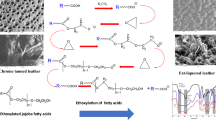
Abstract
Ethylolamides of animal (beef and mutton) fats have been synthesized and modified with H3PO4. The identity of these products was confirmed by IR and NMR spectroscopy. The physical properties of the synthesized surfactants including interfacial tension and critical micelle concentration (CMC) were studied. From these measurements, the maximum surface excess concentration and the minimum area per molecule at the kerosene solution/water interface, the surface pressure at the CMC, and the standard thermodynamic parameters of adsorption and micellization were calculated. Ethylolamides and ethylolamide phosphates were obtained and tested as petroleum-collecting and petroleum-dispersing reagents. Some correlations between these parameters of the ethylol units and their ability to collect thin petroleum films from the water surface were revealed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive carrying of crude oil and its products across the oceans has increased concerns about the effects of accidental spillage of petroleum hydrocarbons in the marine environment. Major marine oil spills highlight the need for cost-effective and environmentally responsible ways for their liquidation. Similar oil spill incidents world wide indicate that the ‘first-minute response’ principle plays a key role as a environmentally safe and cost-effective response to marine oil spills [1]. The economic and environmental impact of oil spills on coastal areas can be immense. Oil spill occurrences may result in loss of habitats for economically important species of fish, other marine animals, and damage to sensitive wetlands along the coast. Recovery of the environment from an oil spill can take many years, so there is a considerable incentive to clean up these areas quickly after a spill, but the efforts can be expensive and themselves destructive [2].
One oil spill response tactic is the use of dispersants. Dispersants are designed to disperse an oil slick chemically so that the oil enters the water column, minimizing the possibility of being washed up on shorelines. As wave energy is applied to the chemically dispersed oil when using the dispersants at sea, the slick breaks up into small droplets that disperse into the water column [3]. Dispersants may enhance oil bio-availability by creating a greater surface area in terms of small oil droplets, allowing for increased biodegradation of the oil [4, 5]. The effects of dispersants on the fate of dispersed oil have often shown conflicting results [6]. Dispersant use in coastal ecosystems has been approached cautiously. Generally, marine ecosystems are sensitive to oil damage, and dispersant use has raised questions about dispersed oil.
A typical commercial oil dispersant is a blend of three types of chemicals, namely surfactant, solvent, and additives. Surfactants are responsible for the decrease in the o/w interfacial tension (γ), which favors the dispersal of oil into tiny droplets in water. Solvents are used for the dissolution of surfactants and additives in order to end up with a homogenous system. The additives serve many purposes such as to increase the biodegradation of the dispersion, to improve the dissolution of the dispersant in the oil slick and to increase the long-term stability of the dispersion.
One of the most interesting classes of non-ionic surfactants includes alkylolamides. Fatty alkylolamides, namely monoalkylolamide and dialkylolamide can be prepared by reacting fatty acids or fatty acid methyl esters with alkanolamine at elevated temperatures [7]. Recently, secondary fatty amides have been synthesized using triacylglycerides from tallow and tripalmitin to react with amine, such as ethanolamine, diethanolamine, ethylenediamine, diethylenediamine, and others [8]. Kolancilar also reported the preparation of ethylolamides from laurel oil of black olive-sized fruits of Laurus nabilis L. [9]. The alkylolamides are usually used as non-ionic surfactant in the household and cosmetic industries. They can be prepared by reacting diethanolamine with natural glycerides, such as soybean, linseed, peanut, and sunflower oils [10]. Fatty alkylolamides are compounds that exhibit low reactivity and high thermal stability. Their chemical properties vary, depending on the lengths of their hydrocarbon chains and the nature of the substituent on the nitrogen atom [11]. Alkylolamides are of great interest for applications requiring relatively stable emulsifiers because their amide linkages are very stable chemically and not easily degraded in alkaline media [12].
Non-ionic surfactants find applications in different operations of petroleum production. They are essentially used to improve the production economics and total recovery of petroleum [13] as well as in environment protection [14].
The main aim of the presented work was to produce new, ecologically safe and efficient oil slick-collecting and dispersing agents based on animal (beef and mutton) fats through their interaction with monoethanolamine (MEA) and diethanolamine (DEA). As a result of such interactions ethylolamide groups will appear in the composition of the compounds produced. These non-ionic surfactants may be converted into their anionic phosphate-containing derivatives which are also intended for a use against oil slicks. The surface properties of both ethylolamides and their phosphates were thoroughly studied.
Experimental Procedures
Materials
Mono- and diethanolamine were from the “Kazanorgsintez” Joint Stock Company (Russia) and were used without further purification. The animal species, namely mutton and beef fats were collected from the local market in Baku, Azerbaijan. ortho-Phosphoric acid (85% wt. solution) was from Moscow’s “Component-Reactant” Joint Stock Company (Russia) production. Kerosene was purified from aromatic compounds by treatment with sulfuric acid up to a surface tension of 24 mN/m. Sea water was Caspian sea water having the following physico-chemical characteristics and composition [15]: ρ20 = 1.0098 g/mL, pH = 7.7, contents of ions and other species (g/kg): Na+ 2.99; K+ 0.09; Ca2+ 0.34; Mg2+ 0.70; Cl− 5.18; SO4 2− 2.98.
Instrumentation
1H-NMR and 13C-NMR spectra were recorded on a Bruker TOP SPIN 300.13 MHz and 75.46 MHz spectrometer with chemical shift values (δ) in ppm downfield from TMS using acetone-d6 and CCl4 as solvents. IR spectra were recorded on a model FT-IR, Spectrum BX spectrometer using KBr disks.
Methods
General Procedure for the Synthesis of Alkylolamides from Animal Fat
Animal fats (0.03 mol) and ethanolamine (0.09 mol) were weighed into a 250-mL round-bottomed flask and reacted with agitation at a temperature of 140–150 °C for 10–12 h. The reaction flask was equipped with a mechanical stirrer, thermometer and condenser. The reaction product was dissolved in water and placed in a separatory funnel. The mixture was washed with 20 mL of 5% aqueous hydrochloric acid to remove excess amine and glycerol. The wax layer was separated, washed with two 100-mL portions of water, boiled with 0.5 g of decolorizing charcoal to remove the yellow color, and filtered. The monoethylolamide was dried over potassium carbonate and the water was rotary-evaporated. Monoethylolamides yields equaled 90–92%.
MFMEA is a yellow wax, insoluble in water. IR ν max (cm−1): 1555 (C–N), 1641 (C=O), 2917 (C–H), 3295 (O–H) (Fig. 1). 1H-NMR (300.13 MHz, acetone-d6 and CCl4), δ (ppm): 0.87 (CH 3 ), 1.20–1.33 (CH 2 chain), 3.39 (NH–CH 2 –CH2), 3.80 (CH2–CH 2–OH). 13C–{1H} NMR (75.46 MHz, acetone-d6 and CCl4), δ (ppm): 13.9–36.5 (saturated alkyl chain), 41.5 (NH–CH 2 –CH2), 61.0 (CH2–CH 2 –OH), 177.5 C(O)N.
BFMEA is a yellow wax, insoluble in water. IR ν max (cm−1): 1560 (C–N), 1643 (C=O), 2924 (C–H), 3303 (O–H). 1H-NMR (300.13 MHz, acetone-d6 and CCl4), δ (ppm): 0.91 (CH 3 ), 1.29–1.33 (CH 2 chain), 3.39 (NH–CH 2 –CH2), 3.79 (CH2–CH 2 –OH). 13C–{1H} NMR (75.46 MHz, acetone-d6 and CCl4), δ (ppm): 14.1–36.5 (saturated alkyl chain), 41.5 (NH–CH 2 –CH2), 61.0 (CH2–CH 2 –OH), 178.0 C(O)N.
During synthesis of diethylolamides, the reaction product was dissolved in acetone, and placed in the condenser at −25 to 28 °C. The wax phase was separated by filtering and then dried. The diethylolamides were recrystallized from acetone. Diethylolamides yields were equal to 87–89%.
MFDEA is a brown yellow wax, soluble in water. IR ν max (cm−1): 1555 (C–N), 1632 (C=O), 2922 (C–H), 3296 (O–H). 1H-NMR (300.13 MHz, acetone-d6 and CCl4), δ (ppm): 0.87 (CH 3 ), 1.26–1.33 (CH 2 chain), 3.39 (N–CH 2 –CH2), 3.79 (CH2–CH 2 –OH). 13C–{1H} NMR (75.46 MHz, acetone-d6 and CCl4), δ (ppm): 13.5–34.9 (saturated alkyl chain), 50.1 (N–CH 2 –CH2), 58.8 (CH2–CH 2 –OH), 177.5 C(O)N.
BFDEA is a brown-yellow wax, soluble in water. IR ν max (cm−1): 1555 (C–N), 1632 (C=O), 2922 (C–H), 3296 (O–H). 1H-NMR (300.13 MHz, acetone-d6 and CCl4) δ (ppm): 0.92 (CH 3 ), 1.26–1.33 (CH 2 chain), 3.39 (N–CH 2 –CH2), 3.79 (CH2–CH 2 –OH). 13C–{1H} NMR (75.46 MHz, acetone-d6 and CCl4), δ (ppm): 13.5–34.9 (saturated alkyl chain), 50.1 (N–CH 2 –CH2), 58.8 (CH2–CH 2 –OH), 178.0 C(O)N (Fig. 2).
General Procedure for the Synthesis of Ethylolamide Phosphates from Alkylolamide
Alkylolamides and H3PO4 were placed in the flask and heated to 55–60 °C. The alkylolamides were then reacted with H3PO4 at a molar ratio of 1:1 (alkylolamide:H3PO4). A water bath was used to maintain a constant temperature. The reaction mixture was stirred continuously for a predetermined reaction period. The progress of the reaction was monitored by analyzing the amount of unreacted H3PO4 in the reaction mixture by a titrimetric method. For purification, the product was dissolved in a mixture of ethanol.
MFMEAPh is a yellow wax, insoluble in water. IR ν max (cm−1): 1108 (P–O), 1550 (C–N), 1637 (C=O), 2918 (C–H).
MFDEAPh is a yellow wax, insoluble in water. IR ν max (cm−1): 1168 (P–O), 1530 (C–N), 1648 (C=O), 2922 (C–H).
BFMEAPh is a yellow wax, insoluble in water. IR ν max (cm−1): 1162 (P–O), 1547 (C–N), 1642 (C=O), 2924 (C–H).
BFDEAPh is a yellow wax, insoluble in water. IR ν max (cm−1): 1167 (P–O), 1563 (C–N), 1620 (C=O), 2922 (C–H).
Interfacial Tension Measurements
All the interfacial tension measurements were carried out using kerosene to make the solutions. The solutions kept at the desired temperature were measured 45 s after transfer to the thermostated measuring dishes. The actual temperature within the dishes was controlled prior to and after the measurement by means of a thermocouple. Deviations from the desired temperature were ±0.2 °C. The interfacial tension as a function of concentration was measured at 25 °C using a drop volume stalagmometer. Interfacial tension values from the two measurements varying by no more than 0.2 mN/m were averaged and reported.
Procedure for Studying Petroleum-Collecting and Petroleum-Dispersing Capacities
Petroleum-collecting properties of the synthesized ethylolamides and their phosphates (in the pure state and in the form of 5% wt. water or alcoholic solution) have been studied on the example of crude oil (density and kinematic viscosity at 20 °C are respectively 0.86 g/cm3 and 0.16 cm2/s) from the Ramany oil field in the Absheron peninsula (Azerbaijan). The surfactant (0.02 g) or its solution was added to a thin film (thickness 0.15–0.16 mm) of this petroleum on the surface of distilled water and the Caspian sea water (separately) in Petri dishes. The maximum values of the petroleum collecting coefficient (K) are calculated using the formula K = S o/S, where S o is an area of the surface of initial petroleum film and S is an area of the surface of accumulated petroleum (as a thickened spot). Since the moment of the surfactant application observations are carried out with measurement of the spot surface area and determination of the K values at fixed time intervals (τ).
Results and Discussion
Synthesis of Ethylolamides
The reaction was carried out using animal (beef and mutton) fats as the starting material. The fat was reacted with MEA and DEA at molar ratio of 1:3 (animal fat:MEA and animal fat:DEA), glycerol being produced as the by-product of the reaction: Scheme 1
Synthesis of Ethylolamide Phosphates
The general scheme of the conducted modification reaction is as following: Scheme 2.
Surface Activity of the Synthesized Surfactants
The critical micelle concentrations (CMC) were determined by the surface balance method. The CMC values of the prepared alkylolamides were determined at 298 K from the change in the slope of the plotted data of interfacial tension versus the natural logarithm of the solute concentration. Some representative plots (Figs. 3, 4) are illustrated here for brevity. The values of the CMC obtained for nonionic surfactants at 298 K temperatures are tabulated in Table 1, together with values for the interfacial tension at CMC. Generally, the increase in the number of ethylol groups in the molecule increases CMC values due to increasing solubility of the surfactants in kerosene. Such an improved solubility lowers the tendency for the surfactant to form micelles.
As a result, a plot of interfacial tension as a function of the equilibrium concentration of the surfactant in one of the liquid phases, rather than an adsorption isotherm, is generally used to describe adsorption at this interface. The concentration of surfactant at the interface may therefore be calculated from interfacial tension data by using the following equation [16]:
where \( \left( {\frac{ - \partial \gamma }{\partial \ln C}} \right)_{T} \) is the slope of the plot of γ versus lnC at a constant temperature (T), and R is the gas constant in J mol−1 K−1. The surface excess concentration at surface saturation (Γmax) can be readily found and it is a useful measure of the effectiveness of adsorption of surfactant at the liquid–gas or liquid–liquid interface, since it is the maximum value to which adsorption can attain.
From the surface excess concentration, the area per molecule at interface is calculated using Eq. 2
where N is Avogadro’s number.
The effectiveness of the interfacial tension reduction, π CMC = γ 0 − γ CMC, where γ 0 is the interfacial tension at the kerosene/water border and γ CMC is the interfacial tension at the kerosene solution of surfactant/water border at CMC, was determined at 298 K. The Γmax, A min and γ CMC values are calculated and listed in Table 1. The data, listed in Table 1, show that the minimum areas per molecule at the kerosene solution/water interface decrease with the increase in the number of ethylol units in the molecule. When changing from the ethylolamide to its phosphate, the value of A min decreases. This can be attributed to the behavior of surfactants with hydrophilic groups at opposite ends of the molecule, which show large area per molecule at the interface and are probably lying flat at the interface with both hydrophilic groups in contact with the kerosene phase. The data of A min and Γmax indicate the dependence of the effectiveness of adsorption at the kerosene solution/water interfaces from the structure of the surfactants. It was found that incorporation of MEA instead of DEA in the surfactant structure appears to cause an unusually small decrease in A min at the interface. This can be attributed to the fact that DEA has a branched hydroxyethyl group, which brings about coiling of the hydrophobic chain with a consequent decrease in A min [17].
As is known [18], in order to possess petroleum-collecting properties one of the obligatory conditions is that the surfactant must have a π CMC higher than 30–35 mN m−1.
The effectiveness of interfacial tension reduction in these compounds shows a steady rise with an increase in the number of ethylol groups.
Standard Free Energies of Micellization and Adsorption at the Kerosene Solution/Water Interface
Standard free energies of micellization ΔG mic, for the synthesized surfactants were calculated using Eq. 3 [19]
where ω = (mol of H2O)/(dm3 of H2O).
Standard free energies of adsorption ΔG ad for these surfactants were determined by using the relationship [19]
where C 30 was the concentration of surfactant when the reduction of surface tension equaled 30 mN m−1.
The values found of ΔG mic and ΔG ad are listed in Table 1. From these data it may be concluded that micellization process has a spontaneous character (ΔG mic < 0). As the number of ethylol groups rises, the values of ΔG mic increase. This can be explained by the fact that an increase in the number of ethylol groups improves the solubility in hydrocarbons. As a result, the CMC increases.
All values found for ΔG ad are negative. Moreover, they are more negative than those of ΔG mic, i.e. adsorption of the mentioned surfactants at the kerosene/water interface is associated with a larger decrease in free energy of the system.
The Prepared Surfactants as Oil Slicks Collection Agents
In this work, petroleum-collecting and petroleum-dispersing properties of the surfactants were studied using as an example thin films of Ramany crude oil on the surface of distilled and sea waters, the surfactant being taken in undiluted form and as 5% wt. alcoholic and water solutions. In Table 2 results of studies of petroleum-collecting and petroleum-dispersing ability of the synthesized surfactants based on animal fats are presented. From Table 2 it can be seen that BFDEA in undiluted form exhibits a better petroleum-collecting action (in distilled and sea waters) than the other ethylolamides. The collected petroleum is held in such a form during the period (τ) 78 h with K max = 24.3. For the 5% aqueous solution form of BFMEA, BFDEA and MFMEA petroleum-collecting effect (K max = 30.4 in distilled water, τ = 140–216 h) is identical. In undiluted form the BFMEAPh shows a better petroleum-collecting effect (K max = 40.5 in distilled water, τ = 168 h) than the other surfactants. In the medium of the sea water, the petroleum-dispersing properties of these surfactants are observed. The influence of the surfactants is maintained for about 26–194 h. In the sea water, in undiluted form, BFDEA demonstrates a value of K max equal to 20.3, τ being 7 h, whereas with MFDEAPh K max is also 20.3 for 26 h. From comparison of colloidal-chemical parameters and petroleum-collecting efficiency, it is obvious that, of the analogous chemicals, a higher efficiency in petroleum collecting is displayed in distilled water by those having larger values of π and smaller values of ΔG mic an ΔG ad.
Conclusions
For the first time, by interaction of beef and mutton fats with MEA and DEA respective ethylolamides were obtained and subsequently phosphated. It was found that at the water-hydrocarbon interface both the ethylolamides and their phosphates form micelles and the micellization process took place spontaneously, occurring more easily in the case of diethylolamides. The A min values for the diethylolamides were slightly less than those of the monoethylolamides whereas the π CMC values of the diethylolamides were higher. The phosphates having higher values of π CMC possessed stronger petroleum-collecting capacities.
Abbreviations
- CMC:
-
Critical micelle concentration
- BFDEA:
-
Beef fat diethylolamide
- BFDEAPh:
-
Beef fat diethylolamide phosphate
- BFMEA:
-
Beef fat monoethylolamide
- BFMEAPh:
-
Beef fat monoethylolamide phosphate
- DEA:
-
Diethanolamine
- MEA:
-
Monoethanolamine
- MFDEA:
-
Mutton fat diethylolamide
- MFDEAPh:
-
Mutton fat diethylolamide phosphate
- MFMEA:
-
Mutton fat monoethylolamide
- MFMEAPh:
-
Mutton fat monoethylolamide phosphate
References
Hua J (2004) Fate of dispersed marine fuel oil in sediment under pre-spill application strategy. Ocean Eng 31:943–956
Cheryl AP, Bonner JS, McDonald TJ, Autenrieth RL (2002) Behavior of a chemically dispersed oil in a wetland environment. Water Res 36:3821–3833
White IC, Nichols JA (1983) The cost of oil spills. In: Proceedings of the 1983 international oil spill conference, Washington DC, pp 541–544
National Research Council (1989) Using oil spill dispersants on the sea. National Academy Press, Washington DC
Hua J (2006) Biodegradation of dispersed marine fuel oil in sediment under engineered pre-spill application strategy. Océan Eng 33:152–167
National Research Council (2002) Fates and effects. National Academy of Science, Washington DC
Shapiro SH (1968) Commercial nitrogen derivatives of fatty acids. In: Pattison ES (ed) Fatty acids and their industrial applications. Marcell Dekker, New York, pp 77–154
Feairheller SH, Bistline RG, Bilyk A Jr, Dudley RL, Kozempel MF, Haas MJ (1994) A novel technique for the preparation of secondary fatty amides. III. Alkanolamides, diamides and arylamides. J Am Oil Chem Soc 71:863–866
Kolancilar H (2004) Preparation of laurel oil alkanolamide from laurel oil. J Am Oil Chem Soc 81:597–598
Wolff P, Kaaber H, Anderson HC (1972) Polyurethane prepared from glyceride reaction products. US Patent 3, 637,539
Bilyk A, Bistline RG, Piazza GJ, Feairheller SH, Haas MJ (1992) A novel technique for the preparation of secondary fatty amide. J Amer Oil Chem Soc 69:488–491
Muargard T, Remaud-Simeon M, Petre D, Monsan P (1997) Enzymatic synthesis of glycamide surfactants by amidification reaction. Tetrahedron 53:5185–5194
Lepain AO (1980) Surface active compositions and method of use in dispersing or collecting oil slick. US Patent 4,224,152
Canevari GP (1969) The role of chemical dispersants in oil cleanup. In: Hoult DP (ed) Oil on the sea. Plenum Press, New York
Peeters F, Kipfer R, Achermann D, Hofer M, Aeschbach-Hertig W, Beyerle U, Imboden DM, Rozanski K, Frohlich K (2000) Analysis of deep-water exchange in the Caspian Sea based on environmental tracers. Deep-Sea Res I 47:621–654
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. John Wiley, New York
Schick MJ (1962) Surface films of nonionic detergents–I. Surface tension study. J Colloid Sci 17:801–813
Humbatov HH, Dashdiev RA (1998) Application of surfactants for liquidation of accidental crude oil spills on the water surface. Elm Publishers, Baku (in Russian)
Murphy DS, Rosen MJ (1988) Effect of the nonaqueous phase on interfacial properties of surfactants–1. Thermodynamics and interfacial properties of zwitterionic surfactant in hydrocarbon/water systems. J Phys Chem 92:2870–2873
Acknowledgments
The authors would like to thank the Institute of Petrochemical Processes of National Academy of Sciences of Azerbaijan for the financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Asadov, Z.H., Rahimov, R.A. & Salamova, N.V. Synthesis of Animal Fats Ethylolamides, Ethylolamide Phosphates and Their Petroleum-Collecting and Dispersing Properties. J Am Oil Chem Soc 89, 505–511 (2012). https://doi.org/10.1007/s11746-011-1931-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1931-8