Abstract
This study investigated the effect of adding platelet-rich plasma (PRP) in semen extender prior cryopreservation on post-thaw quality, kinematics, and in vivo fertility of fertile and subfertile buffalo spermatozoa. Eleven buffalo bulls were classified based on their conception rate (CR) into fertile (n = 8, CR > 55%) and subfertile (n = 3, CR < 35%) groups. Ejaculates were collected with artificial vagina, pooled, and dispensed into 6 aliquots, diluted with Tris-egg yolk-glycerol extender supplemented with different proportions of PRP [0% (control), 5%, 10%, 15%, 20%, and 25%] followed by cryopreservation using standard procedures. Post-thaw sperm quality, kinematics, antioxidant activity, cryosurvival rate, and in vivo fertility were compared between fertile and subfertile groups and among proportions of PRP within each group. The results showed that 15% PRP greatly (P < 0.001) improved sperm characteristics, average path velocity, and curvilinear velocity of the subfertile group. Interestingly, 5%, 10%, and 15% PRP greatly (P < 0.001) reduced malondialdehyde content and improved enzymatic (glutathione peroxidase and superoxide dismutase) and total antioxidant capacity in fertile and subfertile groups. However, these three proportions of PRP significantly (P < 0.001) improved the cryosurvival rate of the subfertile group; only 15% PRP greatly improved CR of subfertile (60.83% vs. 34.17%) animals to be comparable with that of fertile ones treated with 5 (59.17%) and 10% (60.83%) PRP. In conclusion, adding 15% PRP to semen extender before cryopreservation is recommended to improve post-thaw quality, antioxidant activity, and in vivo fertility of buffalo semen particularly of the subfertile animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semen cryopreservation is a simple and practical assisted reproduction technique that has been employed worldwide to improve the reproductive efficiency of both domestic and pet animals. Understanding the mechanisms and challenges of bovine semen cryopreservation is an essential prerequisite for the success of artificial insemination on a commercial basis (Upadhyay et al. 2021). Freezing–thawing procedures have been induced some sperm structural and functional damages, such as reduced sperm motility and damage of sperm membranes (Gómez-Torres et al. 2017).
Osmotic stress, cold shock, and intracellular ice crystals formation, as well as oxidative stress, are implicated in this cryodamage (Yeste 2016). As a result, new protective techniques have been investigated and modified, such as adjusting buffalo semen extenders by incorporating antifreeze protein (Qadeer et al. 2015), antioxidant (Awan et al. 2018), cryoprotectant (Almadaly et al. 2019), fatty acid (Ejaz et al. 2020), and cholesterol-loaded cyclodextrins (Bishist et al. 2021) to ameliorate this inevitable cryodamage. Similarly, animal serum (Marco-Jiménez et al. 2006), and platelet-rich plasma (PRP; Bader et al. 2020; Yan et al. 2021) were added to European eel semen extender and human semen extender, respectively to reduce freezing–thawing damage.
PRP has dense granules containing serotonin, ATP, and calcium, as reported by Yamakawa and Hayashida (2019). These granules are important in the acceleration and control of wound healing. Besides, PRP containing α granules that have many secretory proteins, which strongly influence the healing process such as platelet-derived growth factor, transforming growth factor–β, endothelial growth factor, and fibroblast growth factor (FGF) as well as insulin-like growth factors (IGF-1 and IGF-2, Chicharro-Alcántara et al. 2018). Notably, almost all these aforementioned factors have a positive impact on sperm cell quality and fertility (Yan et al. 2021).
Seminal IGF-1 secreted from the Leydig cell and Sertoli cell (Roser 2001) has a pivotal role in the spermatogenesis and steroidogenesis processes (Lee et al. 2016). There are many interesting findings concerning the relationship between high IGF-1 concentration in either serum or seminal plasma (SP) and male fertility. For instance, it yielded greater fertility in stallions (Macpherson et al. 2002), correlated to sperm quality and fertility in humans (Lee et al. 2016), and improved sperm motility and membrane integrity in buffalo bull (Kumar et al. 2019, 2021). Thus, quantification of IGF-1 concentration in either serum or SP might be beneficial to predict sperm cell quality and fertility (Rasheed et al. 2019).
Although bovine semen possesses a natural defense mechanism against oxidative stress, it is thought to be unable to prevent lipid peroxidation caused by the cryopreservation process (Patel et al. 2016). Further, buffalo spermatozoa are more vulnerable to thermal changes associated with freezing and thawing procedures (Rastegarnia et al. 2013), and oxidative stress (El-Khawagah et al. 2020) as well. The incorporation of antioxidants into semen extender to neutralize free radicals has been succeeded to preserve the metabolic activity and cellular viability of bull (Tvrdá et al. 2016), and buffalo (Awan et al. 2018) spermatozoa. Based on all these premises, this study aimed to investigate the potential effects of adding different proportions of PRP in the semen extender before cryopreservation on post-thaw sperm characteristics and kinematics, as well as antioxidant activity and in vivo fertility of fertile and subfertile buffalo semen.
Materials and methods
All chemicals used were bought from Sigma-Aldrich (St. Louis, MO, USA) and were of high purity unless otherwise stated.
Experimental animals
In this trial, eleven mature (3 – 4 years old) healthy Egyptian buffalo (Bubalus bubalis) bulls were used. Animals were classified according to their conception rate (CR) obtained following insemination of 330 estrus females during the breeding season (September–2019 to March–2020) using their freshly processed frozen-thawed straws, into fertile (n = 8, CR > 55%) and subfertile (n = 3, CR < 35%, Kumar et al. 2012). Also, three hundred and sixty Egyptian buffalo cows (4 – 5 years old) with a history of normal calving were used for in vivo fertility experiments (September–2020 to March–2021) using our frozen-thawed straws supplemented or non-supplemented with different proportions of PRP.
All Animals were kept in open yards in Mehalet-Mousa Research Station, Kafrelsheikh, Egypt (latitude 31° 06' N and longitude 30° 56' E) and fed concentrated food mixture plus roughages according to National Research Council (NRC 2001) requirements.
Semen collection and evaluation
Ejaculates were collected twice per week at 07:00 − 08:00 am using an artificial vagina (40 °C to 42 °C) for 12 consecutive weeks (24 ejaculates/animal). Directly after collection, the collected ejaculates were observed for color, consistency, and hygienic quality, as well as, ejaculate volume was determined. Unhygienic semen samples, and any sample of abnormal color and/or consistency were excluded. Sperm concentration was estimated (1 × 106/mL) using a density spectrophotometer (SDM-5, Minitub, GmbH, Germany) where an aliquot of semen was diluted (1:100) with 0.9% sodium chloride (Rashid et al. 2015).
Sperm motility, kinematics, and viability
A computer-aided sperm motion analyzer (CASA; Hamilton–Thorne Biosciences, Beverly, MA, USA) system was used to measure sperm motility and kinematics. Fast > 80 m/s, medium > 60 m/s, slow > 20 m/s, and static speed standards were used to calculate sperm motilities. Eight microscopic fields were randomly selected and evaluated by the CASA system for each evaluation. From each ejaculate, an aliquot (5 μL) of semen was loaded into a pre-warmed (37 °C) clean Makler chamber and observed with a 100 × objective to evaluate total motility (%), progressive motility (%), average path velocity (VAP, μm/s), straight linear velocity (VSL, μm/s), curvilinear velocity (VCL, μm/s), straightness (STR, %), linearity (LIN, %) and wobble coefficient (WOB, %, El-Khawagah et al. 2020). Eosin-nigrosin stained semen smear was examined to estimate the proportion of viable spermatozoa (Mortimer 1994). Two hundred spermatozoa were examined under an oil immersion lens (1000 ×) where those with white heads were presented as a percentage (%) of sperm viability.
Functional plasma membrane integrity
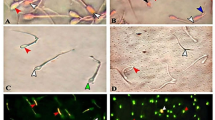
The hypo-osmotic swelling test (HOST) was used to assess the functional integrity of the sperm plasma membrane in either fertile or subfertile buffalo bulls (Kumar et al. 2015). In detail, 100 μL of semen was suspended in 1000 μL hypo-osmotic solution (150 mOsm/kg; 0.735 g sodium citrate and 1.351 g fructose in 100 mL Milli-Q water) and incubated in a water bath of 37 °C for 30 – 60 min. After incubation, sperm tail bending/coiling was assessed by putting 2 μL of a well-mixed sample onto a warm slide (38 °C), covered by a pre-warmed coverslip, and observed under a phase-contrast microscope at 400 × magnifications. After incubation, sperm cells with coiled and/or enlarged tails were judged to have functional plasma membranes (HOST-positive). The proportion of spermatozoa with abnormal tail morphology was determined before HOST and subtracted from the proportion of HOST-positive spermatozoa to obtain the true % of HOST-positive spermatozoa.
Acrosome integrity
The fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) smear staining method was used to determine acrosome integrity, as described before (Almadaly et al. 2012). Briefly, an aliquot (10 μL) of semen was fixed for 30 min at room temperature with 4% paraformaldehyde, then diluted (1:10) with PBS containing 0.1% polyvinyl alcohol and 0.1% polyethylene glycol. Five µL of fixed spermatozoa was smeared, air-dried, and permeabilized with 1% (v/v) Triton X-100 at room temperature for 5 min; then allowed to dry before staining with FITC-PNA (20 μg/mL in PBS) in dark space for 30 min at room temperature. Stained smears were rinsed with PBS, air-dried, and covered with a coverslip before examination under a phase-contrast microscope with fluorescence illumination (Olympus, Tokyo, Japan). Intact-acrosome sperm displaying more intense, uniform, and demarcated green fluorescence in the acrosomal cap, whereas sperm cells showing less intense and/or ill-defined fluorescence or even no fluorescence, were classified as damaged-acrosome (Almadaly et al. 2012).
Preparation of platelet-rich plasma (PRP)
On the day of semen collection and under complete aseptic condition, PRP was harvested from fresh whole blood drawn from the jugular vein of clinically healthy, mature, and fertile 5 buffalo bulls. The blood was collected into 10 mL capacity sterile tubes, containing sodium citrate (BD Vacutainer®, Becton Drive, Franklin Lakes, NJ, USA; Giraldo et al. 2015). As soon as possible, the collected blood samples were centrifuged at 300 g for 5 min at room temperature to separate red blood cells, and then the collected plasma was re-centrifuged at 700 g for 17 min to collect pure PRP without white blood cells (Nazari et al. 2016).
Determination of IGF-1 concentration in the collected PRP
The concentration of IGF-1 in PRP collected from fertile and subfertile groups was quantified using an immuno-radiometric assay kit (Immunotech SAS, Marseille Cedex, France) after acid–ethanol extraction (16 h at 4 °C) as described by Echternkamp et al. (1990) with an intra-assay coefficient of variation of 9.2%.
Semen dilution and cryopreservation
One day before semen cryopreservation, Tris-egg yolk-glycerol diluent consisted of 3.028 g Tris, 1.678 g citric acid, 1.0 g fructose, 20% (v/v) egg yolk, 7% (v/v) glycerol and antibiotics; 1 mg/mL streptomycin sulfate, and 1000 IU/mL penicillin G sodium was prepared, centrifuged (at 3310 g for 20 min) and the intermediate portion was collected, kept at 4 °C overnight and used for semen dilution (Almadaly et al. 2019) during the cryopreservation procedures.
Immediately after semen collection, the collected ejaculates of either fertile (n = 8) or subfertile (n = 3) group were pooled and divided into six equal fractions, and then diluted to 80 × 106 sperm/mL with the intermediate portion of Tris-egg yolk-glycerol extender supplemented with PRP at different proportions: 0% (control), 5%, 10%, 15%, 20% and 25%. After being gradually cooled (equilibrated) to 4 °C for 4 h; the diluted semen was packed in 0.25 mL polyvinyl labeled straws (IMV, L'Aigle, France) with a suction pump in a cold cabinet (Minitub, Germany). The straws were frozen at –140 °C in a programmed biofreezer (Mini Digit-cool, ZH 400, IMV technologies, L'Aigle, France) before being immediately immersed in liquid nitrogen at –196 °C for storage (Dalal et al. 2018).
Preparation of frozen straws supplemented with different proportions of PRP was repeated for 7 occasions. After at least 2 weeks of frozen storage, frozen straws (3 straws/proportion of PRP/animal group/replicate for 4 replicates) were immersed in a water bath of 39 °C for exactly 1 min, thoroughly dried, and gently evacuated in 1.5 mL micro-centrifuge tube (Almadaly et al. 2019) to be examined for post-thaw sperm characteristics and kinematics.
Frozen-thawed semen evaluation
Sperm motility, kinematics, and viability
Five μL of semen was loaded in a pre-warmed (37 °C) clean Makler chamber for motility and kinematics analyses using the CASA system as described above in the evaluation of fresh semen (El-Khawagah et al. 2020). Also, sperm viability was determined according to Mortimer (1994) as described in fresh semen evaluation.
Functional sperm plasma membrane integrity
Likewise, functional plasma membrane integrity of frozen-thawed fertile and subfertile buffalo spermatozoa was assessed using HOST as described in fresh semen evaluation according to Kumar et al. (2015).
Cryosurvival rate
Using the formula: 100 × post-thaw total motile sperm/pre-freeze total motile sperm, the cryosurvival rate of frozen-thawed fertile and subfertile buffalo spermatozoa was estimated (Nagata et al. 2019).
Acrosome integrity
Similarly, acrosome integrity of frozen-thawed spermatozoa of either fertile or subfertile buffalo bulls was evaluated in FITC-PNA stained semen smear as described in fresh semen evaluation in line with our earlier report (Almadaly et al. 2012).
Antioxidant activity and lipid peroxidation
Both fresh (500 μL) and frozen-thawed (three straws per each % of PRP were pooled/animal group) semen were centrifuged at 1000 g for 10 min at room temperature. The collected supernatants were used (No. of replicates = 5) for the estimation of total antioxidant capacity (TAC), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) activity as per instruction of kit manufacturer (Cayman Chemicals Company).
Total antioxidant capacity
As previously stated, TAC was measured using an antioxidant assay kit provided by Cayman Chemicals Company (Michigan, USA; Lone et al. 2016). In brief, 10 μL of standard or sample in duplicate + 10 µL of metmyoglobin + 150 µL of chromogen were added to each well in the plate. To initiate the reaction, a multichannel pipette was used to add 40 µL of hydrogen peroxide to the plate, which was then covered and incubated at room temperature for 5 min. After incubation, the absorbance was measured with a plate reader at 750 nm, and the TAC (Mm) of the samples was calculated using the equation obtained from the linear regression of the standard curve as described by Lone et al. (2016).
Glutathione peroxidase activity
GPx activity was determined using the Cayman GPx assay kit (Cayman Chemicals Company) as described by Kumar et al. (2015). Briefly, to each well in the plate add 100 μL of assay buffer, 50 μL of the co-substrate mixture, and 20 μL standards/samples, then add 20 μL of cumene hydroperoxide to initiate the reaction. A plate reader was used to record the absorbance at 340 nm per min for at least 5 min. Using the GPx standards, the standard curve was drawn, and the GPx activity (nmol/min/mL) for each sample was calculated.
Superoxide dismutase activity
Using the Cayman SOD assay kit, SOD activity was determined according to Kumar et al. (2015). In each well, 200 μL of the diluted radical detector and 10 μL of standards/samples were added, followed by 20 μL of diluted xanthine oxidase, and the plate was incubated for 20 min at room temperature with gentle shaking. Thereafter, using the SOD standards, the standard curve was plotted and the activity of SOD (U/mL) for each sample was determined.
Malondialdehyde content
MDA content was estimated using the TABARS assay kit (Cayman Chemicals Company) following the methodology of Kumar et al. (2015). In brief, 100 μL of samples/standards + 100 μL of sodium dodecyl sulfate solution + 4000 μL color reagent was added into a clean test tube. The tubes were maintained in crushed ice for 10 min after being incubated in a boiling water bath for 60 min. At 4 °C, the suspension was centrifuged for 10 min at 1600 g. Following that, 150 μL of suspension were placed into a colorimetric plate, and the absorbance was measured at 535 nm. The MDA standards were used to plot the standard curve, and the MDA content (μM/mL) of each sample was determined.
In vivo fertility experiment
A total of 360 healthy pluriparous cyclic buffalo cows received (i.m; Day 0) the first dose of prostaglandin F2α (PGF2α; 750 pg Cloprostenol sodium, Estrumate, Berkhamsted, England). Throughout the day and night, 48 h following PGF2α injection, all animals were monitored for signs of estrus. Estrus buffalo cows were ultrasound scanned for the presence of graffian follicle (GF ≥ 12 mm) before being inseminated twice (one on detection of GF, and the other 12 h later) using our frozen-thawed straws supplemented with different proportions of PRP.
On Day 11, non-estrus buffalo cows received the second dose of PGF2α (Borchardt et al. 2017). Likewise, treated females were observed for estrus signs and inseminated twice using frozen-thawed straws supplemented with different proportions of PRP. All inseminated buffalo cows were ultrasound scanned using the linear probe on day 45 post-insemination for pregnancy diagnosis. The fertility data were based on 6 proportions (0% × 60 buffalo cows, 5% × 60 buffalo cows, 10% × 60 buffalo cows, 15% × 60 buffalo cows, 20% × 60 buffalo cows, and 25% × 60 buffalo cows) of PRP supplemented frozen-thawed straws for either fertile or subfertile groups.
Statistical analyses
The data were tabulated as the mean ± SEM. Using the General Linear Model procedures of SAS (2004), the obtained results were subjected to two-way ANOVA. Differences among means were tested using the Range Multiple tests of Duncan (1955). All proportions were subjected to arcsine transformation before being analyzed by Chi-square (χ2) test. Yij = U + Ai + eij was the statistical model, where Yij denoted observed values, U denoted overall mean, Ai denoted animal groupings, and eij denoted random error. At P < 0.05, differences were considered significant in all analyses.
Results
IGF-1 concentration in PRP of fertile and subfertile buffalo bulls
IGF-1 concentrations of PRP were extremely higher in fertile (P < 0.001; 1654 ± 26.09 ng/mL) than subfertile (1350 ± 14.61 ng/mL) buffalo bulls as depicted in Fig. 1.
IGF-1 concentration (mean ± SEM) in PRP of fertile and subfertile buffalo bulls. The concentration of IGF-1 in PRP collected from both fertile and subfertile buffalo bulls was determined using an immuno-radiometric assay kit (Immunotech SAS, Marseille Cedex, France). Bars with different superscripts differ significantly at P < 0.05
Sperm characteristics and kinematics of fertile and subfertile buffalo bulls
As presented in Tables 1 and 2, all sperm characteristics (total motility, progressive motility, viability, membrane integrity, and acrosome integrity) and the majority of sperm kinematics (VAP, VSL, VCL, and STR) of either fresh or frozen-thawed semen were greater (P < 0.01) in the fertile group than their counterparts in the subfertile group. Meanwhile, LIN and WOB were similar between fertile and subfertile groups in either fresh or frozen-thawed semen (Table 2).
Effect of PRP on post-thaw sperm quality and kinematics of fertile and subfertile buffalo bulls
Neither sperm characteristics (Table 1) nor sperm kinematics (Table 2) were affected by adding 5, 10, and 15% PRP in semen extender before cryopreservation of fertile spermatozoa. Whereas, in the subfertile group, the effect of PRP tend to be a dose-dependent effect where 5% improved membrane integrity only; 10% PRP improved all sperm characteristics, except acrosome integrity, and only the VAP variable of sperm kinematics; moreover, 15% PRP greatly improved (P < 0.001) all sperm characteristics, VAP and VCL variables of sperm kinematics. Surprisingly, both 20 and 25% PRP did not improve either sperm characteristics or sperm kinematics of the fertile group, but in the subfertile group, the latter proportion negatively affected (P < 0.001) total motility, progressive motility, VAP, VSL, and VCL as shown in Tables 1 and 2.
Antioxidant activity of fresh and frozen-thawed fertile and subfertile buffalo semen
The values of TAC, GPx, and SOD in either fresh or frozen-thawed semen of the fertile group were greater than their counterparts in the subfertile group as presented in Table 3. On the other hand, MDA content in either fresh or frozen-thawed semen of the subfertile group was greater than their counterparts in the fertile group (Table 3).
Effect of PRP on antioxidant activity of frozen-thawed fertile and subfertile buffalo semen
As shown in Table 3, in either fertile or subfertile groups, adding 5, 10, and 15% PRP in the extender medium before cryopreservation significantly (P < 0.001) increased the values of TAC, GPx, and SOD than their counterparts in frozen-stored samples without PRP (0%). Besides, following 15% PRP supplementation, the values of TAC, GPx, SOD, and MDA in the subfertile group were comparable (P > 0.05) with their counterparts in the fertile group. Meanwhile, 5, 10, and 15% PRP significantly decreased MDA content in comparison with the control (0% PRP) in either fertile or subfertile groups. Unexpectedly, 20 and 25% PRP significantly (P < 0.001) decreased the values of TAC, GPx, and SOD, while increasing MDA content compared with their corresponding values following 15% PRP supplementation in either fertile or subfertile groups (Table 3).
Cryosurvival rate and in vivo fertility of frozen-thawed fertile and subfertile buffalo spermatozoa
As depicted in Fig. 2, adding 5, 10, and 15% PRP into the extender medium used for cryopreservation of the subfertile buffalo spermatozoa greatly (P < 0.001) improved their cryosurvival rates in comparison with those of 0, 20, and 25% PRP, and also with their counterparts of fertile spermatozoa. Unfortunately, all proportions of PRP did not improve cryosurvival rates of the fertile group meanwhile 25% PRP significantly (P < 0.001) decreased cryosurvival rate of the subfertile group as compared to that of the control (Fig. 2).
Effect of PRP on cryosurvival rate of frozen-thawed fertile and subfertile buffalo spermatozoa. Cryosurvival rate of fertile and subfertile buffalo spermatozoa frozen stored with or without PRP was calculated from the following equation: Cryosurvival rate = 100 × post-thaw total motile sperm/pre-freeze total motile sperm. Means bearing one similar superscript were similar (P ≥ 0.05)
Incorporation of 5, 10, and 15% PRP into the semen extender before cryopreservation yielded a dose-dependent increase in CR% with greater in vivo fertility following insemination with 15% PRP-supplemented frozen-thawed straws of either fertile (67.50 ± 1.71% vs. 57.5 ± 1.71%) or subfertile (60.83 ± 0.40% vs. 34.17 ± 2.01%) buffalo bulls. On contrary, using 20% or 25% PRP-supplemented frozen-thawed straws in the insemination of estrus buffalo cows yielded low CR% with or without significant difference in comparison with the control straws of fertile and subfertile groups, respectively (Fig. 3).
Effect of PRP on conception rate of frozen-thawed fertile and subfertile buffalo spermatozoa. Pluriparous buffalo cows were received (i.m; Day 0) the first dose of prostaglandin F2α (PGF2α; 750 pg Cloprostenol sodium, Estrumate, Berkhamsted, England). Exactly, 48 h after PGF2α injection all animals were observed for estrus signs. Estrus buffalo cows were ultrasound scanned for the presence of graffian follicle (GF ≥ 12 mm) before being inseminated twice (one on detection of GF, and the other 12 h later) using our frozen-thawed straws supplemented with different proportions of PRP. On Day 11, non-estrus buffalo cows received the second dose of PGF2α (750 pg Cloprostenol sodium), observed for estrus signs and ultrasound scanned for the presence of GF, and then finally inseminated twice (one on detection of GF, and the other 12 h later) using frozen-thawed straws supplemented with different proportions of PRP. Means bearing one similar superscript were similar (P ≥ 0.05)
Discussion
In the present study, sperm characteristics and kinematics, as well as antioxidant activity of fresh and frozen-thawed fertile and subfertile buffalo spermatozoa cryopreserved in the presence of different proportions of PRP were compared. In either fresh or frozen-thawed semen, sperm characteristics as well as VAP, VSL, VCL, and STR were greater in the fertile buffalo bulls than subfertile ones, supporting the findings of Singh et al. (2016) who reported that the proportion of motile spermatozoa, as well as the values of VAP, VSL, and VCL in bulls of high fertility, were higher than those of low fertility. Furthermore, our findings revealed that proportions of intact-acrosome and intact-plasma membrane in the fertile animals were greater than their counterparts in subfertile animals which might be one of the plausible reasons for this clear difference in their in vivo fertility. These findings corroborated the findings of Hirose et al. (2020), who demonstrated that an intact plasma membrane and intact acrosome are required for oocyte penetration and successful fertilization.
Doubtless, freezing and thawing procedures disturb sperm membranes and reduce sperm motility, viability, and fertility (Kumar et al. 2019). Moreover, buffalo spermatozoa seem to be more sensitive to freezing–thawing damage leading to reduced post-thaw motility and subsequently low fertility (Selvaraju et al. 2016). According to our findings, frozen-thawed semen has low sperm characteristics, kinematics, and antioxidant activity as compared to fresh semen, this might be related to cryopreservation-associated stressors (osmotic, dilution, and oxidative) and subsequent functional, and structural changes which inhibit glycolysis and ATP production (Yeste 2016; Gómez-Torres et al. 2017).
To the best of our knowledge, our study is unique to determine the effect of PRP on post-thaw quality and in vivo fertility of frozen-thawed buffalo spermatozoa. Herein, we found that the addition of 15% PRP before cryopreservation of subfertile spermatozoa improved their post-thaw sperm characteristics and some of their sperm kinematics. This might be attributed to the buffering effect of PRP that prevents osmotic shock as its protein component mechanically protects sperm membranes by lowering the risk of crystallization or melting during the various steps of the cryopreservation process (Taher-Mofrad et al. 2020). Also, this protective function of PRP might be attributed to the presence of multiple bioactive components in PRP according to Saucedo et al. (2015) who found that FGF, a component of PRP, increases the phosphorylation of FGF receptors on sperm flagella and activates the extracellular signal-regulated kinase and protein kinase B signaling pathways, resulting in a significant increase in the proportions of total and progressive sperm motility, as well as some sperm kinematics. In line with our study, Hernández-Corredor et al. (2020) have been proven that the inclusion of PRP in ram semen improves sperm motility and morphometry, the action of FGF, which enhances sperm motility, contributes to this improvement.
Another possibility for this improvement in post-thaw sperm quality and function especially of subfertile buffalo bulls might be attributed to antioxidant activities of IGF-1 (Selvaraju et al. 2016) which is a major component in PRP that has been proven to improve sperm plasma membrane and acrosomal membrane integrities as well as DNA stability of human spermatozoa (Yan et al. 2021). IGF-1 increases intracellular calcium ions concentrations by improving calcium transport, resulting in increased sperm progressive motility (Miah et al. 2008), and also has an important role in the energy metabolism of buffalo spermatozoa (Selvaraju et al. 2009). Moreover, it reduces cryopreservation-induced damage through maintaining sperm membrane proteins such as calmodulin, dermcidin, and acrosomal membrane-associated proteins (Selvaraju et al. 2016).
Based on our result that found IGF-1 concentration in PRP collected from fertile buffalo bulls is ranged from 1295 to 2009 ng/mL with an average of 1652 ng/mL. Thus, adding 15% PRP (≈ 250 ng/mL IGF-1) to buffalo semen improved post-thaw quality and/or in vivo fertility in the current study, similar to Kumar et al. (2019), who found that adding 250 ng/mL IGF-1 to buffalo spermatozoa before cryopreservation improved their post-thaw motility, longevity, and membrane integrity. Hence, it is recommended to measure IGF-1 concentration in PRP before adding it to the semen extender. Notably, herein, PRP collected from subfertile buffalo bulls has lower IGF-1 levels than that collected from fertile animals which assumes that IGF-1 concentration in PRP might be associated with male fertility at least in buffalo bulls.
Even though, 20 and 25% PRP should be containing higher concentrations of IGF-1 compared to 15% PRP; both failed to improve sperm quality and function of either fertile or subfertile animals which might be attributed to the fact that higher proportions of PRP containing higher serum concentrations that leading to head-to-head agglutination of spermatozoa in accord with Dong et al. (2007). This should be the most plausible reason because 25% PRP adversely affects motility (total and progressive), velocity (VAP, VSL, and VCL), and cryosurvival rate (which is motility-dependent) of subfertile buffalo spermatozoa without any effect on their viability, membrane integrity, and acrosome integrity.
PRP has an antioxidant and anti-apoptotic effect on mammalian cells; it also can aid muscle recovery by regulating antioxidant enzymes and reducing radiation-induced kidney histological abnormalities (Lai et al. 2016). Our results revealed that adding 15% PRP to semen extender before cryopreservation significantly increased TAC, GPx, and SOD levels, and decreased MDA content either in fertile or subfertile buffalo semen, but negatively affected when increased to 20 and 25% PRP suggesting that PRP has a potent antioxidant activity in accord with Bader et al. (2020) who had proved that adding 2% PRP was capable to reverse the negative effect of H2O2-induced oxidative stress on human spermatozoa but, no difference was noted when PRP concentration was increased to 5%, and when the concentration was further enhanced to 10% showing decreased percentages of viability, progressive, total motile spermatozoa and a higher percentage of dead spermatozoa compared to control (P < 0.001) group. Our findings, as well as those of Bader et al. (2020), are consistent with Bucak et al. (2007), who concluded that antioxidant additives had cryoprotective activity on sperm in moderate doses, but that higher doses of antioxidant additives would result in a hypertonic property of extender, impairing sperm functions. Also, Yan et al. (2021) found that human semen supplemented with lower proportions of PRP has a low level of reactive oxygen species and patent mitochondrial membrane.
Cryosurvival rate is more accurate in determining post-thaw sperm quality than either total motility or progressive motility (Saleh et al. 2018). Fertile and subfertile buffalo semen varies widely in terms of freezability outcome (Nagata et al. 2019), herein, adding either 10 or 15% PRP before cryopreservation successfully ameliorates such variation through improving cryosurvival rate of subfertile buffalo spermatozoa. This striking finding highlights the novelty of adding PRP before cryopreservation of subfertile spermatozoa, at least in buffalo bulls.
It is worth noting that, in the current study PRP improves in vivo fertility (in terms of CR%) of fertile and subfertile buffalo spermatozoa which suggests that, the effect of PRP might be also expressed on the female genitalia because Pasch et al. (2021) found that PRP has anti-inflammatory and regenerative capabilities that have been used to treat degenerative changes in the endometrium of subfertile mares. Furthermore, PRP has antimicrobial properties to reduce the chances of uterine infection after breeding and it improves immune response which is likely one of the major factors contributing to enhancing embryonic survival and consequent greater embryonic recovery obtained in mare (Segabinazzi et al. 2021). Furthermore, PRP has been proven to reduce intrauterine adhesions and improve ovarian reserve (Yazhini and Kanchana 2021), as well as increase pregnancy and live birth rates in women (Sharara et al. 2021). All these promising results reveal that 15% PRP containing ≈ 250 ng/mL IGF-1 is the optimal proportion to improve post-thaw quality and function of frozen-thawed buffalo spermatozoa which could be the originality of this study.
Conclusion
In summary, adding 15% PRP into the extender medium used for cryopreservation of buffalo spermatozoa is recommended to ameliorate their post-thaw sperm characteristics, kinematics, antioxidant activity, cryosurvival rate, and in vivo fertility, particularly of subfertile spermatozoa. This study opens a new area of research on the subfertility of frozen-thawed spermatozoa in farm animals.
Data availability
All data has been provided.
References
Almadaly E, El-Kon I, Heleil B, El-S F, Mukoujima K, Ueda T, Hoshino Y, Takasu M, Murase T (2012) Methodological factors affecting the results of staining frozen-thawed fertile and subfertile Japanese Black bull spermatozoa for acrosomal status. Anim Reprod Sci 136:23–32. https://doi.org/10.1016/j.anireprosci.2012.10.016
Almadaly E, Tawfik F, El-Kon I, Heleil B, Fattouh El-S (2019) Effect of different cryoprotectants on the post-thaw sperm characteristics and in vivo fertility of buffalo (Bubalus bubalis) bull semen. Slov Vet Res 56:541–551. https://www.slovetres.si/index.php/SVR/article/view/792. Accessed 5-9 Mar 2021
Awan MA, Akhter S, Husna AU, Ansari MS, Rakha BA, Azam A, Qadeer S (2018) Antioxidant activity of Nigella sativa seeds aqueous extract and its use for cryopreservation of buffalo spermatozoa. Andrologia 50:e13020. https://doi.org/10.1111/and.13020
Bader R, Ibrahim JN, Moussa M, Mourad A, Azoury J, Alaaeddine N (2020) In vitro effect of autologous platelet-rich plasma on H2O2-induced oxidative stress in human spermatozoa. Andrology 8:191–200. https://doi.org/10.1111/andr.12648
Bishist R, Raina VS, Bhakat M, Lone SA, Mohanty TK, Sinha R, Raj Kumar R (2021) Effect of cholesterol loaded cyclodextrins on cryosurvivability of buffalo spermatozoa. Buffalo Bull 40:115–121. https://kuojs.lib.ku.ac.th/index.php/BufBu/article/view/633. Accessed 20 Apr 2021
Borchardt S, Haimerl P, Pohl A, Heuwieser W (2017) Evaluation of prostaglandin F2α versus prostaglandin F2α plus gonadotropin-releasing hormone as presynch methods preceding an ovsynch in lactating dairy cows: a meta-analysis. J Dairy Sci 100:4065–4077. https://doi.org/10.3168/jds.2016-11956
Bucak MN, Atessahin A, Varıslı O, Yuce A, Tekin N, Akcay A (2007) The influence of trehalose, taurine, cysteamine and hyaluronan onram semen: microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology 67:1060–1067. https://doi.org/10.1016/j.theriogenology.2006.12.004
Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda J, Cuervo-Serrato B, Peláez-Gorrea P, Sopena-Juncosa J (2018) Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater 9:10–20. https://doi.org/10.3390/jfb9010010
Dalal J, Kumar A, Kumar P, Honparkhe M, Malik VS, Singhal S, Kaur S, Brar PS (2018) Improvement in cryosurvival of buffalo bull (Bubalus bubalis) sperm by altering freezing rate within critical temperature range. Asian Pac J Reprod 7:72–78. https://doi.org/10.4103/2305-0500.228016
Dong Q, Huang C, Tiersch TR (2007) Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology 54:87–98. https://doi.org/10.1016/j.cryobiol.2006.11.007
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Echternkamp SE, Spicer LJ, Gregory KE, Canning SF, Hammond JM (1990) Concentrations of insulin-like growth factor-I in blood and ovarian follicular fluid of cattle selected for twins. Biol Reprod 43:8–14. https://doi.org/10.1095/biolreprod43.1.8
Ejaz R, Qadeer S, Ansari MS, Rakha BA, Shamas S, Azam A, Maqsood I, Husna AU, Akhter S (2020) In vitro supplementation of linoleic acid improves quality of cryopreserved buffalo semen. Indian J Anim Res (In press). https://doi.org/10.18805/ijar.B-1182
El-Khawagah ARM, Kandiel MMM, Samir H (2020) Effect of quercetin supplementation in extender on sperm kinematics, extracellular enzymes release, and oxidative stress of Egyptian buffalo bulls frozen-thawed semen. Front Vet Sci 7:604460. https://doi.org/10.3389/fvets.2020.604460
Giraldo CE, Álvarez ME, Carmona JU (2015) Effects of sodium citrate and acid citrate dextrose solutions on cell counts and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet Res 11:60 67.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4364319/ Accessed Dec 2015
Gómez-Torres MJ, Medrano L, Romero A, Fernández-Colom PJ, Aizpurúa J (2017) Effectiveness of human spermatozoa biomarkers as indicators of structural damage during cryopreservation. Cryobiology 78:90–94. https://doi.org/10.1016/j.cryobiol.2017.06.008
Hernández-Corredor L, León-Restrepo S, Bustamante-Cano J, Báez-Sandoval G, Jaramillo X (2020) Effect of the incorporation of plasma rich of platelets on the spermatozoa physiology of ram semen. J Dairy Vet Anim Res 9:34–38. https://doi.org/10.15406/jdvar.2020.09.00275
Hirose M, Honda A, Fulka H, Tamura-Nakano M, Matoba S, Tomishima T, Mochida K, Hasegawa A, Nagashima K, Inoue K, Ohtsuka M, Baba T, Yanagimachi R, Ogura A (2020) Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proceed Nat Acad Sci 117:2513–2518. https://www.pnas.org/content/pnas/117/5/2513.full.pdf. Accessed 4 Feb 2020
Kumar D, Kumar P, Singh P, Yadav SP, Yadav PS (2012) Buffalo bull semen-fertility evaluation in relation to motility and integrity of acrosome, plasma membrane, and sperm DNA. Reprod Fertil Dev 25:180–180. https://www.publish.csiro.au/rd/RDv25n1Ab66. Accessed 4 Dec 2012
Kumar P, Kumar D, Sikka P, Singh P (2015) Sericin supplementation improves semen freezability of buffalo-bulls by minimizing oxidative stress during cryopreservation. Anim Reprod Sci 152:26–31. https://doi.org/10.1016/j.anireprosci.2014.11.015
Kumar P, Suman Pawaria S, Dalal J, Bhardwaj S, Patil S, Jerome A, Sharma RK (2019) Serum and seminal plasma IGF-1 associations with semen variables and effect of IGF-1 supplementation on semen freezing capacity in buffalo bulls. Anim Reprod Sci 204:101–110. https://doi.org/10.1016/j.anireprosci.2019.03.010
Kumar A, Singh G, Jerome A, Kumar P, Arjun V, Bala R, Verma N, Sharma RK (2021) IGF-1 supplementation in semen affects mitochondrial functional and calcium status of buffalo sperm following cryopreservation. Anim Reprod Sci 231:106783. https://doi.org/10.1016/j.anireprosci.2021.106783
Lai L, Mohamed MNA, Ali MRM, Keen KT, Yusof A (2016) Effect of platelet-rich plasma treatment on antioxidant enzymes’ activity following hamstring injury among Malaysian athletes. Sains Malays 45:769–775. https://www.researchgate.net/publication/305176873. Accessed May 2016
Lee HS, Park Y-S, Lee JS, Seo JT (2016) Serum and seminal plasma insulin-like growth factor-1 in male infertility. Clin Exp Reprod Med 43:97–101. https://doi.org/10.5653/cerm.2016.43.2.97
Lone SA, Prasad JK, Ghosh SK, Das GK, Balamurugan B, Sheikh AA, Verma MR (2016) Activity of enzymatic antioxidants and total antioxidant capacity in seminal plasma of murrah bulls during Cryopreservation. J Anim Res 6:405–410. https://doi.org/10.5958/2277-940X.2016.00038.3
Macpherson ML, Simmen RCM, Simmen FA, Hernandez J, Sheerin BR, Varner DD, Loomis P, Cadario ME, Miller CD, Brinsko SP, Rigby S, Blanchard TL (2002) Insulin-like growth factor-I and insulin-like growth factor binding protein-2 and -5 in equine seminal plasma: association with sperm characteristics and fertility. Biol Reprod 67:648–654. https://doi.org/10.1095/biolreprod67.2.648
Marco-Jiménez F, Garzón DL, Peñaranda DS, Pérez L, Viudes-de-Castro MP, Vicente JS, Jover M, Asturiano JF (2006) Cryopreservation of European eel (Anguilla anguilla) spermatozoa: effect of dilution ratio, foetal bovine serum supplementation, and cryoprotectants. Cryobiology 53:51–57. https://doi.org/10.1016/j.cryobiol.2006.03.011
Miah AG, Salma U, Takagi Y, Kohsaka T, Hamano K-I, Tsujii H (2008) Effects of relaxin and IGF-1 on capacitation, acrosome reaction, cholesterol efflux and utilization of labeled and unlabeled glucose in porcine spermatozoa. Reprod Med Biol 7:29–36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5904611/. Accessed Mar 2008
Mortimer D (1994) Sperm recovery techniques to maximize fertilizing capacity. Reprod Fertil Dev 6:25–31. https://doi.org/10.1071/rd9940025
Nagata MB, Egashira J, Katafuchi N, Endo K, Ogata K, Yamanaka K, Yamanouchi T, Matsuda H, Hashiyada Y, Yamashita K (2019) Bovine sperm selection procedure prior to cryopreservation for improvement of post-thawed semen quality and fertility. J Anim Sci Biotechnol 10:91–105. https://doi.org/10.1186/s40104-019-0395-9
Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L (2016) Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: a pilot study. Int J Reprod Biomed 14:625–628. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5124324/. Accessed Oct 2016
NRC (2001) Nutrient requirements of dairy cattle, 7th edn. National Academies Press, Washington, DC.
Pasch L, Schmidt A, King W (2021) Clinical observations after prebreeding intrauterine plasma infusion in 18 mares inseminated with thawed frozen semen. J Equine Vet Sci 99:103389. https://doi.org/10.1016/j.jevs.2021.103389
Patel HA, Siddiquee GM, Chaudhari DV, Suthar VS (2016) Effect of different antioxidant additives in semen diluent on cryopreservability (–196 °C) of buffalo semen. Vet World 9:299–303. https://doi.org/10.14202/2Fvetworld.2016.299-303
Qadeer S, Khan MA, Ansari MS, Rakha BA, Ejaz R, Iqbal R, Younis M, Ullah N, DeVries AL, Akhter S (2015) Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim Reprod Sci 157:56–62. https://doi.org/10.1016/j.anireprosci.2015.03.015
Rasheed AI, Abdelfattah NS, El Shennawy DE (2019) Evaluation of seminal IGF-1 and Α2-macroglobulin measurement in predicting testicular sperm output. Ain Shams Med J 70:1–3. https://asmj.journals.ekb.eg/article_145119_46ec128ccccf0510aad5216f23caeba4.pdf. Accessed Jul 2019
Rashid MM, Hoque MA, Huque KS, Bhuiyan AKFH (2015) Effect of semen collection frequency and scrotal circumference on semen quality parameters in Brahman x Local crossbred bulls. Adv Anim Vet Sci 3:677–684. https://doi.org/10.14737/journal.aavs/2015/3.12.677.684
Rastegarnia A, Shahverdi A, Topraggaleh TR, Ebrahimi B, Shafipour V (2013) Effect of different thawing rates on post-thaw viability, kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell J 14:306–313. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3593936/
Roser JF (2001) Endocrine and paracrine control of sperm production in stallions. Anim Reprod Sci 68:139–151. https://doi.org/10.1016/s0378-4320(01)00151-8
Saleh R, Assaf H, Abd-El Maged WM, Elasuity M, Fawzy M (2018) Increased cryosurvival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin Exp Reprod Med 45:177–182. https://doi.org/10.5653/2Fcerm.2018.45.4.177
SAS (2004) SAS user’s guide: statistics, version, 8th edn. SAS Inst. Inc., Cary North Carolina
Saucedo L, Buffa GN, Rosso M, Guillardoy T, Gongora A, Munuce MJ, Vazquez-Levin MH, Marín-Briggiler C (2015) Fibroblast growth factor receptors (FGFRs) in human sperm: expression, functionality and involvement in motility regulation. PLoS ONE 10:e012729. https://doi.org/10.1371/journal.pone.0127297
Segabinazzi LGTM, Canisso IF, Podico G, Cunha LL, Novello G, Rosser MF, Loux SC, Lima FS, Alvarenga MA (2021) Intrauterine blood plasma platelet-therapy mitigates persistent breeding-induced endometritis, reduces uterine infections, and improves embryo recovery in mares. Antibiotics 10:490–500. https://doi.org/10.3390/antibiotics10050490
Selvaraju S, Reddy IJ, Nandi S, Rao SB, Ravindra JP (2009) Influence of IGF-I on buffalo (Bubalus bubalis) spermatozoa motility, membrane integrity, lipid peroxidation and fructose uptake in vitro. Anim Reprod Sci 113:60–70. https://doi.org/10.1016/j.anireprosci.2008.08.011
Selvaraju S, Binsila BK, Santhanahalli SA, Janivara JP (2016) IGF-1 stablizes sperm membrane proteins to reduce cryoinjury and maintain post-thaw sperm motility in buffalo (Bubalus bubalis) spermatozoa. Cryobiology 73:55–62. https://doi.org/10.1016/j.cryobiol.2016.05.012
Sharara FI, Lelea LL, Rahman S, Klebanoff JS, Moawad GN (2021) A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assi Reprod Genet 38:1003–1012. https://doi.org/10.1007/s10815-021-02146-9
Singh RK, Kumaresan A, Chhillar S, Rajak SK, Tripathi UK, Nayak S, Datta TK, Mohanty TK, Malhotra R (2016) Identification of suitable combinations of in vitro sperm function test for the prediction of fertility in buffalo bull. Theriogenology 86:2263–2271. https://doi.org/10.1016/j.theriogenology.2016.07.022
Taher-Mofrad SMJ, Topraggaleh TR, Ziarati N, Bucak MN, Nouri M, Seifi S, Esmaeili V, Rahimizadeh P, Shahverdi A (2020) Knockout serum replacement is an efficient serum substitute for cryopreservation of human spermatozoa. Cryobiology 92:208–214. https://doi.org/10.1016/j.cryobiol.2020.01.013
Tvrdá E, Tušimová E, Kováčik A, Paál D, Greifová H, Abdramanov A, Lukáč N (2016) Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim Reprod Sci 172:10–20. https://doi.org/10.1016/j.anireprosci.2016.06.008
Upadhyay VR, Ramesh V, Dewry RK, Kumar G, Raval K, Patoliya P (2021) Implications of cryopreservation on structural and functional attributes of bovine spermatozoa: an overview. Andrologia 53:e14154. https://doi.org/10.1111/and.14154
Yamakawa S, Hayashida K (2019) Advances in surgical applications of growth factors for wound healing. Burns Trauma 7:10–21. https://doi.org/10.1186/s41038-019-0148-1
Yan B, Zhang Y, Tian S, Hu R, Wu B (2021) Effect of autologous platelet-rich plasma on human sperm quality during cryopreservation. Cryobiology 98:12–16. https://doi.org/10.1016/j.cryobiol.2021.01.009
Yazhini S, Kanchana M (2021) Rejuvenation of ovary and thin endometrium by autologous PRP injection in POR and recurrent implantation failure. Adv Sexual Med 11:1–15. https://doi.org/10.4236/asm.2021.111001
Yeste M (2016) Sperm cryopreservation update: cryodamage, markers and factors affecting the sperm freezability in pigs. Theriogenology 85:47–64. https://doi.org/10.1016/j.theriogenology.2015.09.047
Acknowledgements
The authors would like to express their gratitude to all of the staff at Mehalet-Mousa Research Station, as well as the staff at the International Livestock Management Training Center in Sakha, Kafrelsheikh, Egypt, for their invaluable assistance with the collection, freezing, and evaluation of buffalo bull sperm.
Funding
This study was not funded by any governmental, private, or non-profit funding agencies.
Author information
Authors and Affiliations
Contributions
EAA, IMI, MSS, MAA, FMS, IIE, TKA, and WBE: conceptualization, methodology, investigation, and data curation. IMI, FMS, and MAA perform the statistical analysis being supervised by EAA. Writing, review, and editing of the manuscript, supervision, formal analysis, and visualization were performed by EAA, IMI, MSS, MAA, FMS, IIE, TKA, and WBE. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Ethical Committee of the Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt, approved all animal experiments.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors have declared that there is no conflict of interest concerning this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almadaly, E.A., Ibrahim, I.M., Salama, M.S. et al. Effect of platelet-rich plasma (PRP) on post-thaw quality, kinematics and in vivo fertility of fertile and subfertile buffalo (Bubalus bubalis) spermatozoa. Vet Res Commun 47, 61–72 (2023). https://doi.org/10.1007/s11259-022-09928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-09928-1