, as described by ASTM D3987-85 method (ASTM 1985), does not work properly to obtain synthetic leachates from the studied tailings because it did not allow the complete transition of water-soluble PTEs of tailing samples to the synthetic leachates.
The poor extraction efficiency may be due to the short duration (18 h) of the shaking period of tailings-water suspension, i.e., it was not enough to reach equilibrium. This hypothesis is supported by reported results in previous studies conducted in the same tailings dam (Romero 2004; Romero et al. 2007).
Romero et al. (2007) generated synthetic leachates from superficial oxidized tailing samples (0- to 20-cm deep) in this same tailings dam, and they used a shaking time of 8 days to reach equilibrium of tailings-water suspension. The dissolved concentrations of Zn (107–432 mg l−1), Cd (1.1–4.5 mg l−1), and Cu (2.1–18.3 mg l−1) reported in these synthetic leachates are similar to those determined in natural leachate discharges from oxidized tailings reported in this article.
These same investigators stated that the PTEs, as Fe, Zn, Cd, and Cu released from the oxidized acidic tailings near the surface, have partially migrated downward through the tailings dam and, therefore, leachates from deeper oxidized tailings contain higher dissolved concentrations of PTEs compared with tailing leachates near the surface. This implies that if any runoff is infiltrated through the deeper oxidized tailings, there is possible generation of tailing leachates with higher dissolved concentrations of PTEs than those reported in this article. Therefore, this should be taken into account in the sampling strategy for further studies in these tailings dam.
In addition to, it is important to emphasize that As and Pb concentrations in natural leachate discharges from tailings are low, as in the synthetic leachates, which implies that dissolved concentrations of these elements have been naturally attenuated. According to the results of the MEB-EDS analyses, this is likely to be due to precipitation of beudantite and scavenging of As and Pb by secondary Fe minerals (Fig. 2).
The decrease in the dissolved concentrations of Zn, Cd, and Cu in the tailing leachates may be due to jarosite precipitation, which was identified in the studied tailings by XRD analysis. Jarosite can act as a sink for heavy metals by way of sorption processes (McGregor et al. 1998; Romero et al. 2007).
Batch Leaching Test
Results of acidic mine leachates treatment in BR1 and BR2 are listed in Table 2.After treatment, the pH values in mine leachates increased from 2.5 to 2.9 to 6.7 to 7.4 (BR1) and from 6.6 to 7.3 (BR2) due to the fast dissolution of calcite, which was identified by DRX (Fig. 3a), which gives indigenous calcareous shale an excellent neutralizing potential. The dissolution of calcite is reflected in the relatively high concentrations of Ca and bicarbonate in leachates after treatment (Table 2).
a XRD patterns of indigenous calcareous shale before batch leaching test Mnt = montmorillonite; Qz = quartz; Ab = albite; Ca = calcite. b SEM-EDS analysis of indigenous calcareous shale after batch leaching test. B1 shows particles composed mainly of Fe, Si, Al, with traces of Cu, Ca, K, and Mg. B2 shows calcite particles that do not show evidence of being coating by metal precipitates. Arrows show location of analyzed particles (B1 and B2) by SEM-EDS
Removal efficiency of indigenous calcareous shale in both batch reactor systems (BR1 and BR2) followed the sequence As = Pb = Cu = Fe > Zn > Cd > SO4 2−. This geologic material has maximum removal efficiency (100%) for As, Pb, Cu, and Fe. Nevertheless, Cd and Zn were the most mobile elements with respect to the other metal ions studied, and their average percentage removal was 87% and 89%, respectively.
Eh values of acid mine leachates before treatment varied between 280 and 646 mV, indicating oxidation conditions. Under acidic and oxidizing conditions, dissolved Fe occurs as ferric ion (Fe III) (Doye and Duchesne 2003). As pH increases due to the dissolution of calcite, in batch reactors systems, ferric ion is subjected to hydrolysis, which results in the precipitation of amorphous Fe-oxyhydroxides (Gazea et al. 1996). According to MINTEQA2 calculation, the treated mine leachates are oversatured with regard to ferryhidrite (SI 4.0–5.3), goethite (SI 8.4–9.7), hematite (SI 21.7–24.4), H-jarosite (SI 4.6–12.2), maghemite (SI 11.3–14.0), and lepidocrocite (SI 7.5–8.8). Possible precipitation of these minerals explains the successful removal of Fe from the leachates after treatment. Several investigators have demonstrated that the neutral conditions reached in passive-treatment systems after calcite dissolution trigger precipitation of Fe-oxyhydroxides (Xu et al. 1997; Cravotta and Trahan 1999; Sasowsky et al. 2000; Simon et al. 2005).
Precipitation of Fe-oxyhydroxides and near-neutral pH values in treated leachates promoted the sorption of dissolved As, Cd, Cu, Pb, and Zn on the surface of Fe-oxyhydroxides as indicated by decreased concentrations of these PTEs in leachates after treatment. SEM–EDS analysis showed the presence of traces of Cu in Fe oxyhydroxide grains (Fig. 3b1), which suggests that dissolved PTEs may be sorbed onto the surface of Fe-oxyhydroxides. Many investigators have reported that Fe-oxyhydroxides play an important role on the removal of trace metals (e.g., Cu, Zn, Cd, Pb, and As) from mine drainage (Courtin-Nomade et al. 2003; Dold and Fontbote 2001; Foster et al. 1998). One recent study has actually trialed this as a passive-treatment application for Zn removal from circumneutral mine waters using pelletised hydrous ferric oxide (Mayes et al. 2009a).
In addition, quartz and montmorillonite, which are two of the major minerals identified in calcareous shale, may play an important role in heavy-metal sorption under the practically neutral conditions (pH 6.6–7.4) of mine leachates after treatment. The reported point of zero charge of quartz and montmorillonite is 2.9 and 2.5, respectively (Appelo and Postma 2005). This means that under neutral conditions of the mine leachates, the surface charge of quartz and montmorillonite is negative, and, therefore, heavy-metal sorption on the surface of these minerals is favored.
In addition to metal sorption onto mineral surfaces, precipitation of some solid phases could be another important control in metal removal from the treated leachates. Some investigators have reported that the neutral conditions reached in passive-treatment systems after calcite dissolution triggered precipitation of Cu(OH)2 and Zn(OH)2 (Xu et al. 1997; Doye and Duchesne 2003). The MINTEQA2 calculation indicates that the near-neutral treated leachates are undersaturated with regard to Cd (OH)2 (SI –0.6 and –0.1), Cu(OH)2 (SI –0.1 and –0.6), Zn(OH)2 (SI –1.8 and –3.5), as well as all discrete Pb- and As-bearing secondary minerals included in the database (Table 3), and, therefore, it can be assumed that concentrations of Cd, Cu, Zn, Pb, and As are mainly removed by sorption onto mineral surfaces. However, the calculated SI values show that precipitation of CuO (SI 0.4–1.3) and otavite (CdCO3; SI 1.1–1.9) may contribute to the removal of Cu and Cd, respectively.
Saturation indices calculated for Zn carbonates indicate that near-neutral treated leachates are undersaturated (SI –1.3 and –0.7) and near saturated (SI 0.3 and 0.5) with respect to smithsonite (ZnCO3), so we could assume that ZnCO3 precipitation is not an important control in the removal of Zn from treated leachates (pH 6.7–7.4). This supports the hypothesis that the main mechanism for removal of Zn in treated leachates from study site is sorption onto mineral surfaces.
In circumneutral mine sulfate-rich water, Zn may be present as a carbonate complex (ZnCO3º) and sulfate complex (ZnSO4º) (Mann and Deutscher 1980; Nuttall and Younger 2000). Because these complexes are zero-charged, they have a small tendency to be retained by sorption processes. In contrast, under near-neutral conditions, Zn-sulfate solid phases are extremely soluble (Mann and Deutscher 1980), and Nuttall and Younger (2000) reported that Zn can form a stable carbonate mineral (ZnCO3) over a narrow pH range (approximately pH 8.0–8.5). This could explain that although the removal efficiency of Zn in treated leachate discharges is relatively high (53% to 100%), the residual concentration of dissolved Zn after treatment still remains still high (≤284.7 mg l−1) (Table 2).
Considering that these concentrations of Zn can be toxic to aquatic life (Mayes et al. 2009), it is necessary to explore options for the treatment of residual Zn in near-neutral leachates that are formed after treatment. According to Nuttall and Younger (2000), to remove Zn from circumneutral mine leachates by ZnCO3 precipitation, it is necessary to increase the pH of the treatment system to approximately to 8.2. We are currently conducting research to assess the optimum way to increase pH to remove residual Zn from the solution produced after treatment of acid leachates with calcareous shale from Taxco mine area.
Finally, our results indicate that calcareous shale has a low capacity of sulfate removal. The percentage of sulfate removal varied between 0.5 and 15.4 (BR1) and between 0.6 and 4.7 (BR2) in mine tailings leachates, whereas in natural leachates discharged from underground mine sulfate removal was 53% (BR1) and 54% (BR2). According to MINTEQA2 calculation, the treated leachates are near saturated (SI 0.1–0.4) with respect to gypsum. However, high dissolved concentrations of residual sulfates in treated leachates (1416–2528 mg l−1) indicate that the potential precipitation of gypsum is not important within the treatment system to achieve significant sulfate removal.
Potential Efficiency of Indigenous Calcareous Shale for Treating AMD in the Taxco Mine Area
Passive-treatment systems based on the dissolution of calcite are widely used to remediate AMD. However, they tolerate only low metal concentrations or acidity loads because they are prone to passivation when surfaces of calcite particles become less reactive as they are progressively coated by Fe-precipitates (Johnson and Hallberg 2005; Simon et al. 2005; Santomartino and Webb 2007).
The use of calcareous shale in the treatment of the acid leachates from Taxco mine area can help overcome the problems related whith calcite-based acid-drainage treatment systems because our mineralogical data indicate that calcareous shale from the studied area is composed of quartz, calcite, and aluminosilicate minerals (Fig. 3a). In this solid mixture, calcite provides an alkalinity source, whereas quartz and aluminosilicate minerals can play the role of precipitating agents that serve as preferred nuclei of deposition for the metals precipitated by the neutralizing agent, i.e., metals precipitated from the aqueous solution during neutralization.
However, in this study, the effects of the solid mixture of quartz, calcite, and aluminosilicate minerals on removal efficiency were not evident due to the short duration of the experiment. We speculate that quartz and aluminosilicate minerals serve to preferentially attract the precipitating metals to a greater degree than calcite (neutralizing agent), with the effect that the neutralizing agent is at least less rapidly coated by the precipitating metals and can therefore continue its neutralizing function for a more significant amount of time. A study by Xu et al. (1997) reported interactions between typical acid mine water and a solid mixture of calcite and quartz, and they concluded that quartz surfaces become a preferred place for precipitation of produced metal oxides.
SEM–EDS analysis of calcareous shale after treatment did not show evidences of coated calcite grains by Fe-precipitates or gypsum (Fig. 3b2). However, these analyses allowed the identification of small (<2 μm) dense particles composed mainly of Fe, Si, and Al, with traces of Cu, Ca, K, and Mg (Fig. 3a), suggesting that aluminosilicate minerals can serve as a preferred locus of metal deposition.
Calcite is the main neutralizing agent present in calcareous shale and due to its fast dissolution, we consider that when calcite minerals are depleted, identified aluminosilicate minerals, e.g., muscovite (KAl2(AlSi3O10)OH)2) and albite (NaAlSi3O8), become significant H+-consuming phases.
The role of aluminosilicate minerals in neutralizing acid mine leachates has been generally underestimated. This is probably due to the fact that aluminosilicate mineral reaction rates are slow and thus more difficult to assess using tests of short duration as those carried out in this study. Therefore, this phenomenon must be further studied to better support the previously mentioned considerations. It is thus necessary to conduct a kinetic study of long duration.
The results presented in this article will be used for the experimental design of long-term laboratory tests to estimate the longevity of calcareous shale in treating AMD in the Taxco mining area, which is essential to implement this passive-treatment technology at the field scale.
Conclusion
Leachates from the Taxco mine area are acidic and are characterized by high contents of sulfates and dissolved PTEs, such as Zn, Fe, Cu, Cd, and As. The highest metal enrichment occurs in leachates discharging from underground mine. In the acidic tailings, leachates had low concentrations of As and Pb, which implies that dissolved concentrations of these elements have been naturally attenuated. This is likely due to precipitation of beudantite and scavenging of As and Pb by secondary Fe-precipitates of oxidized tailings.
This investigation has demonstrated that under laboratory batch conditions, indigenous calcareous shale is efficient for the neutralization and removal of As and heavy metals of acid mine leachates in the Taxco mining area, Mexico. After treatment, pH values in mine leachates increased to values between 6.6 and 7.4. At these pH values, the maximum removal efficiency (100%) for As, Pb, Cu, and Fe was obtained. However, the average percentage removal for Cd and Zn was 87% and 89%, respectively. Although the removal efficiency of Zn in treated leachates discharges is relatively high, the residual concentration of dissolved Zn after treatment is still quite high (≤284.7 mg l−1). Considering that fact that these Zn concentrations can be toxic to aquatic life, it is necessary to explore options for the elimination of residual Zn in near-neutral leachates produced after treatment.
Calcite from indigenous calcareous shale plays the role of neutralizing agent, providing a source of alkalinity to promote metal precipitation, and quartz and aluminosilicatate minerals possibly serve as precipitating or scavenging agents that preferentially remove metals from the mine leachates.
Additional work is needed to demonstrate the importance of quartz and aluminosilicate minerals to minimize the metal hydroxide armoring of calcite such that calcite is not coated with precipitating metals and can therefore continue its neutralizing function for a longer period of time.
References
Abollino O, Aceto M, Malandrino M, Sarzanini C, Mentasti E (2003) Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res 37:1619–1627
Allison JD, Brown DS, Novogradac KJ (1991) MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems: version 3.11. EPA/600/3-91/021, Washington, DC, USA
American Society for Testing and Materials (1985) Standard test method for shake extraction of solid waste with water. Designation: D 3987–85:14–17
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A. A. Balkema, Leiden, The Netherlands
Armienta MA, Villaseñor G, Rodríguez R, Ongley LK, Mango H (2001) The role of arsenic-bearing rocks in groundwater pollution at Zimapán Valley, Mexico. Environ Geol 40:571–581
Armienta MA, Talavera O, Morton O, Barrera M (2003) Geochemistry of metals from mine tailings in Taxco, Mexico. Bull Environ Contam Toxicol 71:387–393
Armienta MA, Micete S, Flores-Valverde E (2008) Feasibility of arsenic removal from contaminated water using indigenous limestone. In: Bundschuh J, Armienta MA, Bhattacharya P, Matschullat J, Mukherjee AB (eds) Natural arsenic in groundwater of Latin America: occurrence, health impact and remediation. Press/Balkema Publisher, Leiden, The Netherlands, pp 505–510
Bain JG, Blowes DW, Robertson WD, Frind EO (2000) Modeling of sulfide oxidation with reactive transport at a mine drainage site. J Contam Hydrol 41:23–47
Benner SG, Herbert RB, Blowes DW, Ptacek CJ, Gould D (1999) Geochemistry and microbiology of a permeable reactive barrier for acid mine drainage. Environ Sci Technol 33:2793–2799
Carrillo A, Drever JI (1998) Adsorption of arsenic by natural aquifer material in the San Antonio–El Triunfo mining area, Baja California, México. Environ Geol 35:251–257
Costello C (2003) Acid mine drainage: innovative treatment technologies. National Network of Environmental Management Studies Fellow for U.S. Environmental Protection Agency, Washington, DC
Courtin-Nomade A, Bril H, Neel C, Lenain JF (2003) Arsenic in iron cements developed within tailings of a former metalliferous mine―Enguiales, Aveyron, France. Appl Geochem 18:395–408
Cravotta CA (2008) Laboratory and field evaluation of a flushable oxic limestone drain for treatment of net-acidic drainage from a flooded anthracite mine, Pennsylvania, USA. Appl Geochem 23:3404–3422
Cravotta CA, Trahan MK (1999) Limestone drains to increase pH and remove dissolved metals from acidic mine drainage. Appl Geochem 14:581–606
Dold B, Fontbote L (2001) Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J Geochem Explor 74:3–55
Doye I, Duchesne J (2003) Neutralization of acid mine drainage with alkaline industrial residues: laboratory investigation using batch-leaching tests. Appl Geochem 18:1197–1213
Foster AL, Brown GE, Tingle TN, Parks GA (1998) Quantitative arsenic speciation in mine tailings using X-ray absorption spectroscopy. Am Mineral 83:553–568
Galan E, Carretero MI, Fernandez-Caliani JC (1999) Effects of acid mine drainage on clay minerals suspended in the Tinto River (Rio Tinto, Spain): An experimental approach. Clay Miner 34:99–108
Gazea B, Adam K, Kontopoulos A (1996) A review of passive systems for the treatment of acid mine drainage. Miner Engineer 9:23–42
Holmstrom H, Salmon UJ, Carlsson E, Petrov P, Ohlander B (2001) Geochemical investigations of sulfide-bearing tailings at Kristineberg, northern Sweden, a few years after remediation. Sci Total Environ 273:111–133
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Jung MC (2001) Heavy metal contamination of soils and waters in and around the Imcheon Au-Ag mine, Korea. Appl Geochem 16:1369–1375
Kwong YTJ, Whiteley WG (1992) Natural acid rock drainage at Macmillan Pass, Yukon. DIAND MEND Project No. 11, NHRI Contribution No. 92046, p 41
Lin Z (1997) Mobilization and retention of heavy metals in mill-tailings from Garpenberg sulfide mines, Sweden. Sci Total Environ 198:13–31
López Pamo E, Aduvire O, Barettino D (2002) Tratamientos pasivos de drenajes ácidos de mina: Estado actual y perspectivas de futuro. Boletín Geol Miner 113:3–21
Mann AW, Deutscher RL (1980) Solution geochemistry of lead and zinc in water containing carbonate, sulphate and chloride ions. Chem Geol 29:293–311
Mayes WM, Potter HAB, Jarvis AP (2009) Zinc removal from circum-neutral mine waters using pelletised recovered ochre. J Hazard Mater 162:512–520
McGregor RG, Blowes DW, Jambor JL, Robertson WD (1998) The solid-phase controls on the mobility of heavy metals at the Copper Cliff tailings area, Sudbury, Ontario, Canada. J Contam Hydrol 33:247–271
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci USA 96:3455–3462
Nuñez Alvarez L (2008) Análisis de residuos mineros en Pinar del Río, Cuba, y Taxco, México. Trabajo de investigación para la obtención del Diploma de Estudios Avanzados. Universidad Complutense de Madrid, Facultad de Ciencias Geológicas
Nuttall Ch, Younger PL (2000) Assessment and experimental passive treatment of zinc-rich net alkaline minewaters, Nent Valley, UK. Wat Res 34:1262–1268
Romero FM (2004) Procesos geoquímicos que controlan la movilidad de metales y metaloides en jales de sulfuros metálicos. “El Fraile”, Taxco– Guerrero. Tesis de Doctorado, Posgrado en Ciencias de la Tierra, UNAM, México
Romero FM, Armienta MA, González-Hernández G (2007) The solid-phase control on the mobility of PTEs in an abandoned lead/zinc mine tailings impoundment, Taxco, México. Appl Geochem 22:109–127
Rötting TS, Cama J, Ayora C, Cortina JL, De Pablo J (2006) Use of caustic magnesia to remove cadmium, nickel, and cobalt from water in passive treatment systems: Column experiments. Environ Sci Technol 40:6438–6443
Santomartino S, Webb JA (2007) Estimating the longevity of limestone drains in treating acid mine drainage containing high concentrations of iron. Appl Geochem 22:2344–2361
Sasowsky ID, Foos A, Miller CM (2000) Lithic controls on the removal of iron and remediation of acidic mine drainage. Water Res 34:2742–2746
Shaw S, Wels C, Robertson A, Fortin S, Walker B (2003) Background characterisation study of naturally occurring acid rock drainage in the Sangre de Cristo Mountains, Taos County, New Mexico. Published in the in 6th ICARD: Cairns, Queensland, Australia, pp 605–616
Simon M, Martin F, García I, Bouza P, Dorronsoro C, Aguilar J (2005) Interaction of limestone grains and acidic solutions from the oxidation of pyrite tailings. Environ Pollut 135:65–72
Takahasi Y, Imai H (1983) Adsorption of heavy metals in montmorillonite. Soil Sci Plant Nut 29:111–122
Talavera O, Yeti M, Moreno Tovar R, Doctor Amazon A, Flores Mundo N, Duarte Gutierrez C (2005) Mineralogy and geochemistry of sulfide-bearing tailings from silver mines in the Taxco, Mexico area to evaluate their potential environmental impact. Geofísica Int 44:49–64
Talavera Mendoza O, Armienta Hernandez MA, Garcia Abundis J, Flores Mundo N (2006) Geochemistry of leachates from the El Fraile sulfide tailings piles in Taxco, Guerrero, Southern Mexico. Environ Geochem Health 28:243–255
United States Environmental Protection Agency (1994) Method 3051 microwave assisted acid disgestion/sludges, soils. Test methods for evaluating solid waste, physical/chemical methods
USEPA (1996) Test methods for evaluating solid wastes. Physical/chemical methods. SW-846 manual, EPA method 6010B: inductively coupled plasma atomic emission spectroscopy. United States Environmental Protection Agency, Washington, DC
Xu CY, Schwartz FW, Traina SJ (1997) Treatment of acid-mine water with calcite and quartz sand. Environ Eng Sci 14:141–152
Younger P (2000) The adoption and adaptation of passive treatment technologies for mine waters in the United Kingdom. Mine Water Environ 19:84–97
Ziemkiewicz PF, Skousen JG, Simmons J (2003) Long-term performance of passive acid mine drainage treatment systems. Mine Water Environ 22:118–129
Acknowledgements
We thank I. Puente Lee (Facultad de Química, UNAM), T. Pi, and O. Zamora (Instituto de Geología, UNAM) for their laboratory assistance. The authors are indebted to anonymous reviewers for their invaluable suggestions and comments, which greatly enriched an earlier version of this article. Funding was provided by DGAPA (Dirección General de Asuntos del Personal Académico, UNAM) project PAPIIT IN 105108, for which the authors are grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, F.M., Núñez, L., Gutiérrez, M.E. et al. Evaluation of the Potential of Indigenous Calcareous Shale for Neutralization and Removal of Arsenic and Heavy Metals From Acid Mine Drainage in the Taxco Mining Area, Mexico. Arch Environ Contam Toxicol 60, 191–203 (2011). https://doi.org/10.1007/s00244-010-9544-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9544-z