Abstract
Borrelia theileri is a relapsing fever group Borrelia that is transmitted to cattle by ticks of the genus Rhipicephalus. In this study, we describe the first molecular detection of B. theileri subclinical infection in a cow in Brazil. During the examination of stained blood smears of 10 cows from a farm with a recent history of fatal Trypanosoma vivax trypanosomiasis, spirochete-like structures were incidentally detected in one of the cows. The animal presented good body score, normal hematocrit and normal-colored ocular mucosa. Temperature, heart rate and respiratory rate were all normal. The animal was infested by ticks, which were morphologically identified as Rhipicephalus microplus. The diagnosis was confirmed by testing DNA extracted from a blood sample using a PCR targeting a ≈ 650 bp fragment of the flagellin B (flaB) gene of Borrelia spp. The partial flaB sequence obtained showed 99.83% similarity with B. theileri. Phylogenetically, the flaB partial sequence generated herein clustered with other B. theileri sequences, being separated from B. lonestari. This is the first molecular detection of B. theileri subclinical infection in a cow in Brazil. The possible implications of this finding are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Borrelia theileri is a relapsing fever group Borrelia known to infect cattle, horses, sheep, goats, deer, impala and other wild ruminants (Theiler 1904; Dodd 1906; Neitz 1935; Callow 1967; Aouadi et al. 2017; Morel et al. 2019; Scoles et al. 2021; Abdullah et al. 2021; Qiu et al. 2021). This bacterium has been reported in Africa, Australia, Europe and South America, where it is transmitted by Rhipicephalus ticks of the subgenus Boophilus, including Rhipicephalus microplus (Theiler 1905, 1909; Brumpt 1919; Callow 1967; Trees 1978; Faccini-Martínez et al. 2022).
Bovine borreliosis caused by B. theileri is considered a mild disease associated with fever, lethargy, hemoglobinuria, anorexia and anemia (Theiler 1905; Callow 1967; Trees 1978; Smith et al. 1978; Faccini-Martínez et al. 2022). The disease usually occurs in association with other vector-borne diseases such as babesiosis and anaplasmosis (Theiler 1904; Van Heerden and Reyers 1984; Koch et al. 1990; Sharma et al. 2000; Abanda et al. 2019). The circulation of B. theileri may be of concern for products derived from bovine blood, such as live vaccines against anaplasmosis and bovine babesiosis (Martins et al. 1996).
Information regarding bovine borreliosis in Latin America is scant. For instance, the presence of B. theileri in a bovine from Argentina was only recently confirmed (Morel et al. 2019). Furthermore, while B. theileri has been molecularly detected in ticks (Cordeiro et al. 2018), this bacterium has never been molecularly detected in cattle in Brazil. In this context, the objective of this study is to report the microscopic and molecular detection of B. theileri in a cow from the Cerrado biome of Brazil.
In April 2020, blood samples were collected by jugular venipuncture in sterile EDTA tubes from 10 cows in a farm in the municipality of Hidrolândia, Goiás State, Brazil. These samples were primarily collected to investigate the presence trypanosomes, due to a recent history of fatal Trypanosoma vivax trypanosomiasis in some animals of this farm. Samples were sent to the Laboratory of Parasitic Diseases of the Veterinary and Animal Science School of the Federal University of Goiás. From each sample, stained blood smear slides (Panótico Rápido rapid hematology stain, Laborclin®, Brazil) and Woo’s test (hematocrit centrifuge technique) were performed. The hematocrit was determined by the microhematocrit method (reference range: 24–46%) (Radostits et al. 2000).
All animals were negative for trypanosomes, but spirochete-like structures were incidentally found in the blood smear of one cow (Fig. 1). This animal was clinically evaluated by measuring rectal temperature (reference range: 38-39.5 ºC) (Robinson 1999; DuPreez 2000), respiratory rate [reference range: 24–36 respiratory movements per minute (mpm)] (Stöber 1993) and heart rate [reference range: 60–80 beats per minute (bpm)] (Detweiler 1996; Naas and Arcaro Júnior 2001). The animal had good body score, normal-colored ocular mucosa, normal hematocrit value (25%), rectal temperature (39.3 °C), heart rate (84 bpm), and respiratory rate (62 mpm). A total of 20 ticks (two males and 18 females) were collected and morphologically (Barros-Battesti et al. 2006) identified as R. microplus; the remaining nine animals were not evaluated for tick infestation.
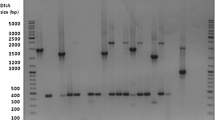
An aliquot (200 µl) of the blood sample taken from the infected cow was subjected to DNA extraction using DNeasy Blood & Tissue Kit (Qiagen, Valencia, California), following the manufacturer’s instructions. The extracted DNA was tested by a conventional PCR targeting a ≈ 650 bp fragment of the flagellin B (flaB) gene from Borrelia spp., using primers FlaLL and FlaRL (Stromdahl et al. 2003). PCR products of the expected size were purified with ExoSap (USB, Cleveland, OH, USA) and DNA sequenced in an ABI automated sequencer (Applied Biosystems/Thermo Fisher Scientific, model ABI 3500 Genetic Analyzer, Foster City, California, USA) with the same primers used for PCR. The obtained sequence was submitted to BLAST analyses (www.ncbi.nlm.nih.gov/blast) to infer the closest similarities available in GenBank. An alignment with our sequence and a subset of relapsing fever and Lyme group Borrelia spp. was constructed with MAFFT (Katoh and Standley 2013), and a phylogeny using the maximum likelihood method implemented in PhyML (Guindon and Gascuel 2003) with the HKY85 substitution model as selected using the Bayesian Information Criterion in MEGA 5 (Tamura et al. 2011).
PCR testing was positive and the flaB partial sequence obtained showed 99.83% identity (592/593 bp) to a sequence of B. theileri (KF569936) detected in Rhipicephalus geigyi from Mali (McCoy et al. 2014). These sequences differed by a single nucleotide that corresponds to a silent mutation (i.e., a nucleotide change in the third codon position which does not alter the encoded amino acid). The partial flaB sequence of B. theileri generated in this study is deposited in GenBank (accession number: ON191583). Phylogenetically, the partial flaB sequence generated in this study clustered with other B. theileri sequences, being separated (100% bootstrap value support) from B. lonestari, its closest congener (Fig. 2).
More than a century ago, Brumpt (1919) reported the presence of B. theileri-like spirochetes (referred to as Spirochaeta theileri) in cattle and transmission through ticks, after these animals were infected by successive generations of R. microplus ticks (referred to as Margaropus australis) from Brazil. After several decades, B. theileri was reported in R. microplus ticks from Brazil through incidental findings in hemolymph smears (Martins et al. 1996; Rezende et al. 2008). In addition, Yparraguirre et al. (2007) reported the molecular detection of a Borrelia sp. in a R. microplus tick in the southeastern Brazil, which was highly correlated with B. theileri and B. lonestari. More recently, Cordeiro et al. (2018) used morphological, molecular and phylogenetic data to confirm the presence of B. theileri in an engorged R. microplus female removed from a bovine in the state of Rio de Janeiro, Brazil.
We detected spirochetes in the stained blood smear from only one out of 10 cows, which could be related to the low parasitemia observed in B. theileri infections. According to experimental studies, B. theileri becomes detectable in blood smears 2–4 weeks after exposure to infected ticks (Theiler 1905; Callow 1967; Trees 1978; Smith et al. 1978). Occasionally, new peaks of parasitemia may occur, but without signs of illness; the short periods of fever usually coincide with the presence of observable spirochetes on blood smears (Trees 1978; Smith et al. 1978; Van Heerden and Reyers 1984).
During the parasitemia, cattle infected with B. theileri may exhibit a mild rise in temperature, fever, anorexia, depression, and anemia (Theiler 1905; Callow 1967), with the presence of hemoglobinuria (Callow 1967). The infected animal in the present study did not present any clinical signs.
Borrelia theileri is transmitted by Rhipicephalus ticks belonging to the subgenus Boophilus (e.g., R. annulatus, R. microplus, R. decoloratus, and R. evertsi) that preferentially parasitize cattle (Theiler 1905, 1909; Brumpt 1919; Callow 1967; Trees 1978). This agrees with our finding of R. microplus ticks parasitizing the infected cow. In addition to transmit B. theileri, R. microplus is reputed to be a vector of Babesia bigemina, Babesia bovis and Anaplasma marginale in some tropical regions (Theiler 1904; Neitz 1956; Callow and Hoyte 1961; Smith et al. 1978; Scoles et al. 2021).
Previous studies reported serologic cross-reactivity of cattle anti-Borrelia IgG antibodies to whole-cell antigens of B. theileri, Borrelia burgdorferi and Borrelia coriaceae (Ji et al. 1994; Rogers et al. 1999). Thus, caution is needed when using serological tests for diagnosing borreliosis in regions where B. theileri co-exists with other tick-borne Borrelia species (Rogers et al. 1999).
In a recent study, Scoles et al. (2021) tested cattle blood samples using a PCR assay targeting the flagellin gene and confirmed the presence of B. theileri in three (out of 135) stray Mexico origin cattle captured in Texas (Scoles et al. 2021). To our knowledge, our study reports the first molecular detection of B. theileri subclinical infection in cattle in Brazil. Further research is needed to assess the prevalence of B. theileri and its possible impact on the livestock industry in Brazil.
Data availability
The data presented in this study are available within the article.
References
Abanda B, Paguem A, Abdoulmoumini M, Kingsley MT, Renz A, Eisenbarth A (2019) Molecular identification and prevalence of tick-borne pathogens in zebu and taurine cattle in North Cameroon. Parasites Vectors 12:448. https://doi.org/10.1186/s13071-019-3699-x
Abdullah HHAM, Amanzougaghene N, Dahmana H, Louni M, Raoult D, Mediannikov O (2021) Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Negl Trop Dis 15:e0009767. https://doi.org/10.1371/journal.pntd.0009767
Aouadi A, Leulmi H, Boucheikhchoukh M, Benakhla A, Raoult D, Parola P (2017) Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp Immunol Microbiol Infect Dis 50:34–39. https://doi.org/10.1016/j.cimid.2016.11.008
Barros-Battesti DM, Arzua M, Bechara GH (2006) Carrapatos de importância médico-veterinária da região neotropical: um guia ilustrado para identificação de espécies. Vox/ICTTD-3/Instituto Butantan, São Paulo
Brumpt A (1919) Existence de la spirochétose des bovidés ao Brásil. Transmission de cette affection par la tique: Margaropus australis (Fuller). Bull Soc Path Exot 12:748–757
Callow LL, Hoyte HMD (1961) Transmission experiments using Babesia bigemina, Theileria mutans, Borrelia sp and the cattie tick Boophilus microplus Aust Vet J 37:381–390. https://doi.org/10.1111/j.1751-0813.1961.tb03790.x
Callow LL (1967) Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J 123:492–497. https://doi.org/10.1016/S0007-1935(17)39704-X
Cordeiro MD, Bahia M, Magalhães-Matos PC, Cepeda MB, Guterres A, Fonseca AH (2018) Morphological, molecular and phylogenetic characterization of Borrelia theileri in Rhipicephalus microplus Rev Bras Parasitol Vet 27:555–561. https://doi.org/10.1590/S1984-296120180083
Detweiler D (1996) Regulação cardíaca. In: Dukes RS (ed) Fisiologia dos animais domésticos, 11th ed. Guanabara Koogan, Rio de Janeiro, pp 157–169
Dodd S (1906) A preliminary note on the identity of the Spirochjetje found in the horse, ox, and sheep. J Comp Pathol Ther 19:318–322
DuPreez JH (2000) Parameters for the determination and evaluation of heat stress in dairy cattle in South Africa. Onderstepoort J Vet Res 67:263–271
Faccini-Martínez ÁA, Silva-Ramos CR, Santodomingo AM, Ramírez-Hernández A, Costa FB, Labruna MB, Muñoz-Leal S (2022) Historical overview and update on relapsing fever group Borrelia in Latin America. Parasit Vectors 15:196. https://doi.org/10.1186/s13071-022-05289-5
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate Large phylogenies by Max- imum Likelihood. Syst Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Ji B, Thomas CB, Collins MT (1994) Evaluation of an enzyme-linked immunosorbent assay that uses the 41-kd flagellin as the antigen for detection of antibodies to Borrelia burgdorferi in cattle. Am J Vet Res 55:1213–1219
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Koch HT, Kambeva L, Ocama JGR, Munatswa FC, Franssen FFJ, Uilenberg G, Dolan TT, Norval RAI (1990) Immunization of cattle against Theileria parva bovis and their exposure to natural challenge. Vet Parasitol 37:185–196. https://doi.org/10.1016/0304-4017(90)90002-S
Martins JR, Ceresér VH, Corrêa BL, Smith RD (1996) Borrelia theileri: Observation on Boophilus microplus ticks in Guaíba. RS Brazil Cienc Rural 26:447–450. https://doi.org/10.1590/S0103-84781996000300018
McCoy BN, Maïga O, Schwan TG (2014) Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick Borne Dis 5:401–403. https://doi.org/10.1016/j.ttbdis.2014.01.007
Morel N, De Salvo MN, Cicuttin G, Rossner V, Thompson CS, Mangold AJ, Nava S (2019) The presence of Borrelia theileri in Argentina. Vet Parasitol Reg Stud Rep 17:100314. https://doi.org/10.1016/j.vprsr.2019.100314
Naas IA, Arcaro Júnior I (2001) Influence of the ventilation and sprinkling in artificial Influence of the ventilation and sprinkling in artificial shading system for dairy cows in hot climate. Rev Bras Eng Agric Ambient 5:139–142. https://doi.org/10.1590/S1415-43662001000100026
Neitz WO (1935) The Transmission of Spirochaeta theileri to a Blesbuck (Damaliscus albifrons). Onderstepoort J Vet Sci Anim Ind 25:7
Neitz WO (1956) A consolidation of our knowledge of the transmission of tick-borne diseases. Onderstepoort J Vet Res 27:115–163
Qiu Y, Squarre D, Nakamura Y, Lau ACC, Moonga LC, Kawai N, Ohnuma A, Hayashida K, Nakao R, Yamagishi J, Sawa H, Namangala B, Kawabataet H (2021) Evidence of Borrelia theileri in wild and domestic animals in the Kafue Ecosystem of Zambia. Microorganisms 9:2405. https://doi.org/10.3390/microorganisms9112405
Radostits OM, Gay CC, Blood DC, Hinchcliff KW (2000) Veterinary medicine, 9th edn. W.B. Saunders, London, pp 1819–1822
Rezende J, Kessler RH, Soares CO, Martins OP (2008) Occurrence of Borrelia spp. in culture of embryonic cells of the tick Boophilus microplus (Acari: Ixodidae) in the State of the Mato Grosso do Sul, Brazil. Rev Bras Parasitol Vet 17:50–52. https://doi.org/10.1590/S1984-29612008000100011
Robinson EN (1999) Termorregulação. In: Cunningham JG (ed) Tratado de fisiologia veterinária 2nd ed. Guanabara Koogan, Rio de Janeiro, pp 427–435
Rogers AB, Smith RD, Kakoma I (1999) Serologic cross-reactivity of antibodies against Borrelia theileri, Borrelia burgdorferi, and Borrelia coriaceae in cattle. Am J Vet Res 60:694–697
Scoles GA, Lohmeyer KH, Ueti MW, Bonilla D, Lahmers KK, Piccione J, Rogovskyy AS (2021) Stray Mexico origin cattle captured crossing into Southern Texas carry Babesia bovis and other tick-borne pathogens. Ticks Tick Borne Dis 2:101708. https://doi.org/10.1016/j.ttbdis.2021.101708
Sharma SP, Amanfu W, Losho TC (2000) Bovine borreliosis in Botswana. Onderstepoort J Vet Res 67:221–223
Smith RD, Brener J, Osorno M, Ristic M (1978) Pathobiology of Borrelia theileri in the tropical cattle tick, Boophilus microplus. J Invertebr Pathol 32:182–190. https://doi.org/10.1016/0022-2011(78)90028-9
Stöber M (1993) Identificação, anamnese, regras básicas da técnica de exame clínico geral. In: Dirksen G, Gründer HD, Stöber M. Exame clínico dos bovinos 3rd ed. Guanabara Koogan, Rio de Janeiro, pp 44–80
Stromdahl EY, Williamson PC, Kollars TMJ, Evans SR, Barry RK, Vince MA, Dobbs NA (2003) Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J Clin Microbiol 41:5557–5562. https://doi.org/10.1128/JCM.41.12.5557-5562.2003
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Theiler A (1904) Spirillosis of cattle. J Comp Path 17:47–55. https://doi.org/10.1016/S0368-1742(04)80003-1
Theiler A (1905) Transmission and inoculability of Spirillum theileri (Laveran). Proc R Soc Lond B 76:504–506
Theiler A (1909) Transmission des spirilles et des piroplasmes par différentes espèces de tiques. Soc Pathol Exot 2:293–294
Trees AJ (1978) The transmission of Borrelia theileri by Boophilus annulatus (Say, 1821). Trop Anim Health Prod 10:93–94. https://doi.org/10.1007/BF02235315
Van Heerden J, Reyers F (1984) Borrellia sp. infection in a horse. J S Afr Vet Assoc 55:41–43
Yparraguirre LA, Machado-Ferreira E, Ullmann AJ, Piesman J, Zeidner NS, Soares CAG (2007) A hard tick relapsing fever group spirochete in a Brazilian Rhipicephalus (Boophilus) microplus. Vector Borne Zoonotic Dis 7:717–721. https://doi.org/10.1089/vbz.2007.0144
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, CNPq (Conselho Nacional de Desenvolvimento Científco e Tecnológico) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG). SML was funded by Fondecyt Iniciación 11220177.
Author information
Authors and Affiliations
Contributions
WVFP, MBL and FSK conceived and designed the study, and critically revised the manuscript. WVFP, MBL, FDT, SML and FSK performed the experiment, analyzed the data, and drafted the manuscript. WVFP, LCN, LGFP, MCAS, FPO helped in the implementation and execution of the study. WVFP, MBL, FDT, SML and FSK performed and interpreted the laboratory analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The farm was visited during routine veterinary care of the Veterinary School of the Federal University of Goiás. Animal examination and blood collection were performed under a signed consent of the farmer.
Consent to participate
All authors give their consent to participate in this article.
Consent of publication
All authors consent to publication of this manuscript.
Competing of interest
Authors declare they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paula, W.V.d., Neves, L.C., de Paula, L.G.F. et al. First molecular detection of Borrelia theileri subclinical infection in a cow from Brazil. Vet Res Commun 47, 963–967 (2023). https://doi.org/10.1007/s11259-022-10020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-10020-x