Abstract
An estimated 4.9 million barrels of crude oil and natural gases was released into the Gulf of Mexico during the Deepwater Horizon oil spill of 2010. The Deepwater Horizon oil spill affected the aquatic species in the Gulf of Mexico, vegetation, and the human population along the coast. To reduce the effect of the spilled oil on the environment, different remediation strategies such as chemical dispersant, and mechanical booms and skimmers were utilized. Over 2.1 million gallons of dispersants was applied to minimize the impact of the spilled oil. However, environmental and human toxicity issues arose due to the perceived toxicity of the dispersant formulations applied. After the Deepwater Horizon oil spill, various studies have been conducted to find alternative and environmentally benign oil spill response strategies. The focus of this manuscript is to demonstrate an objective and an overall picture of current research work on oil spill response methods with emphasis on dispersant and oil sorbent applications. Current trends in oil spill sorbent and dispersant formulation research are presented. Furthermore, strategies to formulate environmentally benign dispersants, as well as the possible use of photoremediation, are highlighted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
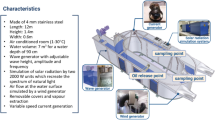
The Deepwater Horizon oil rig exploded and sank on April 20, 2010, resulting in the continuous flow of oil for 3 months into the Gulf of Mexico (Fig. 1). The blowout preventer failed to seal the Macondo well located 1.0–1.5 km below the sea surface, setting off the worst marine oil spill in the USA with the release of approximately 4.9 million barrels of crude oil into the Gulf of Mexico (Kostka et al. 2011; McNutt et al. 2012; Reddy et al. 2012). The spill was ultimately contained when the well was capped on July 15, 2010. A large amount of the oil was transported to the sea surface and stretched over 1600 km of shoreline. The Deepwater Horizon platform was owned by Transocean and was leased to British Petroleum (BP) as a fifth-generation dynamic positioned semi-submersible oil rig. The Horizon cost $365 million to build and was designed to operate in water as deep as 2.4 km and to drill down 9.1 km. The platform was located 66 km off the Louisiana coast and was 121 m long and 78 m wide. It was observed that not only crude oil was spilled but also a large amount of natural gas such as methane, propane, and ethane. Since the source of the spill was located approximately 1.0–1.5 km below the sea’s surface, the water dissolvable component of the oil (Whitehead et al. 2011) as well as the natural gases (Yvon‐Lewis et al. 2011) remained in the ocean endangering the life of aquatic species (Valentine et al. 2010).
a Fire boats try to extinguish the blaze on the Deepwater Horizon oil rig (www.nola.com). b Deepwater Horizon oil slick footprint (red) (www.eoearth.org)
The Deepwater Horizon oil spill had an adverse effect on aquatic species and the vegetation along the coast (Mason et al. 2012). Permanent marsh area loss was observed after the Deepwater Horizon oil spill (Silliman et al. 2012) depicting the detrimental effect of the spill on the environment. The population of the fish that lived in the oil spilled areas was also affected. Killifish collected from the oil-spill-affected areas had divergent gene expression in the liver and gill tissues coinciding with the environmental contamination from oil (Dubansky et al. 2013). The oil spill also affected the human population along the coast in the following ways: some children experienced either physical symptoms or mental health distress, household income reduced since some lost their jobs, and others moved their families from the oil spill area (Abramson et al. 2010). In addition to these, most of the fishing grounds in the areas affected were closed and, as a result, productivity of the Gulf fisheries was reduced. Environmental restoration of fishery habitats and Gulf ecosystems is needed to support the long-term recovery and productivity of the Gulf fisheries (Upton 2011).
Whenever oil spills occur, different remediation strategies are adopted to minimize their impact on the environment. Some of the most commonly used oil spill response options are in situ burning, mechanical containment and recovery, and chemical dispersant application. Recently, oil absorbents as an oil spill response option has attracted the attention of many researchers since this allows for the recovery of the spilled oil (Chen et al. 2013; Gui et al. 2013; Gu et al. 2014). Among these oil spill response methods, the chemical dispersant application is one of the few response measures that when adopted under the right circumstances and with judicious use of dispersants lead to reduced environmental and economic impact (Chapman et al. 2007). Dispersants are also one of the only feasible response options for minimizing the impact of large-scale oil spills.
After the Deepwater Horizon oil spill, containing the leak, preventing the leaked oil from reaching the shoreline and removing the spilled oil from the surface of the sea became a priority. Installing the sealing cap prevented the continuous leakage of the oil from the Macondo well. Mechanical booms and skimmers were used to skim the oil from the surface of the sea and to prevent the oil from flowing ashore (Petroleum 2011). EPA-approved dispersants Corexit 9500A and 9572A were then applied. Approximately 2.1 million gallons of dispersants was applied to the surface and the well head. In both modes, dispersant was added to lower the interfacial tension between the oil and the water and thereby reducing the size of oil droplets formed by wave action (Kujawinski et al. 2011). The shoreline in the spilled areas was surveyed by a team of scientists to access oiling conditions. Shoreline cleanup spearheaded by the coast guards also helped removed the oil from the coastline.
A lot of concerns were raised with respect to the use of chemical dispersants after the oil spill. Corexit 9527A contains 2-butoxyethanol, organic sulfonate, and propylene glycol. 2-Buoxyethanol is known to be carcinogenic and was identified as the causal agent in the health problems experienced by cleanup workers after the 1989 Exxon Valdez oil spill (SCHOR 2010). The use of Corexit during the spill cleanup caused respiratory, nervous, kidney, liver, and blood disorders in humans (Lustgarten 2010). As a result, the Corexit lines of product have been banned in the UK (D’Andrea and Reddy 2013).
Critical analysis of published articles revealed that limited studies were conducted into oil spill remediation strategies before the occurrence of the Deepwater Horizon oil spill of 2010. Only a handful of research articles on oil spills were published before 2010. This handful of publications includes patents filed by oil production companies such as ExxonMobile. The number of published research articles increased enormously after the Deepwater Horizon oil spill. To help address the challenges created by the Deepwater Horizon oil spill, British Petroleum (BP) established the Gulf of Mexico Research Initiative (GOMRI). Research grants have been awarded to various institutions by GOMRI to conduct studies into the effect of the Deepwater Horizon oil spill on the environment, formulate and design new oil spill response strategies, and also understand on the molecular level how existing dispersant formulations work and suggest mechanisms for improving their efficiency. As a result, research into the effect of oil spills and the formulation of environmentally benign oil spill response strategies has improved remarkably over the past 5 years. This manuscript therefore covers the advancements made in oil spill response research in the past 5 years with oil spill sorbents and dispersant as the main focus.
2 Oil Spill Sorbents
Oil spill economic and environmental impacts are increasing global concerns. The absorption of oil by sorbents is one of the quickest means of removing spilled oil from the surface of water and is normally used for small-scale oil spills. The oil spill sorbents are placed on or around the oil. They can be used to recover the oil through the mechanism of absorption, adsorption, and/or both (Karan, et al. 2011). A sorbent material that works by absorption mechanism absorbs and retains the oil. The absorbed oil is subsequently removed from the surface of the water. The oil can also adsorb to the sorbent material by weak van der Waal forces and transform the oil into a “semi-solid” mass which can be removed from the surface of the water. The absorbed/adsorbed oil can then be recovered from the sorbent material by either chemical or mechanical means and recycled. One major limitation of this response method is the possible absorption of water in addition to the spilled oil. To address this problem, the sorbents are treated to be oleophilic (oil loving) and hydrophobic (water hating). The sorbent should therefore be oleophilic, hydrophobic, biodegradable, reusable, inexpensive, and easily handled by untrained oil spill personnel while also having a good oil retention over time and a high oil uptake capacity (Yuan and Chung 2012). Oil spill sorbents can be classified into two major categories, namely, natural organic sorbents, and synthetic sorbents (EPA; Karakasi and Moutsatsou 2010; Zhu et al. 2011).
2.1 Natural Organic Sorbents
In the last 5 years, renewable resources such as kapok fiber, cotton fiber, and lignocellulosic waste materials have received increasing attention as oil spill sorbent materials due to their high oil uptake and retention capacity (Hussein et al. 2011; Wang et al. 2012b; Shahi 2014). Natural sorbents present important advantages such as their biodegradability, reusability, low density, and good mechanical properties (Rubasinghe et al. 2013). Compared to synthetic sorbent products, natural organic sorbents are abundant, renewable, and low in cost.
Treated and untreated cotton fibers as oil spill sorbents have been studied extensively and their oil sorption capacity reported to be dependent on their surface area, alignment of the individual fibers, maturity, and cellulose content. In addition to these, the oil sorption capacity of cotton fibers is dependent on the type of cotton used, fiber size, bulk density, fiber lumen, surface properties of the cotton fibers (oleophilic and hydrophobic), and pore volume (Hubbe et al. 2011). An oil sorption capacity of 22 g oil/g of sorbent has been reported for low-grade raw cotton fibers with over 90 wt% cellulose content (Hussein et al. 2011). The high cellulose content may be responsible for the relatively low sorption capacity since the hydrophilicity of cotton fibers has been ascribed to their cellulosic content (Wang et al. 2013a). Water molecules can therefore fill the inter-fiber spaces in the low-grade cotton and impact negatively on its oil sorption capacity. A much higher oil sorption capacity can be obtained by using low-maturity raw cotton fibers. Low-maturity raw cotton fibers have low cellulose content, making them more hydrophobic. High hydrophobicity can result in high oil sorption capacity. Singh et al. (2013) reported 30.5 g/g sorption capacity for low-micronaire cotton fibers. The high oil sorption capacity was attributed to the enhanced fineness, increased surface area, and the lower cellulose content of the immature (low micronaire) cotton fibers. These properties of the low-micronaire cotton fibers favor inter-fiber capillary sorption, adsorption, and absorption within the fiber. This is because the oil attaches to the surface of the cotton fiber by van der Waals forces as can be seen in Fig. 2.
Crude oil sorption by raw cotton fibers (Singh et al. 2013)
Carding of the low-micronaire cotton fibers into individualized fibers and aligning them in one direction can increase oil sorption capacity with an approximately 65 % improvement reported (Singh et al. 2014). The low bulk density of the aligned cotton fibers and the fact that the oil can fill the lumen of the cotton fiber are responsible for the increase in oil sorption capacity. Modification of the cotton fiber to increase its specific surface area, hydrophobicity, and oleophilicity can result in a significant improvement in its oil sorption capacity. Pyrolysis of raw cotton fibers in N2 atmosphere to modify their structure and increase their specific surface area resulted in an oil sorption capacity of 76.6 ± 1.5 g/g (Wang et al. 2013a). Pyrolysis of the cotton fiber increased the specific surface area and resulted in large-sized macropores which impacted positively on its oil sorption capacity (Fan et al. 2010).
In addition to cotton fiber, another natural material that has been used as an oil sorbent is kapok fiber. Kapok fiber is a naturally renewable material with high hydrophobicity and large lumen. It has light weight and a high flammability while being very buoyant, resilient, and resistant to water (Karan, et al. 2011). It is typically made up of approximately 64 wt% cellulose and 13 wt% lignin (Reddy and Yang 2009), has a hollow structure (Fig. 3) with a very large lumen volume, and is less dense than water (∼290 kg/m3). These properties make it a useful sorbent material for oil spills. The sorption capacity of kapok fiber has been reported to be dependent on its physicochemical properties and that of the oil to be absorbed. Abdullah et al. (2010) reported that high sorption capacity can be attained with kapok fibers of low packing density, low contact angle between the kapok and the oil to be absorbed, and high-viscosity oil. A maximum oil sorption capacity of 50.8 g/g was recorded with low-density kapok fiber and highly viscous engine oil.
Rengasamy et al. (Rengasamy et al. 2011) investigated the oil sorption capacity, porosity, and the contact angle of the kapok fibers. A contact angle of 44° and 34° was reported for high-density oil and diesel oil, respectively. This observation can be attributed to the low cellulose content of kapok fibers. High porosity was observed in the kapok fiber and was responsible for the high sorption capacity of 52.7 and 61.6 g/g for diesel and high-density oils, respectively. Altering the properties of the kapok through chemical treatment can also improve the oil sorption capacity. Acid treatment of the kapok resulted in the hydrolysis of the cellulose content in kapok, and this enhanced the oil sorption capacity up to a maximum acid concentration of 1.5 wt%. The sorption capacity decreased by ∼5 g/g at a higher acid concentration of 3 wt% due to the collapse of the tubular structure of kapok (Wang et al. 2012b).
Plant-derived cellulosic and lignin fibers have also been examined for possible application as oil sorbents. An oil sorption capacity of approximately 6.5 g/g has been reported for bleached softwood fibers (Payne et al. 2012). The relatively low oil sorption capacity of this cellulosic fiber can be ascribed to its high water wettability due to the presence of carboxylic acid groups. The high water wettability decreases the oil uptake since the fibers prefer absorbing water instead. A similar observation was made when moss which has a high water absorption capacity was used as an oil spill sorbent (Paulauskienė et al. 2014). The ability of natural sorbents to remove oil from the surface of the water is also dependent on the salinity of the water. The oil and water sorption capacities of moss, sawdust, peat, straw, and wool have been reported to be different in the presence of salt ions. The oil sorption capacity of straw, moss, and peat increased in the presence of salt ions while that of wool decreased (Paulauskienė et al. 2014). This can be attributed to the effect of neutral electrolytes on the hydrophilic–oleophilic properties of the natural sorbents.
The oil sorption capacity of these natural sorbents can be improved through surface modification. Predeposition of lignin onto the surface of bleached kraft fibers improved their ability to take up oil. The aromatic nature of lignin and the π–π interaction between the oil and the lignin aromatic group are responsible for the high oil uptake after lignin predeposition (Payne et al. 2012). Hydrothermal and acetylation treatment of populus fibers increased their oil sorption capacity. The oil sorption capacity of the treated fibers was dependent on the acetylation time and temperature. The oil sorption capacity of the treated fibers increased due to an increase in its hydrophobicity which can be associated with the breaking of hydrogen bonds and the swelling of the fiber. Modification of raw jute fiber by the acetylation process also resulted in a dramatic increase in its oil sorption capacity from 2.58 to 21.08 g/g (Teli and Valia 2013). The hydrothermally treated fibers recorded an increase in their oil uptake capacity due to dissolution of hemicellulose and deposition of lignin droplets (Zhang et al. 2014b). Acetylation of curaua fibers has resulted in an oil sorption capacity of approximately 12.38 g/g (Elias et al. 2015) which is relatively higher than the oil sorption capacity values of 4.14 and 4.35 g/g recorded for chemically and thermally treated palm kernel powder, respectively (Al Zubaidy 2012).
Agricultural waste can also be used as an oil sorbent either in the raw state or after it has been modified. The unique properties of luffa, a sub-tropical and climbing plant, have made it possible for it to be used as an oil sorbent for crude oil spill remediation. Raw luffa is able to retain about 15 g of heavy crude oil per gram of raw luffa. Luffa has a higher oil sorption capacity than water implying that raw luffa is oleophilic, and recorded approximately 50 % oil removal efficiency (Abdelwahab 2014). A much higher oil removal efficiency of 67.38 % has been reported for raw sugar bagasse as an oil spill sorbent. The high oil removal efficiency for raw sugar bagasse can be ascribed to its low density and high specific surface area. Sugar cane bagasse has a high sorption capacity and quick oil uptake and is readily available and cheaper when compared with synthetic sorbents (Abdelwahab 2014). Fiber complexes of coir (from coconut) and banana have been examined as the next generation of oil sorbents. The oil sorption is higher than the water sorption in coir fibers while the vice versa is the case for the banana fiber. The higher lignin content in coir fiber increases the π–π interaction between the coir fiber and the oil resulting in the high oil sorption. However, lower lignin content in banana decreased the interaction between its fiber and the oil. A moderately higher oil sorption (28 g/g) and a much lower water sorption (3 g/g) can be attained by coir–banana fiber complexes made up of different weight percent of coir and banana fibers (Rubasinghe et al. 2013).
The oil sorption capability of untreated and treated wheat straw has also been reported. Treated and untreated wheat straw have been used as an oil spill sorbent. The oil can be held due to capillary action in the straw tissue and can also exist as oil bridges between the stalk. Data reported by Konstantinou and Sidiras (2014) indicated that both hydrolyzed and unhydrolyzed wheat straw have higher affinity for the aqueous phase than the oil phase. The oil and water retention capacity is therefore dependent on hydrolysis possibly due to structural changes that occur in the wheat straw at very high hydrolysis temperature (>160 °C).
Rice husks, an agricultural waste product, can also be used as an oil spill sorbent with an oil sorption capacity of 2 g/g reported in literature (Kenes et al. 2012). The oil sorption capacity of rice husks can be improved by thermal treatment (pyrolysis). With such treatment, the oil sorption capacity will depend on the treatment temperature and time, as well as on the density of the spilled oil to be absorbed (Kenes et al. 2012). Pyrolysis of rice husks at 700 °C for 25 min increased the oil sorption capacity by 750 %. Pyrolysis of the rice husks further created new micropores and mesopores, resulted in the formation of graphite and amorphous silica, reduced its density, and increased its hydrophobicity and specific surface area (Angelova et al. 2011; Kenes et al. 2012). All of the above positively impacted on the oil sorption capacity. Higher pyrolysis temperatures (>700 °C) will decrease the oil sorption capacity due to the phase transformation of amorphous silica to a more crystalline form (Kenes et al. 2012).
A low-density (0.048 g/cm3) melon carbon aerogel prepared by hydrothermal and post-pyrolysis of winter melon has shown high efficiency as an oil spill sorbent (Li et al. 2014). The treatment of winter melon is necessary due to its high hydrophilicity which can have a negative impact on the oil sorption capacity. Crude oil absorption capacity of 25 g/g has been recorded for winter melon aerogels due to their high hydrophobicity (water contact angle of 135°), low density, highly porous structure (Fig. 4), and high pore volume after hydrothermal and post-pyrolysis treatment (Li et al. 2014).
SEM images of winter melon after a hydrothermal treatment and b pyrolysis (Li et al. 2015)
The sorption capacities of natural sorbent materials reported in literature are summarized in Table 1 below.
2.2 Synthetic Sorbents
In addition to natural sorbent materials, synthetic materials can be used as oil sorbent. The properties of synthetic materials can be tuned during synthesis to achieve desired properties that may enhance their oil sorption capacity. Synthetic materials such as spongy graphene, carbon nanotubes, silica xerogels (xerogel is a solid formed from a gel by drying with unhindered shrinkage), and polyurethane foams have been studied as oil spill sorbents. These synthetic materials can therefore serve as a supplement to the already enormous natural sorbents. As already stated, the ability to tune the properties (light weight, high porosity, large surface area, hydrophilicity, and oleophilicity) of these materials during synthesis has made them a promising candidates for oil spill cleanup.
Various allotropes of carbon have been studied as oil spill sorbents. These carbon allotropes can be nanostructured or macrostructured, with the nanomaterials recording high sorption capacities. The high sorption capacity of the nanocarbons can be attributed to the unique properties of nanomaterials such as high surface-to-volume ratio. Hollow carbon beads fabricated by the phase inversion method have been examined as oil spill sorption material. However, due to the large size of these carbon beads (∼3 mm), a relatively lower oil sorption capacity (40 g/g) was recorded when compared to those obtained from nanocarbon materials (Zeng et al. 2014). Carbon nanotube sponges synthesized by chemical vapor deposition (CVD) with a precursor solution of ferrocene in dichlorobenzene recorded an oil sorption capacity of 180 g/g (Gui et al. 2010). The high oil sorption capacity can be attributed to its light density, high porosity, structural flexibility, and high oleophilicity.
Needle-like and cottony structured spongy graphene synthesized by reducing graphene oxide platelets have also been studied as a possible oil sorption material. Due to the unique properties of the spongy graphene such as its high hydrophobicity, high surface area (∼423 m2/g), and high microporosity, a high oil sorption capacity of 86 g/g was attained (Bi et al. 2012). A much higher oil sorption capacity of 100 g/g has been reported for hydrophobic reduced graphene oxide foams (He et al. 2013). The hydrophobic reduced graphene foams were synthesized by thermally reducing hydrophilic graphene oxide foams. Thermal reduction reduced the amount of the polar head groups making the reduced graphene oxide foams hydrophobic. The thermal reduction also increased the porosity of the reduced graphene oxide foams.
The oil sorption capacity of polymeric material such as polyurethane can be improved by modifying it with carbon material. Modification of polyurethane with either graphene oxide or lauryl methacrylate increases its water contact angle and surface roughness but reduces its surface energy. This implies that the hydrophobicity of the modified polyurethane increases when it is modified with graphene. It is interesting to note that such modification does not affect the porosity since the porosity of the polyurethane before and after modification was constant. Graphene-coated polyurethane has a higher oil sorption capacity of 90 g/g (Li et al. 2012a; Liu et al. 2013a; Liu et al. 2013b).
Superhydrophobic and superoleophilic textile sorbent has been synthesized by coating textile material with a diamond-like carbon film via plasma-enhanced chemical vapor deposition (PECVD). The diamond-like coating increased the surface roughness of the material imaged by SEM in Fig. 5. The surface roughness contributed to the superhydrophobicity of the coated textile. The water contact angle of the textile increased from 0° to 143° after the coating, improving its oil sorption capacity by ∼300 % (Cortese et al. 2014).
SEM images of a cotton fibers without plasma pre-treatment, b fiber after plasma pre-treatment, and (c) diamond-like coating after fiber plasma pre-treatment (Cortese et al. 2014)
Microspheres and gels have also been explored as possible sorbents for oil spills. Hollow and porous microspheres prepared by a suspension polymerization method have been examined for their oil spill remediation capability. Oleophilic β-cyclodextrin and iron-oxide-based microsphere with high porosity has recorded approximately 18.2 and 8.42 g/g oil sorption capacities, respectively (Song et al. 2013; Mao et al. 2014). Despite the relatively low oil sorption capacity of these microspheres, the magnetic nature of the iron-oxide-based microsphere allows for the easy removal of the oil after the absorption process.
Gels have some unique properties compared to other sorbents. Gels are highly porous and have the lowest density and highest surface area per unit volume. Poly(alkoxysilane) organogels (Kizil et al. 2015) and silica aerogels (Wang et al. 2011; Wang et al. 2012a) have been studied as possible oil spill absorbents. Examining the possibility of using poly(alkoxysilane) three-dimensional cross-linked organogels as an oil spill absorbent revealed a maximum oil sorption capacity of 250 % for diesel oil. The oil sorption capacity increased with an increasing carbon number in the poly(alkoxysilane). This can be attributed to the formation of a dense three-dimensional network structure of the higher carbon poly(alkoxysilane) when compared with that of the lower carbon. Silica aerogels, on the other hand, revealed a maximum oil absorption capacity of 4 (Wang et al. 2011) and 11.7 g oil/g silica aerogel (Li et al. 2012a) for toluene and crude oil, respectively. Three-dimensional interconnected porous structures of poly(dimethylsiloxane) (PDMS) have also being examined for their oil absorption capabilities (Zhang et al. 2013). The three-dimensional interconnected PDMS showed high oil absorption capacity and excellent reusability. The relatively high crude oil sorption capacity of 9 g/g can be attributed to the hydrophobic and oleophilic nature of the synthesized three-dimensional interconnected PDMS.
Other materials such as aromatic amino acid (phenylglycine) (Basak et al. 2012) and lithium tetraalkoxyborates (Kumpanenko et al. 2014) have been found to form organogels in fuel hydrocarbon solvents. Such compounds should fulfill the following requirements: (i) selectively and efficiently gelate the oil, (ii) synthesized easily from a cheaper raw material, (iii) instant gelation of the oil at room temperature, (iv) should be reusable without a reduction in its efficiency, and (v) easy recovery of the oil from the gel phase (Jadhav et al. 2010). Recovery of the oil from these gelators may be difficult due to their spilled oil recovery mechanism, unlike the silica aerogels where the oil can easily be recovered by mechanical means, and the application of an external pressure is needed.
Different polymeric materials such as polypropylene (Rengasamy et al. 2011; Sek et al. 2013; Zhao et al. 2013), poly(butyl methacrylate-co-hydroxyethyl methacrylate) (Zhao et al. 2013), and poly(methyl methacrylate-butyl methacrylate) (Ji et al. 2011) have been studied for their sorption properties. The principal advantages of such synthetic polymeric materials are their high hydrophobicity, simplicity of absorbed oil recovery, sufficient reusability, excellent imbibition and retention capacity, and high buoyancy (Zhu et al. 2011). The oil sorption behavior of these polymeric materials is influenced by the polymerization method adopted in their synthesis, the ratio of the monomers, the effective network volume, and the amount of cross-linker used. An addition of excessive amount of cross-linker results in a significant reduction in the oil absorption capacity. Poly(methyl methacrylate-butyl methacrylate) prepared by a suspended emulsion polymerization method has a much higher absorption capacity (17.57 g/g) when compared with the same polymeric material synthesized by suspension polymerization (9.68 g/g) and emulsion polymerization (6.93 g/g) (Ji et al. 2011).
The possible application of novel miniature devices for oil spill remediation has recently been studied (Wang et al. 2014a, b). These miniature devices are characterized by high superhydrophobicity, superoleophilicity, and porosity. These novel devices serve as oil containments to separate the oil from the seawater during application. Since the operation of these devices is similar to mechanical booms, the collected oil is likely to contain some amount of water. Near-complete recovery of oil from the collected oil–water mixture can be attained by flowing the oil–water mixture through superhydrophobic steel meshes (Deng et al. 2013; Song et al. 2014). The superhydrophobic meshes are able to separate the oil from the oil–water mixture with a separation efficiency of ∼100 %. The devices discussed above were made hydrophobic by treating with either xylene, CuCl2, or CuSO4 solution. In addition to the point elucidated above, the oleophilicity of oleophilic materials can be enhanced by increasing the roughness of their surface. This has been achieved by coating the steel mesh with ZnO nanorods. The coated mesh showed high oleophilicity resulting from the hierarchical microstructured/nanostructured ZnO rod arrays on the stainless steel mesh. Coating with ZnO nanorods improve not only the oleophilicity but also the corrosion resistance (Li et al. 2013).
2.3 Nano-oil Sorbents
Many studies have been conducted into the possible application of nanotechnology in oil spill remediation. This can be attributed to the unique properties exhibited by nanomaterials such as their high surface area, high aspect ratio, and high surface-to-volume ratio. Although many of the work reported in literature deals with the possible application of nanocomposites in oil spill remediation, individual nanomaterials such as carbon nanotube (CNT) sponges (Zhu et al. 2013) and magnetic coconut oil nanofluid (Nabeel Rashin et al. 2014) have also been studied. Randomly interconnected CNTs (Fig. 6) synthesized by chemical vapor deposition with a ferrocene catalyst and 1,2-dichlorobenzene have been studied as oil spill absorbents. A high oil sorption capacity of 92.30 g/g (Zhu et al. 2013) was recorded and can be attributed to the hydrophobic nature of CNT sponges coupled with their high internal volume. A novel magnetic coconut oil nanofluid synthesized by the phase-transfer method recorded 91 % oil removal efficiency. The phase-transfer method was carried out by using an aqueous magnetic nanofluid obtained by mixing ferrous chloride and ferric chloride solutions (Nabeel Rashin et al. 2014).
Morphology of CNT sponges; a photograph of a macroscopic CNT sponge with hollow cylindrical structure; b, c SEM images at different magnifications; d TEM images (Zhu et al. 2013)
One major limitation for the application of nanomaterials as oil spill absorbent is the challenge associated with their removal after the application. To facilitate the removal of oil-absorbed nanomaterials, magnetic nanomaterials or nanomaterials coated with magnetic materials are used so that they can easily be removed with the aid of a strong magnet. Oil spill absorbing capabilities of different nanostructures and morphologies of magnetic iron oxide nanoparticles have been studied and found to have around 95 wt% oil removal efficiency (Saber et al. 2015). The magnetic iron oxide nanoparticles with an average diameter of 13 nm were prepared by a microemulsion method. The nanosize, low density, high surface area, and super paramagnetic nature of the Fe3O4 particles enhanced their oil absorption capacity.
In addition to these, interconnected CNTs with rich Fe encapsulation synthesized by the chemical vapor deposition (CVD) have been investigated for oil spill remediation (Gui et al. 2013). The interconnected CNTs-Fe have high mechanical strength and, hence, the absorbed oil can easily be removed by compression. The magnetic CNTs-Fe is light and highly porous with pore sizes from several nanometers to a few micrometers (Gui et al. 2011). These pores provide sufficient space for the storage of absorbed oil and for capillary action to spontaneously drive the spilled oil into the pores (Fig. 7)
Removal of spilled oil from water surface by magnetic carbon nanotube sponges. a Optical image of microscopic magnetic carbon nanotube sponges; b SEM image of the magnetic carbon nanotube sponges; c TEM image of a carbon nanotube filled with magnetic Fe nanowire; d, e, f oil spill cleanup with the magnetic carbon nanotube sponges (Gui et al. 2013)
Absorption of both water and oil by these nanomaterials complicates the oil separation process after the application. To improve water repellent capability of magnetic nanomaterials in oil spill remediation, a novel and environmentally friendly core–shell composite material based on hollow magnetic Fe3O4 particle has been synthesized and its oil removal capacity examined. Hollow magnetic Fe3O4 particles compose the core of this material with a hydrophobic polystyrene layer acting as a shell. These magnetic particles with an average diameter of 200–300 nm were synthesized via the hydrothermal method and coated with the polystyrene layer (Chen et al. 2013). The chemical reactions for synthesizing the hollow Fe3O4 nanoparticles are as follows:
Coating of the Fe3O4 with polystyrene increased its hydrophobicity and oleophilicity and reduced the saturation magnetization from 79.79 to 61.25 emu/g. Due to the hollow nature of the nanocomposite, the absorption mechanism was controlled by molecular diffusion of the oil (Chen et al. 2013). The shell can also be a combination of different polymeric materials such as polystyrene–divinylbenzene and methacrylate–polystyrene–divinylbenzene. The hydrophobic and superoleophilic shell will enhance the oil separation rate (Gu et al. 2014). A well-studied shell material for polymer-coated iron oxide nanoparticles is polyvinylpyrrolidone. Polyvinylpyrrolidone-coated iron oxide nanoparticles have recorded a near-complete oil removal on a laboratory scale (Palchoudhury and Lead 2014). Despite the near-complete oil removal capability of these particle, it is likely that such values may not be attained in real oil spill application due to the solubility of polyvinylpyrrolidone in water. The hydrophobicity of the polymer-coated magnetic particles will diminish with time as the polyvinylpyrrolidone dissolves in water. The particles may therefore absorb water and the oil. A new oil–water separation apparatus will therefore have to be developed, and this will increase the cost of remediation.
Nanofibrous membranes with high oil sorption capacity have also been reported in literature (Raza et al. 2014b). These nanofibrous membranes were made from polyethylene glycol diacrylate (Raza et al. 2014a), polystyrene and polyvinyl chloride (Zhu et al. 2011; Lin et al. 2012), and polystyrene-polyurethane (Lin et al. 2013). The polymeric materials used in synthesizing these nanofibrous membranes are responsible for their high hydrophobicity and oleophilicity. The fibrous nature also creates large internal volume and specific surface area for oil absorption and hence the high oil sorption capacity recorded for these materials. For instance, composite polystyrene–polyurethane fibrous membranes registered an oil sorption capacity of 64.40 g/g for motor oil (Lin et al. 2013).
Polymer–clay nanocomposite made up of organo-attapulgite and poly(butylmethacrylate), synthesized by suspended emulsion polymerization, exhibits high oil sorption capacities (Wang et al. 2013b). Treatment of the organo-attapulgite with quaternary ammonium salts enhanced the compatibility of the magnesium aluminosilicate mineral with the polymer matrix. The treatment can enhance the oil absorption and swelling rate.
The application of oil sorbents in crude oil spill remediation is limited to small scale oil spills. Since an oil spill can occur at any time, a stock pile of oil sorbent materials should be available and easily accessible. Another perceived issue is the toxicity of the oil sorbents. Surprisingly, the toxicological data of the oil sorbent materials reported are not available in the literature. Since some of the sorbent materials may be lost to the ocean during the application, it would be necessary for toxicological studies to be conducted on the materials. The lives of the aquatic species may be endangered by these oil sorbent materials. In addition to these, most if not all of the analysis was conducted on laboratory scale. Field trials should be conducted to examine the feasibility of the reported sorbent materials under real-life oil spill conditions.
The sorption capacities of natural sorbents materials reported in literature are summarized in Table 2 below.
3 Dispersants
After the Deepwater Horizon oil spill of 2010 in which over 200 million gallons of crude oil was spilled into the Gulf of Mexico, an enormous amount of resources have been allocated to researching into various oil spill remediation strategies. The use of dispersants for crude oil spill remediation has gained a lot of attention in publications. These publications focused on (a) understanding the emulsification behavior of some of the most commonly used surfactants such as Tween 80 and DOSS, (b) investigating the toxicity of existing dispersants, and (c) formulating new dispersants. The publications on new dispersant formulations can be categorized into three categories: (i) formulating dispersant particles, (ii) formulating solubilized dispersants using new surfactants, and (iii) formulating new dispersants using biosurfactants. Although chemical dispersants have been defined as a liquid blend of surfactants, hydrocarbon-based solvent, and additives (Kujawinski et al. 2011), some of the commonly investigated dispersants are particulates. The active ingredients in dispersant formulations are the surfactants. A surfactant is an amphiphilic molecule with a hydrophobic tail and a hydrophilic head. Hence, in the presence of water, surfactant molecules arrange themselves with the head group aligned toward the water molecules while the tail is aligned away from the water molecules. Such an arrangement reduces the overall energy of the system since the surfactant molecules replace the highly energetic oil/water molecules at the oil–water interface.
When chemical dispersants are applied to an oil spill and sufficient energy is exerted to the dispersant–oil–water mixture, the slick is broken down into smaller droplets due to the reduction of the oil–water interfacial tension (Fig. 8) (Sorial et al. 2004). The oil droplets diffuse both vertically and horizontally by the action of the sea waves and remain in the water column because they possess very little rising velocity. The increased surface area that arises from the smaller oil droplet size helps to accelerate biodegradation of the oil by bacterial action and hence reduces the environmental impact of the spilled oil (Chapman et al. 2007).
Motivated by the need to find alternative dispersants for emulsifying oil after oil spill, many researchers have examined the emulsion forming capability of other materials. The next section reviews some of the dispersant research findings after the Gulf of Mexico oil spill of 2010.
3.1 Particulate Dispersants
Emulsion formed solely by particle arrangement at the oil droplet–water interface (Pickering emulsion) was observed by Pickering (1907) and Ramsden (1903) in 1907 and 1903, respectively. Although commercial use of Pickering emulsion is not common, potential application areas that take advantage of the properties of the particles are emerging. For a Pickering emulsion to form, the particles must be smaller in size than the emulsion droplet. With this criteria, particle size in the micrometer and nanometer ranges is required.
One of the most widely studied Pickering emulsions was formed with carbon black. The interfacial tension, surface elasticity, and emulsion forming capability of carboxyl terminated carbon black have also been reported (Saha et al. 2013; Powell and Chauhan 2014). The functionalized carbon black stabilized the emulsion by forming multiple layers of closely packed aggregates at the oil droplets–water interface as shown in the Cryo-SEM in Fig. 9b, d (Saha et al. 2013). The carbon black coverage decreased the interfacial tension by approximately 72 %, and the decrease is accompanied by an increase in the surface elasticity (Powell and Chauhan 2014). The increase in surface elasticity helps to stabilize the emulsion against droplet coalescence.
Cryo-SEM of Octane(O)-in-Water(W) emulsion stabilized by carbon black (CB); a, b pH 3.3, 0.0075 wt% carbon black, c, d 0.6 M NaCl, 0.015 wt% carbon black (Saha et al. 2013)
The behavior of the carbon black at the oil–water interface is dependent on the pH of the aqueous phase with the carbon black behaving differently in acid conditions and in the presence of NaCl (Saha et al. 2013). The features observed at the oil–water interface are related to the hydrophilic–lipophilic balance of the carbon black particles, which in turn are related to the residual charge on the particle surface.
Usually, a combination of surfactants and particles will provide the optimum characteristics for the emulsion. The surfactants often provide the low interfacial tension to facilitate drop formation, and the particles provide enhanced stability (Kraft et al. 2010). The synergy between particle and surfactant mixtures has been exploited to make particle-stabilized emulsions. The behavior of carbon black stabilized emulsions in the presence of different anionic, cationic, and nonionic surfactants has been examined (Katepalli et al. 2013). Findings reveal that depending on the amphiphile’s interaction, interfacial activity, and the bulk concentration, some carbon black particles get displaced from the oil–water interface and are replaced by the surfactants. Despite these happenings at the oil droplet–water interface, the emulsion remains stable. While the anionic and nonionic surfactants bind to the carbon black by hydrophobic interactions, the cationic surfactants bind strongly to the carboxyl group of the functionalized carbon black (Katepalli et al. 2013). Carbon black provides an alternative to dispersants that keeps emulsions stable at high dilutions for months at a time, reduces polyaromatic hydrocarbon partitioning into aqueous phase, and potentially lowers the toxicity of dispersants to bacteria and marine life (Bose et al. 2012; Saha et al. 2012; Rodd et al. 2014).
Graphene oxide which is a product of chemical exfoliation of graphite has been examined for its possible application in oil spill remediation (Kim et al. 2010). The amphiphilic behavior of graphene oxide is dependent on their size with smaller-size graphene oxide being more hydrophilic. This behavior can be attributed to higher charge density resulting from the ionizable carboxyl (–COOH) groups. The degree of ionization of the –COOH groups can be affected by pH. At high pH, deprotonation of the –COOH group is promoted and this increases the charge on the graphene oxide. Due to this, the zeta potential of graphene oxide can vary from −50.2 mV (pH of 10) to −22.7 mV (pH of 2) (Kim et al. 2010). All these factors influence the emulsification behavior of graphene oxide.
Naturally occurring clay particles such as montmorillonite, kaolinite, and laponite are known to disperse oil through the formation of Pickering emulsions. Since these clay particles have negative surface charges, they are highly hydrophilic. The surfaces of these clay particles are typically modified with surfactants to lower their hydrophilic–lipophilic balance (HLB). Montmorillonite clay particles modified with bis(2-hydroxyethylene)oleylamine stabilized oil-in-water emulsion against coalescence (Dong et al. 2014). The stability of the emulsions was attributed to the reduction of the oil–water interfacial tension by the surface modified montmorillonite clay. The emulsion can be stabilized over a long period of time due to the formation of surfactant bilayer at the oil drop–water interface with the surfactant head group pointing outward. Increasing the surfactant-to-clay mass ratio resulted in a transitional inversion, that is, the oil-in-water (o/w) emulsion was converted to a water-in-oil (w/o) emulsion. A similar observation was made when emulsion stabilized by cetyl trimethylammonium bromide (CTAB)-modified montmorillonite was examined at different clay–surfactant mass ratios (Zhang et al. 2014a). A measurement of the montmorillonite/CTAB contact angle revealed a contact angle maxima at 15 mM CTAB concentration after which a significant drop in the contact angle was observed. The hydrophilicity of the modified clay was therefore dependent on the surfactant concentration. A similar observation was made when emulsion stabilized by hydrophilic quaternary amine surfactants such as BerolR648 and Ethoquad C/12 was studied (Cui et al. 2011).
Hydrophilic laponite particles can be rendered to be hydrophobic by surface modification with aliphatic amines (Wang et al. 2010; Li et al. 2012b). The hydrophobicity of the laponite particles was enhanced by the adsorption of the amine at the laponite clay particle surface reducing the overall zeta potential. The reduction of the zeta potential makes it easier for the modified laponite to adsorb at the oil–water interface (Li et al. 2012b). Increase in the amine concentration stabilized the emulsion against coalescence and creaming. The emulsions were also stabilized by the formation of a rigid interconnected layer of laponite at the oil droplet–water interface (Wang et al. 2010).
Naturally occurring halloysite nanotubes (HNTs) have recently been examined for their possible application in crude oil spill remediation (Owoseni, Nyankson et al. 2014; Nyankson et al. 2015b; Owoseni et al. 2016). Naturally occurring halloysite nanotubes are effective in stabilizing o/w emulsions and can serve as interfacially active vesicles for delivering oil-spill-treating agents. The HNTs were loaded with surfactants by using a vacuum suction method. The adsorption of surfactants serves to lower the interfacial tension while the adsorption of particles provides a steric barrier to drop coalescence hence stabilizing the o/w emulsion for a long period of time.
Due to the large volume of water in the ocean, a significant amount of dispersant may not come into contact with the oil. The solubility and miscibility of dispersant components coupled with the ocean waves often make it imperative to apply large amounts of dispersant, with significant economic and environmental consequences. This problem is often associated with the application of traditional liquid dispersants. To address this challenge, dispersant composite particles with paraffin wax as the dissolvable matrix and dioctyl sodium sulfosuccinate (DOSS) as the surfactant have been synthesized and their application for crude oil spills examined (Nyankson et al. 2014; Nyankson 2015). The mechanism of surfactant release is depicted in Fig. 10. The effectiveness of the dispersant composite particles prepared by a spray freezing method to stabilize the emulsion was dependent on the solubility of the dissolvable matrix in the crude oil, the presence of neutral electrolytes, and the particle size distribution.
Mechanism of surfactant release from composite particle (Nyankson et al. 2014)
Oil-in-water emulsions formed and stabilized by silica nanoparticles modified with a zwitterionic surfactant, capramidopropyl betaine, have been investigated (Worthen et al. 2014). The role of the surfactant is to lower the interfacial tension by adsorbing at the oil droplet–water interface. The silica nanoparticles also adsorb at the oil droplet–water interface and form a steric barrier that prevents droplet coalescence and creaming. At the oil droplet–water interface, the positively charged portion of the zwitterionic surfactant is weakly attracted to the negatively charged surface of the silica nanoparticles by electrostatic interactions. The emulsion stability is highly dependent on the particle size distribution of the silica nanoparticles. A similar observation has been reported for silica nanoparticles functionalized with Span 20 (Yu, Dong et al. 2014; Yu et al. 2015) and polyalkyleneoxy moiety (Törncrona et al. 2012).
3.2 Plant- and Animal-Based Dispersants
Exposure of marine species to chemical dispersants has resulted in significant membrane damage (Hook and Osborn 2012). In view of the perceived toxicity of chemical dispersant, a lot of research is being conducted into finding alternative surfactants which are environmentally benign. Dispersants formulated with soybean lecithin have recently been reported (Nyankson et al. 2015a). The dispersion effectiveness of soybean-based phospholipids (phosphatidylcholine and phosphatidylinositol) solubilized in water was reported to be of the same order as Tween 80 solubilized in propylene glycol but higher than DOSS solubilized in propylene glycol at higher surfactant-to-oil ratios. The emulsion forming capability of soybean lecithin was improved upon the addition of Tween 80 (Athas et al. 2014).
In addition to this plant-based dispersant, the emulsion forming capabilities of animal-based surfactants have also been reported. Diethylolamide and monoethylolamide fats which are derived from fish oil, mutton, and beef have been promising in dispersing crude oil on the laboratory scale (Asadov et al. 2012a; Asadov et al. 2014). The efficiency of these animal-derived surfactants was dependent on the concentration of the salt ions such as NaCl, KBr, and MgSO4. Treating the ethylolamides with phosphates (Asadov et al. 2012a) and the complexation of palmitic acid and nitrogen-containing compounds (monoethanolamine and triethylenetetramine) (Asadov et al. 2012b; Asadov et al. 2013) increased their oil dispersing efficiency.
Cactus mucilage, an extract from Opuntia ficus-indica, is able to disperse crude oil slick as well as absorb it while remaining afloat (Alcantar et al. 2012). The powdered surfactant extract can be used in remediating spilled oil on the surface of the sea as well as under the sea as observed in the Deepwater Horizon oil spill. Cactus mucilage therefore acts as a dispersant and, also, as an absorbant. The ability of the cactus mucilage to absorb the oil facilitates the removal of the oil–mucilage aggregate after its application.
3.3 Polymer and Gel-Based Dispersants
Dendritic polymers such as poly(amidoamine) and poly(ethyleneimine) exhibit strong and comparable dispersion capacities for oil (Geitner et al. 2012). The dispersion capacity of these polymers is dependent on their structure. That is, the dispersion capacity of hyperbranched polymers is higher than that of the dendrimers. However, the high cationic charge density of poly(amidoamine) dendrimers has been shown to be cytotoxic (Geitner et al. 2014). Examining the effect of surface charge variation revealed that, although the cytotoxicity can be reduced by either making the poly(amidoamine) uncharged or by making it negatively charged, the dispersion capacity is reduced.
ExxonMobil has recently formulated a positively buoyant viscous dispersant gel for crude oil spill remediation (Nedwed 2010; Nedwed et al. 2011). This new dispersant formulation is made up of roughly 90 % surface-active agents while the current commercial dispersants are at most 40–50 %. The highly viscous nature of this dispersant allows easy delivery as a spray of large droplets that are persistent in breaking wave conditions and adhere to the oil slick instead of being washed away as experienced in the application of the current commercial dispersants. In addition to this, the white gel material visually contrasted with oil allowing response personnel to immediately evaluate slick coverage after a dispersant application. The aforementioned allows for the oil spill remediation with chemical dispersant to be safer, easier, and efficient.
The emulsion forming capabilities of hydrophobically modified chitosan combined with Corexit 9500A have also been reported (Venkataraman et al. 2013). Chitosan was hydrophobically modified by attaching the dodecyl chains to the amine groups on the polymer backbone. The emulsions formed by the chitosan and Corexit 9500A were very stable compared to the emulsions formed by using only Corexit 9500A. The enhanced stability of the droplets is due to the increased electrostatic and steric repulsions provided by the polymeric layering. The polymeric layering prevents droplet coalescence.
3.4 Biosurfactant
Biosurfactants are surface-active chemicals synthesized by a wide variety of microorganisms. They are amphiphilic compounds comprised of glycolipids, polysaccharides, and lipopeptides (Banat et al. 2010). Biosurfactants alter the physico-chemical conditions at the oil–water interface by reducing the interfacial tension. Biosurfactants produced by Candida sphaerica were able to remove 75 and 92 % of oil contained in clay and silty soil, respectively (de Souza Sobrinho, de Souza Sobrinho et al. 2013). The effectiveness of the biosurfactant in removing oil is dependent on the physical properties of the oil and the conditions of the oil spill environment. The emulsification properties of Rhodococcus sp. strain TA6 which consists of extracellular lipids and glycolipids have been studied (Shavandi et al. 2011). An interfacial tension value of 30 mN/m was recorded by this biosurfactant. The emulsification properties of this biosurfactant were unaffected even at an extremely high temperature of 120 °C and a salinity of 10 % NaCl. A biosurfactant produced from Rhodococcus erythropolis can thus be used in oil spill remediation even under harsh environments (Moshtagh and Hawboldt 2014).
Surfactin and fatty acyl-glutamate are biosurfactants produced from genetically modified strains of Bacillus subtilis. The critical micelle concentration (CMC) of these biosurfactants is dependent on salinity; hence, their effectiveness in dispersing crude oil decreased upon increasing salinity. It should be noted that the CMC is the concentration above which micelles form. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant. It is interesting to note that surfactin and the commercial surfactant, sodium laurel sulfate, are more toxic than the biosurfactant fatty acyl-glutamate (Marti et al. 2014). In addition to dispersing crude oil, biosurfactants can be used to increase the biodegradation rate of dispersed oil (Antizar-Ladislao 2010; Ron and Rosenberg 2014). The ability of different bacteria strains such as B. subtilis (Montagnolli et al. 2015), Bioaug-SC (Sheppard et al. 2014), Pleurotus ostreatus (Adedokun and Ataga 2014), and Alcanivorax borakumensis (M 2014) to increase the biodegradation rate of dispersed oil has been examined. These bacteria naturally degrade spilled oil by feeding on it.
3.5 The Behavior of Tween 80, Span 80, and Dioctyl Sodium Sulfosuccinate at the Oil Drop–Water Interface
The commercial Corexit dispersant formulations made up of the surfactants Span 80, Tween 80, and dioctyl sodium sulfosuccinate (DOSS) were applied after the Deepwater Horizon oil spill of 2010 (Campo et al. 2013). The effectiveness of dispersants made up of DOSS, Tween 80, and Span 80 in dispersing crude oil has been shown to be governed by the relative percentages of these surfactants in the dispersant, initial oil–water interfacial tension observed upon dispersant–oil–seawater contact, rate of decrease from the initial interfacial tension as DOSS is rapidly lost to the aqueous phase, and the slowing kinetics of dispersant adsorption at the oil–water interface as the concentration of Span 80 is increased (Riehm and McCormick 2014). The different surfactants present in the commercial dispersant influence the CMC which in turn influences their effectiveness in dispersing crude oil. For instance, the CMC of DOSS solutions has been reported to reduce drastically from 23.5 to 20 % in the presence of salt ions (Steffy et al. 2011). In addition to the effect of salt ions on the effectiveness of these surfactants in dispersing crude oil, temperature has been reported to influence the effectiveness in a significant manner. The effect of temperature on performance of the dispersant is dependent on whether or not the surfactants are anionic or nonionic (Fingas 2010). While the anionic surfactant DOSS readily desorbs from the oil droplet–water interface into the surrounding aqueous phase (Reichert and Walker 2013), the nonionic surfactant Tween 80 irreversibly adsorbs at the oil droplet–water interface (Kirby et al. 2015). The coalescence of emulsion droplets tends to minimize the dispersion effectiveness of dispersants upon application; hence, a stable emulsion must resist coalescence. Two types of coalescence behavior can be observed, namely, fast coalescence at low oil droplet surface coverage by surfactants and slow coalescence at high oil droplet surface coverage by surfactants (Reichert and Walker 2015). Coalescence is influenced by the presence of neutral electrolytes and the interfacial elasticity.
4 Photoremediation
After an oil spill has occurred, some of the spilled oil is lost through photooxidation (Lehr 2001). Most recent studies published have shown that a nanogrid produced from CuO and CuWO4 photocatalysts can remove benzene, toluene, ethylbenzene, and xylene (BTEX) from produced water obtained from hydraulic fracturing or fracking. The findings from this study can be applied as an additional cleaning step after oil spill remediation since studies conducted several months after the Gulf of Mexico oil spill revealed that water-dissolvable organic compounds from the spilled oil were still present in the Gulf waters (Ziervogel et al. 2012). Crude oil is a mixture of smaller volatile and larger nonvolatile hydrocarbon compounds. The smaller volatile hydrocarbon compounds can be remediated through natural evaporation processes while the large nonvolatile compounds can possibly be remediated though photooxidation and by other remediation methods. The classes of compounds present in crude oil are alkanes, cycloalkanes, waxes, benzenes, BTEX, PAHs, naphthenaromatics, resins, and asphaltenes (Fingas 2010). With these organic compounds being the main components of crude oil, photoremediation through photocatalysis may be the new area of attention for most researchers in the near future. Photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. The mechanism of the photocatalytic reaction at the surface of a semiconductor involves multiple steps (Shan et al. 2010): (a) Solar radiation with energy greater than or equal to the band gap of the semiconductor is absorbed, (b) electrons are then excited from the valence band to the conduction band creating an electron/hole pair, and (c) the valence band hole is strongly oxidizing and therefore can oxidize organic compound to innocuous compounds while the conduction band electron is strongly reducing. The process is summarized in Fig. 11.
Photocatalysis can therefore be utilized to oxidize the various organic compounds present in the spilled oil. The catalyst can be in a form of a nanogrid or particles. With the nanogrid, the grid can be placed on the spilled oil while utilizing solar radiation as its main source of energy. For particulate photocatalyst, the particles can form a slurry with the sea water and the spilled oil. Solar energy can then be utilized to carry out the photocatalysis. The second scenario can be limited by the concentration of the particles as lesser solar energy can penetrate and be absorbed when the particle concentration is too high. The advantages of using photoremediation are (a) the remediation cost will be reduced since free solar energy will play a central role in the remediation process, (b) a larger area can be targeted at the same time, (c) an environmentally benign photocatalyst will be used to reduce the toxicity of the remediation process, and (d) the highly toxic organic compounds will be oxidized into innocuous compounds. The perceived disadvantages are (a) the application of photoremediation will be dependent on the location of the spill since the solar insolations at the north and south poles are smaller than at the equator, (b) the time of the year that the oil spill occurred since the solar insolations vary from month to month, and (c) the difficulty in synthesizing a large amount of photocatalyst should a large oil spill like the Deepwater Horizon oil spill occurs. The fact that oxidation of the organic compounds may result in the production of carbon dioxide which is a green house gas is another perceived disadvantage. The CO2 produced can be captured and converted into a more useful and benign products.
5 Conclusions
From this review, it is evident that research has been conducted after the Deepwater Horizon oil spill with the main objective of finding alternative and benign oil spill remediation methods and to also understand existing systems. New oil sorbents and dispersants have been proposed from the findings of many research studies. Natural oil spill sorbent materials are very promising due to their ready availability and cheap cost. On the other hand, environmentally benign plant-based surfactants such as soybean lecithin are the future for oil spill remediation. However, a very effective dispersant formulation with near-complete dispersion effectiveness can only be attained if in-depth research is conducted to understand the behavior of the active ingredient (surfactant) at the oil droplet–water interface. In-depth research will include understanding the arrangement of different surfactant mixtures at the oil droplet–water interface, how emulsion coalescence can be reduced, and how the interfacial excess of a given dispersant formulation can be improved. Removal of spilled oil from the surface of the ocean can also be achieved through photoremediation. Future research in this field may include developing an efficient photocatalyst system to achieve a near-complete oil removal and that carbon-based compounds such as CO2 formed during the photoremediation process can be captured and converted into a more useful material through carbon capture. It is envisioned that continuous research into oil spill remediation methods will help address most, if not all, of the environmental concerns associated with existing oil spill remediation strategies.
References
Abdelwahab, O. (2014). Assessment of raw luffa as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. Alexandria Engineering Journal, 53(1), 213–218.
Abdullah, M., Rahmah, A. U., et al. (2010). Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. Journal of Hazardous Materials, 177(1), 683–691.
Abramson, D. M., I. E. Redlener, et al. (2010). Impact on children and families of the deepwater horizon oil spill: preliminary findings of the coastal population impact study. Report by the National Center for Disaster Preparedness, 2010-08:1–19
Adedokun, O. M., & Ataga, A. E. (2014). Oil spills remediation using native mushroom—a viable option. Research Journal of Environmental Sciences, 8(1), 57.
Al Zubaidy, E. (2012). Effect of activation of date palm kernel powder on the remediation process of oil polluted water. Journal ISSN, 1929, 2732.
Alcantar, N. A., Fox, D. I., & Thomas, S., (2012). Use of cactus mucilage as a dispersant and absorbant for oil in oil-water mixtures. U.S. Patent Application 13/687,595, filed November 28, 2012.
Angelova, D., Uzunov, I., et al. (2011). Kinetics of oil and oil products adsorption by carbonized rice husks. Chemical Engineering Journal, 172(1), 306–311.
Antizar-Ladislao, B. (2010). Bioremediation: working with bacteria. Elements, 6(6), 389–394.
Asadov, Z. H., Rahimov, R. A., et al. (2012a). Synthesis of animal fats ethylolamides, ethylolamide phosphates and their petroleum-collecting and dispersing properties. Journal of the American Oil Chemists’ Society, 89(3), 505–511.
Asadov, Z. H., Tantawy, A. H., et al. (2012b). Surfactants based on palmitic acid and nitrogenous bases for removing thin oil slicks from water surface. Chemistry Journal, 2(04), 136–145.
Asadov, Z. H., Tantawy, A. H., et al. (2013). Synthesis of new surface-active ammonium-type complexes based on palmitic acid for removing thin petroleum films from water surface. Egyptian Journal of Petroleum, 22(2), 261–267.
Asadov, Z. H., Zarbaliyeva, I. A., et al. (2014). Petroleum-collecting and dispersing chemicals for cleaning sea surface from thin petroleum slicks. Bulletin of the Chemical Society of Ethiopia, 28(2), 205–214.
Athas, J. C., Jun, K., et al. (2014). An effective dispersant for oil spills based on food-grade amphiphiles. Langmuir, 30(31), 9285–9294.
Banat, I. M., Franzetti, A., et al. (2010). Microbial biosurfactants production, applications and future potential. Applied Microbiology and Biotechnology, 87(2), 427–444.
Basak, S., Nanda, J., et al. (2012). A new aromatic amino acid based organogel for oil spill recovery. Journal of Materials Chemistry, 22(23), 11658–11664.
Bi, H., Xie, X., et al. (2012). Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Advanced Functional Materials, 22(21), 4421–4425.
Bose, A., John, V. T., & Nikova, A. T., (2012). Oil emulsification and polycyclic aromatic hydrocarbon adsorption using fine particles as dispersants. U.S. Patent Application 14/131,323, filed July 10, 2012.
Campo, P., Venosa, A. D., et al. (2013). Biodegradability of Corexit 9500 and dispersed South Louisiana crude oil at 5 and 25 C. Environmental Science & Technology, 47(4), 1960–1967.
Chapman, H., Purnell, K., et al. (2007). The use of chemical dispersants to combat oil spills at sea: a review of practice and research needs in Europe. Marine Pollution Bulletin, 54(7), 827–838.
Chen, M., Jiang, W., et al. (2013). Synthesis of highly hydrophobic floating magnetic polymer nanocomposites for the removal of oils from water surface. Applied Surface Science, 286, 249–256.
Cortese, B., Caschera, D., et al. (2014). Superhydrophobic fabrics for oil–water separation through a diamond like carbon (DLC) coating. Journal of Materials Chemistry A, 2(19), 6781–6789.
Cui, Y., Threlfall, M., et al. (2011). Optimizing organoclay stabilized Pickering emulsions. Journal of Colloid and Interface Science, 356(2), 665–671.
D’Andrea, M. A., & Reddy, G. K. (2013). Health consequences among subjects involved in gulf oil spill clean-up activities. The American Journal of Medicine, 126(11), 966–974.
de Souza Sobrinho, B., de Souza Sobrinho, H. B., et al. (2013). Application of biosurfactant from Candida sphaerica UCP 0995 in removal of petroleum derivative from soil and sea water. Journal of Life Sciences, 7(6), 559–569.
Deng, D., Prendergast, D. P., et al. (2013). Hydrophobic meshes for oil spill recovery devices. ACS Applied Materials & Interfaces, 5(3), 774–781.
Dong, J., Worthen, A. J., et al. (2014). Modified montmorillonite clay microparticles for stable oil-in-seawater emulsions. ACS Applied Materials & Interfaces, 6(14), 11502–11513.
Dubansky, B., Whitehead, A., et al. (2013). Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident Gulf killifish (Fundulus grandis). Environmental Science & Technology, 47(10), 5074–5082.
Elias, E., Costa, R., et al. (2015). Oil-spill cleanup: The influence of acetylated curaua fibers on the oil-removal capability of magnetic composites. Journal of Applied Polymer Science, 132(13), 41732–41739.
EPA, U. S. (09/30/2014). Emergency Response. Retrieved April 9, 2015, 2015, from http://www2.epa.gov/emergency-response/sorbents.
Fan, Z., Yan, J., et al. (2010). Oil sorption and recovery by using vertically aligned carbon nanotubes. Carbon, 48(14), 4197–4200.
Fingas, M. (2010). Oil spill science and technology. Houston: Gulf professional publishing.
Geitner, N. K., Bhattacharya, P., et al. (2012). Understanding dendritic polymer–hydrocarbon interactions for oil dispersion. RSC Advances, 2(25), 9371–9375.
Geitner, N. K., Wang, B., et al. (2014). Structure–function relationship of PAMAM dendrimers as robust oil dispersants. Environmental Science & Technology, 48(21), 12868–12875.
Gu, J., Jiang, W., et al. (2014). Facile removal of oils from water surfaces through highly hydrophobic and magnetic polymer nanocomposites. Applied Surface Science, 301, 492–499.
Gui, X., Wei, J., et al. (2010). Carbon nanotube sponges. Advanced Materials, 22(5), 617–621.
Gui, X., Li, H., et al. (2011). Recyclable carbon nanotube sponges for oil absorption. Acta Materialia, 59(12), 4798–4804.
Gui, X., Zeng, Z., et al. (2013). Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Applied Materials & Interfaces, 5(12), 5845–5850.
He, Y., Liu, Y., et al. (2013). An environmentally friendly method for the fabrication of reduced graphene oxide foam with a super oil absorption capacity. Journal of Hazardous Materials, 260, 796–805.
Hook, S. E., & Osborn, H. L. (2012). Comparison of toxicity and transcriptomic profiles in a diatom exposed to oil, dispersants, dispersed oil. Aquatic Toxicology, 124, 139–151.
Hubbe, M. A., Hasan, S. H., et al. (2011). Cellulosic substrates for removal of pollutants from aqueous systems: a review. 1. Metals. BioResources, 6(2), 2161–2287.
Hussein, M., Amer, A., et al. (2011). Heavy oil spill cleanup using law grade raw cotton fibers: trial for practical application. Journal of Petroleum Technology and Alternative Fuels, 2(8), 132–140.
Jadhav, S. R., Vemula, P. K., et al. (2010). Sugar‐derived phase‐selective molecular gelators as model solidifiers for oil spills. Angewandte Chemie, 122(42), 7861–7864.
Ji, N., Chen, H., et al. (2011). Synthesis of high oil‐absorption resins of poly (methyl methacrylate‐butyl methacrylate) by suspended emulsion polymerization. Polymers for Advanced Technologies, 22(12), 1898–1904.
Karakasi, O., & Moutsatsou, A. (2010). Surface modification of high calcium fly ash for its application in oil spill clean up. Fuel, 89(12), 3966–3970.
Karan, C. P., Rengasamy, R., et al. (2011). Oil spill cleanup by structured fibre assembly. Indian Journal of Fibre & Textile Research, 36(2), 190–200.
Katepalli, H., John, V. T., et al. (2013). The response of carbon black stabilized oil-in-water emulsions to the addition of surfactant solutions. Langmuir, 29(23), 6790–6797.
Kenes, K., Yerdos, O., et al. (2012). Study on the effectiveness of thermally treated rice husks for petroleum adsorption. Journal of Non-Crystalline Solids, 358(22), 2964–2969.
Kim, J., Cote, L. J., et al. (2010). Graphene oxide sheets at interfaces. Journal of the American Chemical Society, 132(23), 8180–8186.
Kirby, S. M., Anna, S. L., et al. (2015). Sequential adsorption of an irreversibly adsorbed nonionic surfactant and an anionic surfactant at an oil/aqueous interface. Langmuir, 31(14), 4063–4071.
Kizil, S., Karadag, K., et al. (2015). Poly (alkoxysilane) reusable organogels for removal of oil/organic solvents from water surface. Journal of Environmental Management, 149, 57–64.
Konstantinou, I., & Sidiras, D. (2014). Oil spill cleaning using modified wheat straw in the presence of chemical dispersant. World Journal of Environmental Research, 4(2), 66–73.
Kostka, J. E., Prakash, O., et al. (2011). Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Applied and Environmental Microbiology, 77(22), 7962–7974.
Kraft, D. J., de Folter, J. W., et al. (2010). Conditions for equilibrium solid-stabilized emulsions. The Journal of Physical Chemistry B, 114(32), 10347–10356.
Kujawinski, E. B., Kido Soule, M. C., et al. (2011). Fate of dispersants associated with the Deepwater Horizon oil spill. Environmental Science & Technology, 45(4), 1298–1306.
Kumpanenko, I., Roschin, A., et al. (2014). Method of obtaining structured hydrocarbon gels for increasing the efficiency of oil spill recovery technologies. Russian Journal of Physical Chemistry B, 8(5), 720–725.
Lehr, W. J. (2001). Review of modeling procedures for oil spill weathering behavior. Advances in Ecological Sciences, 9, 51–90.
Li, H., Liu, L., et al. (2012a). Hydrophobic modification of polyurethane foam for oil spill cleanup. Marine Pollution Bulletin, 64(8), 1648–1653.
Li, W., Yu, L., et al. (2012b). Oil-in-water emulsions stabilized by Laponite particles modified with short-chain aliphatic amines. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 400, 44–51.
Li, H., Li, Y., et al. (2013). ZnO nanorod array-coated mesh film for the separation of water and oil. Nanoscale Research Letters, 8(1), 1–6.
Li, Y.-Q., Samad, Y. A., et al. (2014). Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustainable Chemistry & Engineering, 2(6), 1492–1497.
Li, Y., Samad, Y. A., et al. (2015). Lightweight and highly conductive aerogel-like carbon from sugarcane with superior mechanical and EMI shielding properties. ACS Sustainable Chemistry & Engineering, 3(7), 1419–1427.
Lin, J., Ding, B., et al. (2012). Subtle regulation of the micro-and nanostructures of electrospun polystyrene fibers and their application in oil absorption. Nanoscale, 4(1), 176–182.
Lin, J., Tian, F., et al. (2013). Co-axial electrospun polystyrene/polyurethane fibres for oil collection from water surface. Nanoscale, 5(7), 2745–2755.
Liu, H. D., Liu, Z. Y., et al. (2013a). Surperhydrophobic polyurethane foam modified by graphene oxide. Journal of Applied Polymer Science, 130(5), 3530–3536.
Liu, Y., Ma, J., et al. (2013b). Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Applied Materials & Interfaces, 5(20), 10018–10026.
Lustgarten, A. (2010, August 8, 2015). Chemicals Meant To Break Up BP Oil Spill Present New Environmental Concerns. Retrieved August 18, 2015, from http://www.propublica.org/article/bp-gulf-oil-spill-dispersants-0430.
Mao, J., Jiang, W., et al. (2014). Synthesis of P (St-DVB)/Fe 3 O 4 microspheres and application for oil removal in aqueous environment. Applied Surface Science, 317, 787–793.
Marti, M., Colonna, W., et al. (2014). Production and characterization of microbial biosurfactants for potential use in oil-spill remediation. Enzyme and Microbial Technology, 55, 31–39.
Mason, O. U., Hazen, T. C., et al. (2012). Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. The ISME Journal, 6(9), 1715–1727.
McNutt, M. K., Camilli, R., et al. (2012). Review of flow rate estimates of the Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences, 109(50), 20260–20267.
Montagnolli, R. N., Lopes, P. R. M., et al. (2015). Assessing Bacillus subtilis biosurfactant effects on the biodegradation of petroleum products. Environmental Monitoring and Assessment, 187(1), 1–17.
Moshtagh, B. and K. Hawboldt (2014). Production of biodispersants for oil spill remediation in Harsh environment using glycerol from the conversion of fish oil to biodiesel. Oceans-St. John’s, 2014, IEEE. p 1–7.
Nabeel Rashin, M., Kutty, R. G., et al. (2014). Novel coconut oil based magnetite nanofluid as an ecofriendly oil spill remover. Industrial & Engineering Chemistry Research, 53(40), 15725–15730.
Nedwed, T. (2010). New dispersant gel treats marine oil spills more effectively with less product. In SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production. Richardson: Society of Petroleum Engineers.
Nedwed, T., Canevari, G. P., et al. (2011). New dispersant gel effective on cold, viscous oils. In International Oil Spill Conference Proceedings (IOSC). Washington, D.C.: American Petroleum Institute.
Nyankson, E. (2015). Smart dispersant formulations for reduced environmental impact of crude oil spills, Auburn University. Auburn: Auburn University.
Nyankson, E., Ober, C. A., et al. (2014). Comparison of the Effectiveness of solid and solubilized dioctyl sodium sulfosuccinate (DOSS) on oil dispersion using the baffled flask test, for crude oil spill applications. Industrial & Engineering Chemistry Research, 53(29), 11862–11872.
Nyankson, E., DeCuir, M. J., et al. (2015a). Soybean lecithin as a dispersant for crude oil spills. ACS Sustainable Chemistry & Engineering, 3(5), 920–931.
Nyankson, E., Olasehinde, O., et al. (2015b). Surfactant-loaded halloysite clay nanotube dispersants for crude oil spill remediation. Industrial & Engineering Chemistry Research, 54(38), 9328–9341.
Owoseni, O., Nyankson, E., et al. (2014). Release of surfactant cargo from interfacially-active halloysite clay nanotubes for oil spill remediation. Langmuir, 30(45), 13533–13541.
Owoseni, O., Nyankson, E., et al. (2016). Interfacial adsorption and surfactant release characteristics of magnetically functionalized halloysite nanotubes for responsive emulsions. Journal of Colloid and Interface Science, 463, 288–298.
Palchoudhury, S., & Lead, J. R. (2014). A facile and cost-effective method for separation of oil–water mixtures using polymer-coated iron oxide nanoparticles. Environmental Science & Technology, 48(24), 14558–14563.
Paulauskienė, T., Jucikė, I., et al. (2014). The use of natural sorbents for spilled crude oil and diesel cleanup from the water surface. Water, Air, & Soil Pollution, 225(6), 1–12.
Payne, K. C., Jackson, C. D., et al. (2012). Oil spills abatement: factors affecting oil uptake by cellulosic fibers. Environmental Science & Technology, 46(14), 7725–7730.
Petroleum, B. (2011). Gulf of Mexico restoration. Retrieved August 8, 2015, from http://www.bp.com/en/global/corporate/gulf-of-mexico-restoration/deepwater-horizon-accident-and-response/shoreline.html.
Pickering, S. U. (1907). Cxcvi.—emulsions. Journal of the Chemical Society, Transactions, 91, 2001–2021.
Powell, K. C., & Chauhan, A. (2014). Interfacial tension and surface elasticity of carbon Black (CB) covered oil–water interface. Langmuir, 30(41), 12287–12296.
Ramsden, W. (1903). The separation of solid materials on the surface of solutions and suspensions. Observations concerning surface diaphragms, foam blisters, emulsions and mechanical coagulation. Proceedings of the Royal Society.
Raza, A., Ding, B., et al. (2014a). In situ cross-linked superwetting nanofibrous membranes for ultrafast oil–water separation. Journal of Materials Chemistry A, 2(26), 10137–10145.
Raza, A., Ge, J., et al. (2014b). Applications of electrospun nanofibers in oil spill cleanup. In Electrospun nanofibers for energy and environmental applications (pp. 433–447). Berlin: Springer.
Reddy, N., & Yang, Y. (2009). Extraction and characterization of natural cellulose fibers from common milkweed stems. Polymer Engineering & Science, 49(11), 2212–2217.
Reddy, C. M., Arey, J. S., et al. (2012). Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences, 109(50), 20229–20234.
Reichert, M. D., & Walker, L. M. (2013). Interfacial tension dynamics, interfacial mechanics, and response to rapid dilution of bulk surfactant of a model oil–water-dispersant system. Langmuir, 29(6), 1857–1867.
Reichert, M. D., & Walker, L. M. (2015). Coalescence behavior of oil droplets coated in irreversibly-adsorbed surfactant layers. Journal of Colloid and Interface Science, 449, 480–487.
Rengasamy, R., Das, D., et al. (2011). Study of oil sorption behavior of filled and structured fiber assemblies made from polypropylene, kapok and milkweed fibers. Journal of Hazardous Materials, 186(1), 526–532.
Riehm, D. A., & McCormick, A. V. (2014). The role of dispersants’ dynamic interfacial tension in effective crude oil spill dispersion. Marine Pollution Bulletin, 84(1), 155–163.
Rodd, A. L., Creighton, M. A., et al. (2014). Effects of surface-engineered nanoparticle-based dispersants for marine oil spills on the model organism artemia franciscana. Environmental Science & Technology, 48(11), 6419–6427.
Ron, E. Z., & Rosenberg, E. (2014). Enhanced bioremediation of oil spills in the sea. Current Opinion in Biotechnology, 27, 191–194.
Rubasinghe, R., Gunatilake, S., et al. (2013). uitability of fibre complexes of coir and banana as eco-friendly sorbent material for oil spills removals. Proceedings to 29th Technical Sessions of Geological Society of Sri Lanka, 131, 134.
Saber, O., Mohamed, N. H., et al. (2015). Synthesis of magnetic nanoparticles and nanosheets for oil spill removal. Nanoscience & Nanotechnology-Asia, 5(1), 32–43.
Saha, A., Nikova, A., et al. (2012). Oil emulsification using responsive carbon black particles. In Abstracts of papers of the american chemical society (p. 1155). Washington, DC: Amer Chemical Soc.
Saha, A., Nikova, A., et al. (2013). Oil emulsification using surface-tunable carbon black particles. ACS Applied Materials & Interfaces, 5(8), 3094–3100.
Schor, E. (2010). Ingredients of controversial dispersants used on gulf spill are secrets no more. Retrieved August 8, 2015, from http://www.nytimes.com/gwire/2010/06/09/09greenwire-ingredients-of-controversial-dispersants-used-42891.html.
Sek, J., Shtyka, O., et al. (2013). Analysis of the effectiveness of polypropylene fibers usage as sorbents of oil spills resulting from the transport acidents. Mokslas: Lietuvos Ateitis, 5(5), 536.
Shahi, A. (2014). Kraft lignin: a novel alternative to oil spill cleanup recycling industrial waste. Canadian Young Scientist Journal, 2014(3), 42–46.
Shan, A. Y., Ghazi, T. I. M., et al. (2010). Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Applied Catalysis A: General, 389(1), 1–8.
Shavandi, M., Mohebali, G., et al. (2011). Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids and Surfaces B: Biointerfaces, 82(2), 477–482.
Sheppard, P. J., Simons, K. L., et al. (2014). The application of a carrier-based bioremediation strategy for marine oil spills. Marine Pollution Bulletin, 84(1), 339–346.
Silliman, B. R., van de Koppel, J., et al. (2012). Degradation and resilience in Louisiana salt marshes after the BP–Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences, 109(28), 11234–11239.
Singh, V., Kendall, R. J., et al. (2013). Crude oil sorption by raw cotton. Industrial & Engineering Chemistry Research, 52(18), 6277–6281.
Singh, V., Jinka, S., et al. (2014). Novel natural sorbent for oil spill cleanup. Industrial & Engineering Chemistry Research, 53(30), 11954–11961.
Song, C., Ding, L., et al. (2013). β-Cyclodextrin-based oil-absorbent microspheres: preparation and high oil absorbency. Carbohydrate Polymers, 91(1), 217–223.
Song, J., Huang, S., et al. (2014). Self-driven one-step oil removal from oil spill on water via selective-wettability steel mesh. ACS Applied Materials & Interfaces, 6(22), 19858–19865.
Sorial, G. A., Venosa, A. D., et al. (2004). Oil spill dispersant effectiveness protocol. II: performance of revised protocol. Journal of Environmental Engineering, 130(10), 1085–1093.
Steffy, D. A., Nichols, A. C., et al. (2011). Investigating the effectiveness of the surfactant dioctyl sodium sulfosuccinate to disperse oil in a changing marine environment. Ocean Science Journal, 46(4), 299–305.
Teli, M., & Valia, S. P. (2013). Acetylation of jute fiber to improve oil absorbency. Fibers and Polymers, 14(6), 915–919.
Törncrona, A, Holmberg, K, et al. (2012). Modified silica particles, Google Patents.
Upton, HF (2011). The Deepwater Horizon oil spill and the Gulf of Mexico fishing industry, Congressional Research Service, Library of Congress.
Valentine, D. L., Kessler, J. D., et al. (2010). Propane respiration jump-starts microbial response to a deep oil spill. Science, 330(6001), 208–211.
Venkataraman, P., Tang, J., et al. (2013). Attachment of a hydrophobically modified biopolymer at the oil–water interface in the treatment of oil spills. ACS Applied Materials & Interfaces, 5(9), 3572–3580.
Wang, J., Liu, G., et al. (2010). Synergistic stabilization of emulsions by poly (oxypropylene) diamine and Laponite particles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 353(2), 117–124.
Wang, D., McLaughlin, E., et al. (2011). Aqueous phase adsorption of toluene in a packed and fluidized bed of hydrophobic aerogels. Chemical Engineering Journal, 168(3), 1201–1208.
Wang, D., McLaughlin, E., et al. (2012a). Adsorption of oils from pure liquid and oil–water emulsion on hydrophobic silica aerogels. Separation and Purification Technology, 99, 28–35.
Wang, J., Zheng, Y., et al. (2012b). Effect of kapok fiber treated with various solvents on oil absorbency. Industrial Crops and Products, 40, 178–184.
Wang, B., Karthikeyan, R., et al. (2013a). Hollow carbon fibers derived from natural cotton as effective sorbents for oil spill cleanup. Industrial & Engineering Chemistry Research, 52(51), 18251–18261.
Wang, J., Wang, Q., et al. (2013b). Synthesis and oil absorption of poly (butylmethacrylate)/organo‐attapulgite nanocomposite by suspended emulsion polymerization. Polymer Composites, 34(2), 274–281.
Wang, F., Lei, S., et al. (2014a). Superhydrophobic and superoleophilic miniature device for the collection of oils from water surfaces. The Journal of Physical Chemistry C, 118(12), 6344–6351.
Wang, F., Lei, S., et al. (2014b). In situ separation and collection of oil from water surface via a novel superoleophilic and superhydrophobic oil containment boom. Langmuir, 30(5), 1281–1289.
Whitehead, A., Dubansky, B., et al. (2011). Science applications in the Deepwater Horizon oil spill special feature: genomic and physiological footprint of the Deepwater Horizon oil spill on resident marsh fishes. Proceedings of the National Academy of Science, 100, 20298–20302.
Worthen, A. J., Foster, L. M., et al. (2014). Synergistic formation and stabilization of oil-in-water emulsions by a weakly interacting mixture of zwitterionic surfactant and silica nanoparticles. Langmuir, 30(4), 984–994.
Yu, G., Dong, J., et al. (2015). Breakup of oil jets into droplets in seawater with environmentally benign nanoparticle and surfactant dispersants. Industrial & Engineering Chemistry Research, 54, 4243–4251.
Yuan, X., & Chung, T. M. (2012). Novel solution to oil spill recovery: using thermodegradable polyolefin oil superabsorbent polymer (oil–SAP). Energy & Fuels, 26(8), 4896–4902.
Yvon-Lewis, S. A., Hu, L., et al. (2011). Methane flux to the atmosphere from the Deepwater Horizon oil disaster. Geophysical Research Letters, 38(1), 1–5.
Zeng, Y., Wang, K., et al. (2014). Hollow carbon beads fabricated by phase inversion method for efficient oil sorption. Carbon, 69, 25–31.
Zhang, A., Chen, M., et al. (2013). Poly (dimethylsiloxane) oil absorbent with a three-dimensionally interconnected porous structure and swellable skeleton. ACS Applied Materials & Interfaces, 5(20), 10201–10206.
Zhang, J., Li, L., et al. (2014a). Effect of cetyltrimethylammonium bromide addition on the emulsions stabilized by montmorillonite. Colloid and Polymer Science, 292(2), 441–447.
Zhang, Y., Yang, S., et al. (2014b). Preparation and characterization of lignocellulosic oil sorbent by hydrothermal treatment of populus fiber. Materials, 7(9), 6733–6747.
Zhao, J., Xiao, C., et al. (2013). Fabrication and characterization of oil-absorptive fiber by polypropylene and poly (butyl methacrylate-co-hydroxyethyl methacrylate) blends. Journal of Thermoplastic Composite Materials, 27(1), 3–17. 0892705712461519.
Zhu, H., Qiu, S., et al. (2011). Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environmental Science & Technology, 45(10), 4527–4531.
Zhu, K., Shang, Y.-Y., et al. (2013). Oil spill cleanup from sea water by carbon nanotube sponges. Frontiers of Materials Science, 7(2), 170–176.
Ziervogel, K., McKay, L., et al. (2012). Microbial activities and dissolved organic matter dynamics in oil-contaminated surface seawater from the Deepwater Horizon oil spill site. PLoS One, 7(4), 34816.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nyankson, E., Rodene, D. & Gupta, R.B. Advancements in Crude Oil Spill Remediation Research After the Deepwater Horizon Oil Spill. Water Air Soil Pollut 227, 29 (2016). https://doi.org/10.1007/s11270-015-2727-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2727-5