Abstract
Sorption–desorption behavior of the antibiotic tetracycline (TET) and the synthetic estrogen hormone 17α-ethinylestradiol (EE2) with wastewater sludge and sludge-derived humic substances [humic acid (HA) and humin] was investigated. From acidic functional group capacity and elemental analyses, HA had higher polarity, aromaticity, and acidity than humin; humin contained aliphatic chains with high mineral content. The different physicochemical properties of the pharmaceuticals and sludge components yielded different kinds of sorption–desorption interactions. Partitioning coefficients (K d) of TET to sludge were higher (1,552 ± 41–4,667 ± 41 L/kg) than EE2 (534 ± 52–609 ± 47 L/kg). TET sorption was highly pH-dependent and maximal at pH 9. Ca2+ ions enhanced sorption, emphasizing the role of polyvalent metal ions in forming TET–sludge complexes. Humin was the dominant component for TET sorption due to its high inorganic matter content. In contrast, EE2 sorption was independent of solution pH, forming mostly hydrophobic interactions with sludge organic matter. EE2 had a high affinity for HA due to its chemical structure. Desorption of the two pharmaceuticals differed as well. The amount of desorbed TET (18.7 ± 1.3–29.8 ± 2 %) was lower than that of EE2 (60.6 ± 3–62.3 ± 2 %), and the hysteresis index was higher for TET than EE2. While TET desorption tended to be delayed in the solid matrix, EE2 desorbed easily and in accordance with the aqueous equilibrium concentration. The conclusions emphasize the need for further research into frequently used pharmaceuticals with different physicochemical properties and the recognition of sludge application as an important source of distribution for these contaminants in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acute and chronic influences of pharmaceutical compounds (PCs) on the ecological systems and human health are not yet fully understood (Kummerer 2009). Among the thousands of frequently used PCs, antibiotics and estrogenic hormones are of particular importance due to their contribution to the development of antibiotic-resistant bacteria (Schwartz et al. 2002; Auerbach et al. 2007) and endocrine-disruptive phenomena such as feminization of fish (Purdom et al. 1994; Jobling et al. 2002), respectively.
PCs are commonly present in domestic, industrial, and agricultural wastewater reaching wastewater-treatment plants (WWTPs) (Ternes and Joss 2006). Since conventional WWTPs are not designed to efficiently eliminate these so-called “emerging pollutants,” they are continuously distributed in the environment via agricultural irrigation, discharge of secondary and tertiary effluents, and land application of biosolids (Chefetz et al. 2008). In Israel, the EU, and the USA, sludge is a preferred organic amendment for agricultural soils.
During the wastewater treatment process, PCs might (1) be degraded or metabolized, (2) remain in the aqueous phase, or (3) adsorb to the solid fraction (sludge) via diverse physicochemical mechanisms (Carballa et al. 2004). While hydraulic retention time in WWTPs is usually less than most PCs’ half-lives, they are not fully degraded (Halling-Sørensen et al. 1998). Thus, these drugs and their degradation products are frequently found in a variety of environmental matrices (Heberer 2002; Kolpin et al. 2002; Lamm et al. 2009; Shafrir and Avisar 2012; Gozlan et al. 2013). Moreover, since solid retention time is commonly one order of magnitude higher than hydraulic retention time, certain PCs and degradation products might accumulate in the sludge, leading to higher solid concentrations than in raw sewage (Diaz-Cruz et al. 2003; Xia et al. 2005).
Several studies have reported the presence of PCs in wastewater sludge. For example, fluoroquinolone antibiotics found at concentrations as high as 3.1 mg/kg (Golet et al. 2002, 2003), sulfonamide and tetracycline antibiotics found at concentrations as high as 669 and 743 μg/kg, respectively, (Ding et al. 2011), and estrogen hormones found at concentrations as high as 49 μg/kg (Ternes et al. 2002). In Israel, Shafrir and Avisar (2012) found tetracycline antibiotics and their degradation products at concentrations of up to 7.6 and 2.95 mg/kg in secondary sludge and in commercial compost consisting of wastewater sludge, respectively. They also found two natural estrogenic hormones (E1 and E2) at concentrations of 160 and 21 μg/kg, respectively, in the commercial compost sample. These findings emphasize the stability and persistence of PCs, even after the application of common practices for additional sanitary processing so that sludge quality will meet the class A biosolids standard.
Sludge usually consists of organic materials, such as extracellular polymers, fats, proteins, polysaccharides, and humic substances (Clara et al. 2004). Humic substances are defined as the chemical and biological transformation products of plant residues and animal tissues (Stevenson 1994). These amorphous polyelectrolyte macromolecules are operationally divided into three components based on their solubility in acid or alkali solutions: humic acid (HA) forms aggregates and participates under acidic conditions, fulvic acid dissolves at all pHs, and humin does not dissolve at all. Different humic substances from various sources exhibit different physicochemical properties; however, they all share the presence of acidic functional groups, the ability to form complexes with metal ions, the capacity to sorb organic pollutants, and resistance to microbial decomposition. Due to lack of studies regarding the importance of sludge humic components, interacting with PCs, this study was aimed to examine the possible interactions between two PCs and sludge-derived humic substances. Additionally, their physicochemical properties were evaluated in terms of their contribution to sorption–desorption processes.
2 The Selected PCs
The two examined PCs represent two types of widespread prescription drugs: the antibiotic tetracycline (TET) and the synthetic estrogen hormone 17α-ethinylestradiol (EE2). Their physicochemical properties and structures are presented in Table 1.
The TETs are a widespread family of antibiotics that have been authorized for treatment in humans and as growth promoters in livestock (Anderson et al. 2005). They consist of a chain of four cyclic 6-carbon rings and possess three ionizable functional groups that can undergo protonation or deprotonation, depending on solution pH (Chang et al. 2009). Sorption of TET to wastewater sludge has been found to be its primary elimination mechanism in the activated sludge process (Kim et al. 2005). TET is a polar compound that is photodegradable (Thiele-Bruhn 2003) and known for its ability to form complexes with divalent and multivalent cations (Tools 2001; Jin et al. 2007). These complexes can affect photodegradation rates, as self-sensitized degradation of TETs under simulated sunlight in the presence of Ca2+ was enhanced at pH 7.3 as compared to pH 9 (Chen et al. 2011).
EE2 is the active ingredient in contraceptive pills and considered very stable in the environment (Ternes et al. 1999). Its high log K OW and low aqueous solubility characterize it as hydrophobic, having a high potential to sorb to the highly organic content in sludge (Clara et al. 2004; Cirja et al. 2007). EE2 is mostly neutral in the environmental pH range, as the phenolic group undergoes deprotonation only at higher pH (pKa = 10.4) (Table 1).
3 Materials and Methods
3.1 Chemicals, Standards, and Stock Solutions
Analytical TET and EE2 standards were purchased from Sigma-Aldrich Ltd. (Rehovot, Israel). High-performance liquid chromatography (HPLC)-grade solvents were purchased from Bio-Lab Ltd. (Jerusalem, Israel). Concentrated stock solutions of the tested PCs were prepared weekly by dissolving an appropriate amount of the analytical standard in MeOH in a volumetric flask and kept in the dark at 4 °C. Calibration standards and working solutions were prepared by diluting stock solutions with deionized water (DI). MeOH concentrations in sorption–desorption experiments were maintained at less than 0.1 % (v/v) to prevent co-solvent effects.
3.2 Sampling and Extraction Procedures
Secondary untreated sludge was sampled from the “Kolchey Hasharon” municipal WWTP located in the central region of Israel, which treats 11,500 m3/day. The plant operates as an activated sludge system without primary sedimentation, while the treatment of excess sludge includes gravitational thickening, polymer addition, and centrifugation, before sending to a composting facility. The sludge was sampled after gravitational thickening, then dewatered by centrifugation, freeze-dried, and homogenized by passing through a 425-μm sieve.
Humic substances (HA and humin) were isolated from the bulk dry sludge according to the protocol suggested by Swift (1996). Briefly, the sludge was extracted four times in 0.1 N NaOH overnight under N2 atmosphere, and the supernatant was decanted and acidified to pH 1 with 6 N HCl to obtain the HA fraction. The HA was then purified to remove clays, subjected to hydrofluoric acid to digest silicates, dialyzed against DI water (12–14-kDa molecular weight cutoff) to remove salts, and finally, freeze-dried. The extraction procedure was performed four more times with alkali solution to obtain the remaining insoluble fraction known as humin, which was then rinsed with DI water and freeze-dried. The dried bulk sludge, HA, and humin samples were kept in a desiccator until analyses.
3.3 Characterization of Bulk Sludge and Humic Substances
All characterization analyses were carried out after overnight oven-drying of the samples at 65 °C. Organic matter content was calculated as weight percentage loss by ignition at 400 °C for 8 h. C, H, N, and O compositions were determined as weight percentage by a Flash EA 1112 elemental analyzer. Atomic ratios between elements were calculated by dividing the weight percentage of an element by its molecular weight. Acidic functional group capacity was determined by potentiometric titration (Titrino, Metroham 785 DMP) as described by Inbar et al. (1990). Each sample (20 mg) was magnetically stirred in 5 mL 0.01 N NaOH under a N2 stream for 30 min. Carboxyl acidity was calculated as the volume of 0.01 N HCl used for titration from pH 8 to 3, while the volume of acid used for titration from pH 10 to 8 represented half of the phenol acidity. Total acidity was calculated by summing these volumes.
3.4 Sorption–Desorption Experiments
A background solution containing 5 mM CaCl2 (for keeping a constant ionic strength) and the desired concentration of the tested PC were added to a 50-mL Teflon centrifuge tube containing a pre-weighed amount of the sorbent material (bulk sludge, HA, or humin), and the pH was adjusted to 7 with 0.5 N NaOH or 0.5 N HCl. In experiments where pH dependence was being examined, 50 mg/L of NaHCO3 was added as a buffer and the pH was adjusted to 4, 7, or 9. Initial applied concentrations ranged 0.25–15 mg/L for TET and 0.5–5 mg/L for EE2. The tubes were agitated (150 rpm) at 23 °C in the dark for 24 h (the time required to reach equilibrium as determined in preliminary studies) and then centrifuged for 30 min at 3,026g. To initiate desorption, half of the background solution was decanted and replaced with the same background solution without the PC. The tubes were agitated again to reach desorption equilibrium and centrifuged in the same manner. An aliquot of each sorption–desorption equilibrium solution was transferred to a 1.5-mL amber vial for quantitative HPLC analysis. Control and blank tubes were tested with each experiment to confirm stability, loss of PC to the tubes, and interference of other compounds with the HPLC analysis.
3.5 HPLC–UV Analysis
Equilibrium concentrations were determined using an Agilent 1100 series HPLC instrument. TET was quantified after elution from a RP-C8 column (ACE, 250-mm length, 5-μm particle size) with a mobile phase gradient consisting of 0.1 % formic acid in water (pH 2.5) and 0.1 % formic acid in acetonitrile, at a UV wavelength of 360 nm. EE2 was quantified after elution from a RP-C18 column with a mobile phase consisting of water and acetonitrile, at a UV wavelength of 280 nm. External standards were prepared from the working solutions used for quantification. Limit of detection (LODs) were 0.005 mg/L for TET and 0.01 mg/L for EE2, and the limit of quantification (LOQs) were 0.05 mg/L for both.
3.6 Calculations and Models
The partitioning coefficient, K d (L/kg) was calculated as follows:
where C S (mg/kg) is the sorbed concentration, and C eq (mg/L) is the aqueous equilibrium concentration. Significant reduction in variability between different sorbents for sorption of many neutral hydrophobic organic chemicals can be achieved by normalizing K d values to the organic carbon content of the solid sample (Tools 2001). The organic carbon-normalized sorption coefficient, K OC, was calculated as follows:
where f OC represents the fraction of organic carbon in the sorbent. Normalization of K d values to the ash content (K ASH) was calculated as
where f ASH represents the fraction of inorganic matter in the sorbent.
The data obtained from the sorption experiments were fitted to Freundlich or Langmuir models using Sigmaplot 12 (Systat Software, Inc.). The Freundlich model was
where C S is the solid sorbed concentration (mg/kg), C eq is the equilibrium aqueous concentration (mg/L), K F ([(mg/kg)/(mg/L)N]) is the Freundlich sorption coefficient, and N (unitless) is the linearity parameter. The Langmuir model was
where C S,max (mg/kg) is the maximum sorption capacity of the sorbent, and K L (L/kg) is the Langmuir sorption coefficient.
The hysteresis index (HI) was calculated as suggested by Huang et al. (1998):
Higher HI values describe a situation in which the initially sorbed compound is delayed in the solid phase during desorption.
4 Results and Discussion
4.1 Sludge and Humic Substance Characterization
4.1.1 Organic Matter Content
Organic matter content in the bulk sludge sample (Table 2) was typical for untreated secondary sludge (Metcalf and Eddie 2003). Sludge-derived HA had a large organic fraction due to the purification procedures, whereas humin had a smaller organic fraction. The humin sample might be composed of HAs tightly bound to mineral matter such as clays and multivalent metal cations (Stevenson 1994).
4.1.2 Elemental Analysis
The humin sample had a higher H/C atomic ratio than the HA sample (Table 2), indicating that humin is more aliphatic and HA is more aromatic (Rice and MacCarthy 1991). Nevertheless, H/C atomic ratio values higher than 1.3 for both samples indicated the presence of nonhumic material that is mostly aliphatic in nature (Steelink 1985). Higher O/C and (O + N)/C atomic ratios for HA suggested that the HA sample is more polar than the hydrophobic humin sample.
4.1.3 Acidic Functional Group Capacity
Acidic functional group capacity was higher for HA than for humin (Table 2), as can be expected for humic substances extracted from the same source (Bowles et al. 1989). In both samples, carboxyl groups represented most of the acidic functional groups, occupying 66 and 59 % of the total acidities of HA and humin, respectively.
4.2 Batch Sorption–Desorption Experiments
4.2.1 TET Sorption
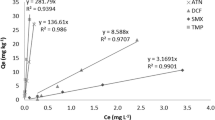
Sorption isotherms for TET with bulk sludge, humin, and HA (pH 7) are presented in Fig. 1. The data obtained for the bulk sludge was well fitted to the Freundlich model (Table 3), indicating a nonlinear isotherm. Calculated K d values ranged between 1,552 ± 41 and 4,667 ± 41 L/kg, comparable to previously reported K d values of TET sorption to sludge (Stuer-Lauridsen et al. 2000; Kim et al. 2005).
Sorption of TET to bulk sludge exhibited a strong pH dependency (Fig. 2a), with enhanced sorption observed at pH 9 as compared to pH 4 and 7. At pH 9, 80 % of the TET molecules exist as monovalent anions (+−−), 10 % exist as divalent anions (0−−) and 10 % exist as zwitterions (+ − 0) (Chang et al. 2009). As a result, enhanced sorption of TET to bulk sludge might be due to two different mechanisms: (1) cation exchange between the positively charged dimethylammonium group in the TET molecule and acidic functional groups in the organic matter and (2) coordination, with divalent or multivalent metal ions serving as a bridge between negatively charged sorbent and sorbate.
The ability of TET to form complexes with multivalent metal cations has been reported previously (Martin 1979; Wessels et al. 1998; Tools 2001; Jin et al. 2007). Moreover, Jin et al. (2007) reported increased complexation with Ca2+ and Mg2+with increasing solution pH. Ca2+ ions originating from the background solution in sorption experiments or other multivalent cations originating from the sludge sample might serve as a metal bridge, enhancing TET sorption at pH 9. To examine this hypothesis, the background solution containing CaCl2 was replaced with NaCl at the same ionic strength. The results of the experiment (Fig. 3) supported the hypothesis as sorption was more enhanced at pH 9 with the CaCl2 solution than with the NaCl solution. As compared to pH 7, raising the pH to 9 resulted in significant enhancement with both background solutions, but this enhancement was less pronounced for the NaCl solution. This might be attributed to multivalent cations originating from the sludge sample itself. At pH 7, no significant differences were observed between the two solutions.
TET sorption to HA fit the Freundlich model (Table 3) with a maximum calculated K d value of 641 ± 23 L/kg. Extremely low affinity of TET to HA was observed for the lower range equilibrium concentration (<1 mg/L), probably due to competition between TET molecules and dissolved HA molecules for available sites on the solid HA. By increasing the ratio of TET to dissolved organic matter (DOM), TET molecules can compete more efficiently with the DOM, resulting in higher K d values.
Sorption of TET to the humin sample fit the Langmuir model as sorption reached saturation. The maximum TET sorption capacity obtained from the model was calculated as 28,462 ± 1,521 mg/kg. K d values for humin ranged between 1,898 ± 124 and 9,236 ± 405 L/kg, the highest among the three sorbents examined. Comparison of K OC values between the three sorbents (Table 3) showed no reduction in the variability between the sorbents, indicating the existence of other sorption mechanisms besides hydrophobic partitioning. At an equilibrium concentration of 0.75 mg/L for example, the K d for humin was more than 3 times higher than the K d for bulk sludge and more than 20 times higher than K d for HA. The K OC for humin was more than 4 times higher than K OC for bulk sludge and more than 30 times higher than K OC for HA. However, variability could be reduced by normalizing to the ash content, as K ASH for humin was only 1.82 and 1.48 times higher than KASH for bulk sludge and HA, respectively. A similar trend was observed for the 5 mg/L equilibrium concentration. These findings further suggest that the mineral matter fraction plays an important role in TET sorption. As seen previously, aside from the metal ions that enable TET complexation, sludge inorganic matter also includes clays. A high affinity of TET antibiotics to clays has been reported in several studies (Sithole and Guy 1987; Pils and Laird 2007; Parolo et al. 2008; Chang et al. 2009; Avisar et al. 2010) and might explain the high affinity to humin, as well as the low affinity to purified HA.
4.2.2 EE2 Sorption
Sorption isotherms of EE2 with bulk sludge, humin, and HA (pH 7) are presented in Fig. 4. The data obtained for the bulk sludge well fitted the Freundlich model (Table 3), indicating only slight deviation from linearity. Calculated K d values ranged from 534 ± 52 to 609 ± 47 L/kg, in accordance with a K d value of 584 L/kg reported by Andersen et al. (2005) and 434 to 757 L/kg reported by Urase and Kikuta (2005) for sorption of EE2 to sludge. Ternes et al. (2004) reported a lower but still comparable K d range, from 278 to 349 L/kg.
As expected for a neutral hydrophobic compound, sorption of EE2 to bulk sludge was not pH dependent (Schwarzenbach et al. 2003) in the pH range tested (Fig. 2b), because the dominant sorption mechanism involves nonspecific hydrophobic partitioning of EE2 between the aqueous and solid phases. However, decreased sorption of EE2 can be expected when the pH is further raised toward the pKa (10.4), due to electrostatic repulsion between the deprotonated phenolic group of EE2 and the deprotonated carboxylic and phenolic groups of the sludge organic matter (Clara et al. 2004).
EE2 sorption to HA and humin samples exhibited a similar degree of nonlinearity, with similar Freundlich N (Table 3). Calculated K d values for HA ranged between 1,228 ± 5 and 1,385 ± 47 L/kg, almost double those for humin (651 ± 5–758 ± 4 L/kg), and more than double those for bulk sludge. As shown in Table 3, normalizing to organic carbon content significantly reduced variability between sorbents. At an equilibrium concentration of 0.5 mg/L, for example, the K d for HA was 1.9 and 2.4 times higher than that for humin and bulk sludge, respectively, while the K OC was only 1.2 and 1.7 times higher, respectively. At the same time, normalizing to the ash content (K ASH) led to the opposite trend. These findings suggest hydrophobic partitioning as a major mechanism for EE2 sorption, as previously deduced by other investigators (Lai et al. 2000; Lee et al. 2003; Sun et al. 2012). However, the affinity of EE2 to the three examined sorbents based on K OC values still followed the trend HA > humin > bulk. These findings might be attributed to the existence of sorption mechanisms other than hydrophobic interactions, which differ among the sorbents according to their chemical structures. The HA sample had the highest degree of aromaticity as reflected by the lowest H/C atomic ratio (Table 2). Aromatic structures might enable π-π interactions with the benzene ring in the EE2 molecule, as suggested by Yamamoto et al. (2003) and Sun et al. (2010). Another possible mechanism is hydrogen bonding, which might occur between the two OH groups in the EE2 molecule and various functional polar groups in the organic matter. The HA sample had higher O/C and (O + N)/C atomic ratios than humin, as well as higher acidic functional group capacity. Sun et al. (2012) suggested that EE2 has a higher likelihood of hydrogen bonding with more polar organic matter, and Yamamoto et al. (2003) suggested hydrogen bonding as a possible mechanism for estrogen hormone sorption to DOM, such as humic and fulvic acids.
4.2.3 TET and EE2 Desorption
Desorption of the tested PCs from bulk sludge was performed in three consecutive steps. As with the sorption stage, TET desorption from bulk sludge was pH dependent (Table 4). These findings also support the sorption mechanisms, as weaker, nonspecific hydrophobic interactions are involved under neutral conditions when TET is zwitterionic, whereas strong specific coordination complexes under basic conditions probably inhibit TET desorption. Nevertheless, even the maximum TET desorption was low compared with EE2 desorption. EE2 desorption from bulk sludge was independent of pH, with a total desorption of approximately 60 %. As seen in the sorption stage, hydrophobic partitioning predominated, enabling quick desorption of EE2 back to the aqueous phase.
The HI values were calculated after one desorption step for two different equilibrium concentrations and based on a comparison between the sorbed concentrations (C S) of the PCs at the end of the sorption or desorption stage. TET HI values for both equilibrium concentrations (0.51 ± 0.14–0.63 ± 0.15) were significantly higher than EE2 HI values (0.136 ± 0.006–0.151 ± 0.007). These findings further emphasize TET’s difficult desorption back into solution after it has been sorbed, exhibiting hysteresis. Other studies have reported TET hysteresis while desorbing from sludge (Kim et al. 2005), marine sediments (Xu and Li 2010), and dissolved HA (Gu et al. 2007). In contrast, EE2 desorbed from the bulk sludge easily and in accordance with the equilibrium aqueous concentration. A similar observation has been reported by Loffredo and Senesi (2006) for EE2 desorption from soil HAs.
5 Conclusions
Land application of wastewater sludge has high potential to expose the environment to PCs. The two PCs examined herein sorb to wastewater sludge via different mechanisms, although TET, despite its polarity, seems to have a higher affinity for the solid phase than EE2. According to the classification (log K d > 2) proposed by Clara et al. (2004), sorption of TET and EE2 to sludge is a relevant removal pathway in WWTPs. The results also highlight the contribution of both organic and inorganic fractions of the sludge to the sorption process, as well as that of solution chemistry. Common practices for sludge treatment and processing may not be useful for eliminating these contaminants; hence, they may accumulate in the upper soil horizon, flow with runoff, or infiltrate toward the groundwater. Since mobility can be enhanced through preferential saturated flow paths (Avisar et al. 2009) and binding to DOM (Chefetz et al. 2008), further investigation is warranted of the thousands of frequently used PCs with their varied physicochemical properties that find their way into wastewater sludge.
References
Andersen, H. R., Hansen, M., Kjolholt, J., Stuer-Lauridsen, F., Ternes, T., & Halling-Sorensen, B. (2005). Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment. Chemosphere, 6, 139–146.
Anderson, C. R., Rupp, H. S., & Wu, W. H. (2005). Complexities in tetracycline analysis—chemistry, matrix extraction, cleanup, and liquid chromatography. Journal of Chromatography A, 1075, 23–32.
Auerbach, E. A., Seyfrid, E. E., & McMahon, K. D. (2007). Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Research, 41, 1143–1151.
Avisar, D., Levin, G., & Gozlan, I. (2009). The processes affecting oxytetracycline contamination of groundwater in a phreatic aquifer underlying industrial fish ponds in Israel. Environmental Earth Sciences, 59, 939–945.
Avisar, D., Primor, O., & Mamane, H. (2010). Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water, Air, & Soil Pollution, 209, 439–445.
Bowles, E. C., Antweiler, R. C., & MacCarthy, P. (1989). Acid–base titration and hydrolysis of Suwannee River, Georgia; interactions, properties and proposed structures. U.S Geological Survey Open File Rep. no. 87–557. Denver: U.S. Geological Survey.
Carballa, M., Omil, F., Llompart, M., Garcia-Jares, C., Rodriguez, I., Gomez, M., & Ternes, T. (2004). Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Research, 38, 2918–2926.
Chang, P. H., Li, Z., Jiang, W. T., & Jean, J. S. (2009). Adsorption and intercalation of tetracycline by swelling clay minerals. Applied Clay Science, 46, 27–36.
Chefetz, B., Mualem, T., & Ben-Ari, J. (2008). Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere, 73, 1335–1343.
Chen, Y., Li, H., Wang, Z., Tao, T., & Hu, C. (2011). Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: Effects of Ca2+ and Mg2+. Journal of Environmental Sciences, 23(10), 1634–1639.
Cirja, M., Zuehlke, S., Ivashechkin, P., Hollender, J., Schaffer, A., & Corvini, P. F. X. (2007). Behavior of two differently radiolabelled 17α-ethinylestradiols continuously applied to a laboratory-scale membrane bioreactor with adapted industrial activated sludge. Water Research, 41, 4403–4412.
Clara, M., Strenn, B., Saracevic, E., & Kreuzinger, N. (2004). Adsorption of bisphenol-A, 17β-estradiole and 17α-ethinylestradiole to sewage sludge. Chemosphere, 56, 843–851.
Diaz-Cruz, M. S., Lopez de Alda, M. J., & Barcelo, D. (2003). Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. Trends in Analytical Chemistry, 22, 340–351.
Ding, Y., Zhang, W., Gu, C., Xagoraraki, I., & Li, H. (2011). Determination of pharmaceuticals in biosolids using accelerated solvent extraction and liquid chromatography/tandem mass spectrometry. Journal of Chromatography A, 1218, 10–16.
Golet, E. M., Strehler, A., Alder, A. C., & Giger, W. (2002). Determination of fluoroquinalone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Analytical Chemistry, 74, 5455–5462.
Golet, E. M., Xifra, I., Siergist, H., Alder, A. C., & Giger, W. (2003). Environmental exposure assessment of fluoroquinalone antibacterial agents from sewage to soil. Environmental Science and Technology, 37, 3243–3249.
Gozlan, I., Rotstein, A., & Avisar, D. (2013). Identification and determination in the aquatic environment: amoxicillin degradation products formed under controlled environmental conditions. Chemosphere, 91, 985–992.
Gu, C., Karthikeyan, K. G., Sibley, S. D., & Pederson, J. A. (2007). Complexation of the antibiotic tetracycline with humic acid. Chemosphere, 66, 1494–1501.
Halling-Sørensen, B., Nors Nielsen, S., Lanzky, P. F., Ingerslev, F., HoltenLützhøft, H. C., & Jørgensen, S. E. (1998). Occurrence, fate and effects of pharmaceutical substances in the environment. Chemosphere, 36, 357–393.
Heberer, T. (2002). Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicology Letters, 131, 5–17.
Huang, W., Yu, H., & Weber, W. J., Jr. (1998). Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments. 1. A comparative analysis of experimental protocols. Journal of Contaminant Hydrology, 31, 129–148.
Inbar, Y., Chen, Y., & Hadar, Y. (1990). Humic substances formed during the composting of organic matter. Soil Science Society of America Journal, 54, 1316–1323.
Jin, L., Amaya-Mazo, X., Apel, M. E., Sankisa, S. S., Johnson, E., Zbyszynaka, M. A., & Han, A. (2007). Ca2+ and Mg2+ bind tetracycline with distinct stoichiometries and linked deprotonation. Biophysical Chemistry, 128, 185–196.
Jobling, S., Beresford, N., Nolan, M., Rodgers-Gray, T., Brighty, G. C., Sumpter, J. P., & Tyler, C. R. (2002). Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biology of Reproduction, 66, 272–281.
Kim, S., Eichhorn, P., Jensen, J. N., Weber, A. S., & Aga, D. S. (2005). Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environmental Science and Technology, 39, 5816–5823.
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, E. M., Zaugg, S. D., Barber, L. B., & Buxton, H. T. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams 1999–2000: a national reconnaissance. Environmental Science and Technology, 36, 1202–1211.
Kummerer, K. (2009). The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. Journal of Environmental Management, 90, 2354–2366.
Lai, K. M., Johnson, K. L., Scrimshaw, M. D., & Lester, J. N. (2000). Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environmental Science and Technology, 34, 3890–3894.
Lamm, A., Rotstein, A., Gozlan, I., & Avisar, D. (2009). Detection of amoxicillin- diketopiperazine-2′,5′ in wastewater samples. Journal of Environmental Science and Health, Part A Environmental Science, 4, 1512–1517.
Lee, L. S., Strock, T. J., Sarmah, A. K., & Rao, P. S. C. (2003). Sorption and dissipation of testosterone, estrogens, and their primary transformation products in soils and sediments. Environmental Science and Technology, 37, 4098–4105.
Loffredo, E., & Senesi, N. (2006). The role of humic substances in the fate of anthropogenic organic pollutants in soil with emphasis on endocrine disruptor compounds. In I. Twardowska, H. E. Allen, M. M. Haggblom, & S. Stefaniak (Eds.), Soil and water pollution monitoring, protection and remediation (Vol. 69, pp. 69–92). Dordrecht: Springer.
Martin, S. R. (1979). Equilibrium and kinetic studies on the interaction of tetracyclines with calcium and magnesium. Biophysical Chemistry, 10, 319–326.
Metcalf, L., & Eddie, H. P. (2003). Wastewater engineering treatment and reuse (4th ed.). New York: McGraw-Hill.
Parolo, M. E., Savini, M. C., Valles, J. M., Baschini, M. T., & Avena, M. J. (2008). Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Applied Clay Sciences, 40, 179–186.
Pils, J. R., & Laird, D. A. (2007). Sorption of tetracycline and chlorotetracycline on K− and Ca− saturated soil clays, humic substances, and clay-humic complexes. Environmental Science and Technology, 41, 1928–1933.
Purdom, C. E., Hardiman, P. A., Bye, V. J., Eno, N. C., Tyler, C. R., & Sumpter, J. P. (1994). Estrogenic effects of effluents from sewage treatment works. Chemistry and Ecology, 8, 275–285.
Rice, J. A., & MacCarthy, P. (1991). Statistical evaluation of the elemental composition of humic substances. Organic Geochemistry, 17, 635–648.
Schwartz, T., Kohnen, W., Jansen, B., & Obst, U. (2002). Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiology Ecology, 1470, 1–11.
Schwarzenbach, R. P., Gschwend, P. M., & Imboden, D. M. (2003). Environmental organic chemistry. New York: Wiley.
Shafrir, M., & Avisar, D. (2012). Development method for extracting and analyzing antibiotic and hormone residues from treated wastewater sludge and composted biosolids. Water, Air, & Soil Pollution, 223, 2571–2587.
Shareef, A., Angove, M. J., Wells, J. D., & Johnson, B. B. (2006). Aqueous solubilities of estrone, 17β-estradiol, 17α-ethynylestradiol, and bisphenol A. Journal of Chemical Engineering Data, 51, 879–881.
Sithole, B. B., & Guy, R. D. (1987). Models for tetracycline in aquatic environments I: Interaction with bentonite clay systems. Water, Air, & Soil Pollution, 32, 303–314.
Steelink, C. (1985). Implications of elemental characteristics of humic substances. In G. R. Aiken, D. M. McKnight, R. L. Wershaw, & P. MacCarthy (Eds.), Humic substances in soil, sediment and water: geochemistry, isolation and characterization (pp. 457–475). New York: Wiley.
Stevenson, F. J. (1994). Humus chemistry: genesis, composition, reactions. New York: Wiley.
Stuer-Lauridsen, F., Birkved, M., Hansen, L. P., Lützhøft, H. C., & Halling-Sørensen, B. (2000). Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere, 40, 783–793.
Sun, K., Gao, B., Zhang, Z., Zhang, G., Liu, X., Zhao, Y., & Xing, B. (2010). Sorption of endocrine disrupting chemicals by condensed organic matter in soils and sediments. Chemosphere, 80, 709–715.
Sun, K., Jin, J., Gao, B., Zhang, Z., Wang, Z., Pan, Z., Xu, D., & Zhao, Y. (2012). Sorption of 17α-ethinyl estradiol, bisphenol A and phenanthrene to different size fractions of soil and sediment. Chemosphere, 88, 577–583.
Swift, R. S. (1996). Organic matter characterization. In D. L. Sparks, A. L. Page, P. L. Helmke, R. H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, & M. E. Sumner (Eds.), Methods of soil analysis. Part 3: chemical methods. Madison: Soil Science Society of America.
Ternes, T. A., & Joss, A. (2006). Human pharmaceuticals, hormones and fragrances: the challenge of micropollutants in urban water management. London: IWA Publishing.
Ternes, T. A., Kreckel, P., & Mueller, J. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants—II. Aerobic batch experiments with activated sludge. Science of the Total Environment, 225, 91–99.
Ternes, T. A., Andersen, H., Gilberg, D., & Bonerz, M. (2002). Determination of estrogens in sludge and sediments by liquid extraction and GC/MS/MS. Analytical Chemistry, 74, 3498–3504.
Ternes, T. A., Herrmann, N., Bonerz, M., Knacker, T., Siegrist, H., & Joss, A. (2004). A rapid method to measure the solid-water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Research, 38, 4075–4084.
Thiele-Bruhn, S. (2003). Pharmaceutical antibiotic compounds in soil. Journal of Plant Nutrition and Soil Science, 166, 145–167.
Tools, J. (2001). Sorption of veterinary pharmaceuticals in soils: a review. Environmental Science and Technology, 35, 3397–3406.
Urase, T., & Kikuta, T. (2005). Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Research, 39, 1289–1300.
Wessels, J. M., Ford, W. E., Szymczak, W., & Schneider, S. (1998). The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: a spectroscopic study. Journal of Physical Chemistry B, 102, 9323–9331.
Xia, K., Bhandari, A., Das, K., & Pillar, G. (2005). Occurrence and fate of pharmaceuticals and personal care products (PPCPs) in biosolids. Journal of Environmental Quality, 34, 91–104.
Xu, X. R., & Li, X. Y. (2010). Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere, 78, 430–436.
Yamamoto, H., Liljestrand, H. M., Shimizo, Y., & Morita, M. (2003). Effects of chemical-physical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environmental Science and Technology, 37, 2646–2657.
Acknowledgments
The authors gratefully acknowledge the Israel Ministry of Environmental Protection, the Israeli Water Authority, and the Porter School for Environmental Studies at Tel Aviv University for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tenenbaum, I., Chefetz, B. & Avisar, D. Physicochemical Behavior of Tetracycline and 17α-Ethinylestradiol with Wastewater Sludge-Derived Humic Substances. Water Air Soil Pollut 225, 2155 (2014). https://doi.org/10.1007/s11270-014-2155-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2155-y