Abstract
The current study was designed to evaluate the effects of central administration of L-arginine (The precursor of nitric oxide), NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide (NO) synthase inhibitor, selective opioid receptor agonists and involvement of central nitrergic/opioidergic systems on feeding behavior in neonatal layer-type chicks. The results of this study showed that the intracerebroventricular (ICV) injection of L-arginine (400 and 800 nmol) significantly decreased food intake (P < 0.001) but the injection of 200 nmol L-arginine had no effect on cumulative food intake in FD3 chickens (P > 0.05). The ICV injection of L-NAME (200 and 400 nmol) increased food intake (P < 0.001) but 100 nmol of L-NAME had no significant effect (P > 0.05). On the other hand, the co-injection of 100 nmol L-NAME significantly attenuated the anorexigenic effect of 800 nmol L-arginine (P < 0.001). Moreover, the food intake of chicks was significantly decreased by ICV injection of DAMGO (μ-opioid receptor agonist, 125 pmol) (P < 0.001) while both DPDPE (δ-opioid receptor agonist, 40 pmol) and U-50488H (κ-opioid receptor agonist, 30 nmol) significantly stimulated food intake (P < 0.001). In addition, the hypophagic effect of DAMGO was significantly amplified by administration of L-arginine (P < 0.001) while the administration of L-NAME attenuated the hypophagic effect of DAMGO (P < 0.001). In contrast, co-injection of L-arginine or L-NAME with DPDPE had no effect on the hyperphagia induced by DPDPE as well as the hyperphagic effect of U-50488H on food intake was not affected by concurrent injection of L-arginine or L-NAME (P > 0.05). These results suggest that nitrergic and opioidergic systems have an important role on feeding behavior in the CNS of neonatal layer-type chicks and it seems that interaction between them is mediated by μ-opioid receptor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays numerous neuropeptides and neurotransmitters have been discovered that regulate food intake. Feeding behavior is modulated by complex neurochemical mechanisms in several parts of brain such as striatum, hypothalamus, amygdala and so on (Parker et al. 2014; Boswell 2005). Several studies propose that many features of feeding regulation in chicks are similar to that in mammals but there are some differences in the neurochemical processes for feeding between them (Furuse 2002). For example, it has been stated that central injection of ghrelin is acting as an anorexigenic peptide in chicken but stimulates feeding behavior in mammals (Zendehdel et al. 2012; Khan et al. 2006). Opioids, as a family of peptides, are recognized to be inhibitory neurotransmitters that take part a key function in reward, pain modulation, respiratory control, neuroendocrine physiology and food intake regulation (Feng et al. 2012; Kaneko et al. 2012). Opioids receptor subtypes, such as mu (MOR), delta (DOR) and kappa (KOR) are associated with G protein-coupled receptors (GPCRs) (Filizola and Devi 2013). Studies have shown that endogenous opioidergic system represent important signals in the modulation of feeding behaviour in chickens (Bungo et al. 2004, 2005; Yanagita et al. 2008; Savory et al. 1989). For example, intracerebroventricular injection of [D-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO) and β-casomorphin (μ-opioid receptor agonists) inhibited food intake in neonatal meat-type chicks whereas ICV injection of [D-Pen2, 5]-enkephalin (DPDPE) (a δ-opioid receptor agonist) and U-50488H (a κ-opioid receptor agonist) elevated the food intake of the chicks (Bungo et al. 2004). Debatable results concerning the role of the opioidergic system on food intake in birds have been reported. For example, central injection of β-endorphin (endogenous ligand for both μ and δ opioid receptors) enhances feeding behavior in neonatal broiler chicks via μ-opioid receptor (Yanagita et al. 2008; Dodo et al. 2005). Another feeding-regulatory factor known to be involved in feeding behavior in avian species is nitric oxide (NO) (Yang and Denbow 2007; Khan et al. 2007). NO, a gaseous messenger molecule, is synthesized by nitric oxide synthases (NOS) from L-arginine and has diverse physiological effects. NOS is a family of enzymes with three major types: neuronal NOS (nNOS), endothelial NOS (eNOS) and the inducible NOS (iNOS) (Guix et al. 2005). It is also reported that NO regulates the feeding behavior in rats (Kamerman et al. 2002) and mice (Morley and Flood 1991; Czech et al. 2003; Han et al. 2013). Furthermore, NO is a critical mediator in food intake (Morley et al. 2011). In chickens, intraperitoneal (IP) administration of NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide (NO) synthase inhibitor, significantly decreased food intake in both broiler and layer chicks while the intracerebroventricular (ICV) injection of L-NAME did not affect food intake in broiler chicks. However, ICV injection of L-NAME increased food intake in layer chicks (Khan et al. 2007). By contrast, the study by Choi et al. (1994) showed that ICV injection of L-NAME decreases food intake in broiler chickens. In this regard, L-arginine (The precursor of NO) increased food intake in mice (Morley and Flood 1991) while in the brain of layer chicks acts as a feeding-inhibitory agent (Khan et al. 2007). Based on the results of previous studies, the effects of factors that regulate feeding behavior are dissimilar between layer and broiler chicks. For example, ICV injection of muscimol, GABAA receptor agonist, stimulates food intake in layer-type chicks but had no effect on meat-type chicks (Bungo et al. 2003) whereas, zendehdel et al. (2009) revealed that muscimol increases food consumption in broilers. Findings have demonstrated that NO might mediate the effect of other orexigenic and anorexigenic factors in neonatal chicks. For instance, ICV co-injection of L-NAME attenuated the anorexigenic effect of corticotropin-releasing hormone (CRH) while NPY-induced feeding behavior was not affected by the L-NAME treatment (Khan et al. 2008). There is also evidence that the leptin-induced hypophagia is mediated by nitric oxide in broilers and Leghorns (Yang and Denbow 2007). In the process of food intake regulation, a widely distributed neural network appears to determine the feeding status of the humans and animals. Intricate physiological mechanisms control feeding behaviors, including those dependent on interaction between neuropeptidergic pathways (Olszewski and Levine 2004). Interaction between nitrergic and opioidergic systems on a number of physiological and pathological processes have been identified. For example, nitric oxide may be involved in the morphine self-administration in rats (Sahraei et al. 2004). Zhu et al. (2004) reported that morphine can motivate the release of NO from limbic tissues. There is also evidence that morphine administration stimulates the release of nitric oxide in rabbit aqueous humor (Dortch-Carnes and Russell 2007). On the other hand, several experiments have shown that relationships between the nitrergic and the opioidergic systems have been paired in many functions including peripheral antinociception induced by codeine (Ortiz et al. 2005), thermoregulation (Benamar et al. 2002), neuropathic pain (Hervera et al. 2011) stress-induced anxiety (Anand et al. 2012), seizure susceptibility (Homayoun et al. 2002) dependence and withdrawal (Cuellar et al. 2000).
Based on findings in the previous literature and considering that NO and opioid have identical effects on a number of physiological and pathological processes, the present study was designed to evaluate the possible NO-opioid interaction on feeding behavior in neonatal layer-type chicks.
Materials and methods
Animals
One-day-old male layer chicks were purchased from a local hatchery (Morghak company, Tehran, Iran). The chicks were maintained in stabilized electrically heated batteries at a temperature of 32 °C ± 1, kept at 40–50 %relative humidity and housed in continuous lighting condition. They were provided with water and a commercial starter diet containing 21 % crude protein and 2850 kcal/kg metabolizable energy (Chineh Co, Tehran, Iran) ad libitum. The chicks were kept as flock for 3 days then were placed in individual cages and had free access to food and water up to the time of the experiments. The experiments started when the chicks were 5 days old. Three hours before initiating the ICV injection, animals were deprived of food (FD3).
Experimental drugs
Drugs used included L-arginine hydrochloride (L-Arg) (The precursor of NO), L-NAME (NO synthase inhibitor), [D-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO) (a μ-opioid receptor agonist), [D-Pen2, 5]-enkephalin (DPDPE) (a δ-opioid receptor agonist) and U-50488H (a κ-opioid receptor agonist) were purchased from Sigma Co. (Sigma, USA). The drugs were dissolved in a 0.1 % Evans Blue solution, which was prepared in 0.85 % saline. Saline containing Evans Blue was also used as the control solution.
ICV injection
Prior to each experiment, chicks were weighed and based on their body weight divided into experimental groups so that the average weight between treatment groups was as uniform as possible. In this study, 8 experiments were conducted on each of the four treatment groups. Each group included at least 11 chicks (n = 44 neonatal chicks in each experiment). The birds were injected intracerebroventricularly using a microsyringe without anesthesia according to the methods of used by Davis et al. (1979) and Furuse et al. (1997). In this method, head of the chick was held with an acrylic device in which the bill holder was 45° and the calvarium was parallel to the surface of table as described by Van Tienhoven and Juhasz (1962). An orifice was made in a plate that was located over the skull immediately over the right lateral ventricle. A microsyringe was inserted into the ventricle through the orifice in the plate and the tip of the needle perforated only 4 mm below the skin of the skull (Jonaidi and Noori 2012). Each chick was injected with saline or drugs solution in a volume of 10 μl. This procedure does not induce any physiological stress in neonatal chicks (Saito et al. 2005). After the injection, chicks were returned to their cages immediately. Then, fresh water and food were supplied and the cumulative food intake (gr) was measured at 30, 60 and 120 min after the injection. Food consumption is expressed as a percentage of body weight that body weight impact on the amount of food intake to a minimum. At the end of the experiments, to recognize accuracy of injection, the chicks were sacrificed by decapitation. Only data from individual chicks were used for analysis that were confirmed by the existence of Evans Blue color in the lateral ventricle.
Feeding experiments
Experiment 1 was designed to examine the effect of ICV injection of L-arginine at doses of 200, 400 and 800 nmol on the food intake of FD3 chickens. In experiment 2, chicks were ICV injected with 100, 200 and 400 nmol of L-NAME. Experiment 3 was performed to evaluate the effect of ICV injection of 800 nmol L-arginine, 100 nmol L-NAME and combined solution of L-arginine and L-NAME.
In Experiment 4, four groups of chicks received a dose of either the control solution, 200 nmol L-arginine, 125 pmol DAMGO or a combination of L-arginine plus DAMGO. Experiment 5 was similar to experiment 4 except that 100 nmol L-NAME was used in place of 200 nmol L-arginine. In Experiment 6, four groups of chicks received a dose of either the control solution, 200 nmol L-arginine, 40 pmol DPDPE or a combination of L-arginine plus DPDPE. Experiment 7 was conducted in a manner similar to Experiment 6 except that the chickens received 100 nmol L-NAME instead of 200 nmol L-arginine. In Experiment 8, four groups of chicks received a dose of either the control solution, 200 nmol L-arginine, 30 nmol U-50488H or a combination of L-arginine plus U-50488H. Experiment 9 was identical to experiment 8 except that chickens received 100 nmol L-NAME instead of 200 nmol L-arginine. These doses of drugs were determined according to the previous (Bungo et al. 2004, 2005; Khan et al. 2007) and pilot studies (un-published).
Statistical analysis
Cumulative food intake was analyzed by repeated measure two-way analysis of variance (ANOVA) and is presented as the mean ± SEM. For treatments found to have an effect according to the ANOVA, mean values were compared with post hoc Tukey-Kramer test. p-values <0.05 were considered to indicate significant differences between the treatments.
Results
The food intake response to ICV injection of L-arginine and L-NAME in chickens is presented in Figs. 1, 2 and 3. Furthermore, the role of L-arginine/NO pathway and opioids interaction on feeding behavior in neonatal layer-type chicks is shown in Figs. 4, 5, 6, 7, 8 and 9.
Effect of intracerebroventricular injection of L-arginine (The precursor of NO) at different doses on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments [F(l,27) = 183.51, P < 0.001]
Effect of intracerebroventricular injection of L-NAME (NO synthase inhibitor) at different doses on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments [F(l,27) = 148.27, P < 0.001]
Effects of intracerebroventricular injection of control solution, L-arginine, L-NAME and a combination of L-arginine plus L-NAME on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,61) = 76.03, P < 0.001; L-arginine, F(l,27) = 183.01, P < 0.001; L-NAME, F(l,27) = 5.318, P > 0.05; L-arginine × L-NAME interaction, F(l,27) = 128.45, P < 0.001. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-arginine, DAMGO(μ-opioid receptor agonist) and a combination of L-arginine plus DAMGO on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,43) = 64.72, P < 0.001; L-arginine, F(l,29) = 0.014, P > 0.05; DAMGO, F(l,29) = 138.36, P < 0.001; L-arginine × DAMGO interaction, F(l,29) = 174.08, P < 0.001. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
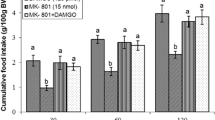
Effects of intracerebroventricular injection of control solution, L-NAME, DAMGO and a combination of L-NAME plus DAMGO on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,79) = 78.072, P < 0.001; L-NAME, F(l,29) = 0.007, P > 0.05; DAMGO, F(l,29) = 164.05, P < 0.0001; L-NAME × DAMGO interaction, F(l,29) = 147.12, P < 0.001. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-arginine, DPDPE (δ-opioid receptor agonist) and a combination of L-arginine plus DPDPE on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,93) = 57.162, P < 0.001; L-arginine, F(l,29) = 0.019, P > 0.05; DPDPE, F(l,29) = 117.92, P < 0.001; L-arginine × DPDPE interaction, F(l,29) = 1.047, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-NAME, DPDPE and a combination of L-NAME plus DPDPE on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,59) = 77.072, P < 0.001; L-NAME, F(l,64) = 0.138, P > 0.05; DPDPE, F(l,64) = 149.04, P < 0.001; L-NAME × DPDPE interaction, F(l,64) = 3.018, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-arginine, U-50488H (κ-opioid receptor agonist) and a combination of L-arginine plus U-50488H on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,13) = 85.162, P < 0.001; L-arginine, F(l,30) = 2.628, P > 0.05; U-50488H, F(l,30) = 128.13, P < 0.001, L-arginine × U-50488H interaction, F(l,30) = 1.005, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-NAME, U-50488H and a combination of L-NAME plus U-50488H on cumulative food intake (% BW) in neonatal layer type chicks. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,59) = 68.072, P < 0.001; L-NAME, F(l,64) = 0.017, P > 0.05; U-50488H, F(l,64) = 138.14, P < 0.001; L-NAME × U-50488H interaction, F(l,64) = 0.081, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
In Experiment 1, ICV injection of 200 nmol L-arginine had no significant effect on cumulative food intake (% BW) in comparison with control group [F(l,27) = 0.068, P > 0.05]; but 400 and 800 nmol of L-arginine significantly decreased cumulative food intake in a dose-dependent manner [F(l,27) = 183.51, P < 0.001] (Fig. 1).
In Experiment 2, ICV administration of L-NAME at dose of 100 nmol had no significant effect on food intake in FD3 chickens [F(l,27) = 0.009, P > 0.05]; while food intake was significantly increased by L-NAME (200 and 400 nmol) compared to control group [F(l,27) = 148.27, P < 0.001] (Fig. 2).
In Experiment 3, the application of 800 nmol L-arginine significantly decreased food intake [F(l,27) = 183.01, P < 0.001]; but the L-NAME treatment at dose of 100 nmol had no significant effect on food intake [F(l,27) = 5.318, P > 0.05]. Considerable hypophagic effect of L-arginine was significantly attenuated by co-injection of L-arginine and L-NAME [F(l,27) = 128.45, P < 0.001] (Fig. 3).
In Experiment 4, ICV injection of DAMGO (125 pmol) significantly decreased food intake compared to control group [F(l,29) = 138.36, P < 0.001]. The 125-pmol DAMGO dose was selected for experiments on the basis of our pilot study, because it was found to induce a decrease in food intake in chickens without affecting other noningestive behavioral parameters such as sedation (e.g., activity of chicks). The hypophagic effect of DAMGO was significantly amplified by administration of 200 nmol L-arginine [F(l,29) = 174.08, P < 0.001], while 200 nmol of L-arginine alone had no effects on food intake in FD3 chickens [F(l,29) = 0.014, P > 0.05] (Fig. 4).
In Experiment 5, the combined administration of L-NAME (100 nmol) and DAMGO (125 pmol) attenuated hypophagic effect of DAMGO [F(l,29) = 147.12, P < 0.001]; but 100 nmol of L-NAME alone could not alter food intake in FD3 chickens [F(l,29) = 0.007, P > 0.05] (Fig. 5).
In Experiment 6, ICV injection of DPDPE (40 pmol) significantly increased food intake when compared with control group [F(l,29) = 117.92, P < 0.001]. However, Co-injection of L-arginine + DPDPE had no effect on the hyperphagia induced by DPDPE in FD3 chickens [F(l,29) = 1.047, P > 0.05] (Fig. 6).
In Experiment 7, the stimulatory effect of DPDPE on food intake was not affected by Co-injection of L-NAME and DPDPE in FD3 chickens [F(l,64) = 3.018, P > 0.05] (Fig. 7).
In Experiment 8, ICV administration of U-50488H (30 nmol) significantly increased food intake compared to the control group [F(l,30) = 128.13, P < 0.001]. The mixture of L-arginine and U-50488H failed to change the hyperphagia induced by U-50488H in FD3 chickens [F(l,30) = 1.005, P > 0.05] (Fig. 8).
In Experiment 9, the hyperphagic effect of U-50488H on food intake was not affected by concurrent injection of L-NAME and U-50488H in FD3 chickens [F(l,64) = 0.081, P > 0.05] (Fig. 9).
All doses that were used for experiments in this study had no behavioral changings such as sedation or hyperactivity by drugs in chicks. In addition, we used from sub effective dose for L-arginine (200 nmol) and L-NAME (100 nmol) in experiments 4–9 for evaluation of interaction between nitrergic and opioidergic systems. Also, in experiment 3 for assessment the effect of L-NAME on L-arginine induced hypophagia, we utilized from L-NAME at dose of 100 nmol plus 800 nmol L-arginine.
Discussion
The present study was designed to investigate the possible involvement of nitrergic and opioidergic systems on food intake in neonatal layer-type chicks. Nitric oxide has been shown to be implicated in the regulation of numerous physiological functions such as the feeding behavior (Morley et al. 2011).
According to the results from Experiment 1 and 2, ICV injection of L-arginine significantly decreases cumulative food intake in a dose-dependent manner while ICV administration of L-NAME at higher doses increases food intake in FD3 chickens (Figs. 1 and 2). These findings propose that NO might act as one of anorexigenic mediators in the brain of layer chicks. Controversial results have been reported about the effect of L-arginine analog on food intake in animals. In accordance with our study, Khan et al. (2007) reported that ICV injection of L-NAME increased food intake in layer chicks while the injection of D-NAME did not affect food intake, suggesting that the effect of L-NAME might be due to NOS inhibition. This result was inconsistent with the data obtained from mammals which ICV injection of L-NAME reduced food consumption (De Luca et al. 1995). Interestingly, food ingestion was depressed by the ICV injection of L-NAME in broiler chickens, then the anorectic effect was reduced by administration of L-arginine (Choi et al. 1994, 1995). It is reported that ICV injection of L-NAME did not affect food intake in broiler chicks. Likewise, the IP administration of L-NAME decreased food intake in both broiler and layer chicks (Khan et al. 2007). The study by Yang and Denbow (2007) revealed that ICV injection of L-NNA, a competitive NOS inhibitor, decreased food intake in both broilers and Leghorns. In addition, L-arginine is demonstrated that might act as an orexigenic factor in mice (Morley and Flood 1991). On the other hand, it was expressed that dietary arginine regulates food intake via the nitric oxide pathway in ducks. In fact, increasing the dietary arginine promoted nitric oxide synthesis and leading to increased of food intake (Wang et al. 2014). The obvious discrepancy between alteration of feeding behavior in animals by L-arginine analog administration may be due to injection site, experimental procedure and species differences.
In experiment 4 we found that ICV injection of μ-opioid receptor agonist (DAMGO) decreased cumulative food intake in neonatal layer-type chicks, which is consistent with previous studies in broiler (Bungo et al. 2004). In line with this result, another study by Bungo et al. (2005) reported that anorexic effect of DAMGO in meat-type chicks attenuated by ICV injection of β-funaltrexamine (β-FNA: μ-opioid antagonist). In the CNS, opioid receptors constitute the most extensive and different peptidergic transmission system and are involved in various functions (Feng et al. 2012). Several reports have shown that endogenous opioid system play an important role in the ingestion of food in chicks. For example, central injection of β-endorphin increased food intake in domestic fowl (McCormack and Denbow 1988) and broiler chicks (Yanagita et al. 2008) through the μ-opioid receptor. Similar to this result, ICV injection of endomorphin-2 into the chick brain produces the orexigenic effect through its interaction with MOR (Bungo et al. 2007). It is suggested that μ-opioid receptor involve in orexigenic effect of metastin in broiler chicks (Khan et al. 2009). In contrast, injection of the DAMGO into the nucleus accumbens stimulated feeding behavior in rats (Zheng et al. 2007). As can be seen, there is various findings regarding the effects of μ-opioid receptor on food intake between birds and mammals.
In our study, the decrease in food intake caused by the injection of DAMGO was amplified by ICV injection of L-arginine (Figs. 4), whereas the combined administration of L-NAME and DAMGO attenuated hypophagic effect of DAMGO (Fig. 5). These results suggest that a possible relationship seems to be exist between opioidergic and nitrergic systems involved in feeding behavior in chickens. In trying to assess the interaction of opioidergic and nitrergic systems, previously (Llorente et al. 2012), in agreement with our results, described that NO enhanced μ-opioid receptor desensitization in rat locus coeruleus neurons. Likewise, there is pharmacological evidence that anxiolytic action of morphine (prototype of μ-opioid receptor agonist) is modulated via nitric oxide. In this regard, co-administration of L-arginine and morphine induced synergistic anxiolytic effects while L-NAME, neutralized the anxiolytic effects of morphine in rats (Anand et al. 2012).
Glutamate N-methyl-D-aspartate (NMDA)- receptors stimulation bring Ca2+ into the cell which then mediates Ca2+-dependent nNOS activation (Southam et al. 1991).
The NO formed by nNOS, diffuses to adjacent nerve terminals to modify neurotransmitter release (Kiss and Vizi 2001).
Interestingly, some studies have shown that NOS staining appears in the hypothalamus that is also abundant in opioid receptors (Bredt and Snyder 1992).
Furthermore, higher numbers of nNOS-positive cells were observed in the hippocampal dentate gyrus treated with morphine (Yoo et al. 2006).
Reports have indicated that NO appears to participate in potentiation of analgesia induced by morphine via μ-opioid receptors and the nitric oxide/cyclic GMP pathway may be involved (Toda et al. 2009).
Glutamate participates in synaptic interactions. It is also mentioned that, there is a dual control comprised of the excitatory NMDA and the inhibitory μ-opioid receptors in modulating cyclic GMP/ NO release in the medial preoptic area (MPOA) of brain and morphine affects NMDA-receptor binding activity and increases nNOS expression (Pu et al. 1997).
Increased activity of excitatory NMDA receptors following to opioid receptor activation have been suggested as possible mechanisms that mediate DAMGO-induced hypophagic effect. Consequently, it is probable that an increase in intracellular Ca+2 concentrations and thereby the release of nitric oxide that modifies the μ-opioidergic receptor hypophagic effect. In this study, L-arginine potentiated and L-NAME inhibited hypophagic effect of DAMGO.
On the other hand, ICV administration of DAMGO to rats increased endogenous NO production. In contrast, administration of the δ-agonist and the κ-agonist had no effect on the production of NO (Schneider and Lysle 1998).
Hence, it seems that μ-opioid receptor within the CNS appears to be involved in the regulation of NO production.
In the current study, we showed that the ICV injection of δ-opioid receptor agonist (DPDPE) increased cumulative food intake in neonatal layer-type chicks in comparison with control group. This result is in agreement with previous reports, which showed that DPDPE stimulated food intake after central administration in neonatal meat-type chicken (Bungo et al. 2004) and mice (Kaneko et al. 2012). Also, ingestive behaviour was enhanced by ICV and intramuscular (IM) administration of metenkephalin (highly specific ligands of the δ-opioid receptor) in cockerels (McCormack and Denbow 1989). Moreover, DPDPE-induced orexigenic activity was blocked by naltrindole, an antagonist of δ-opioid receptor, suggesting that DPDPE stimulates food intake via δ opioid receptor which has been reported to be expressed in the rat CNS (Gray et al. 2006).
In mice, Kaneko et al. (2012) previously reported that the central δ-opioid receptor activation stimulated food intake via the PGD2–NPY system which may be involved in food intake regulation under normal physiological conditions. In addition, we found that co-injection of L-arginine and DPDPE had no effect on the hyperphagia induced by DPDPE. Similar to this condition, the stimulatory effect of DPDPE on food intake was not affected by simultaneous injection of LNAME and DPDPE in FD3 chickens (Figs. 6 and 7). This findings proposed that the orexigenic effect of DPDPE in neonatal chicks was not linked to NO in contrast to mammals which it was observed that the antinociceptive effect of SNC80 (δ-opioid receptor agonist) attenuated by the NO synthase inhibitor in rats. This result suggested that nitric oxide is involved in antinociception of δ-opioid receptor (Pacheco et al. 2005).
In experiment 8, we ascertained the effect of κ-opioid receptor on cumulative food intake in neonatal layer-type chickens. According to our data, ICV injection of κ-opioid receptor agonist (U-50488H) increased food intake in FD3 chickens. This finding is in accordance with the result reported by Bungo et al. (2004) and Koch et al. (1992) which explained that ICV injection of κ-opioid receptor agonist evoked hyperphagic responses in broiler chiks and rats, respectively. By contrast, injection of mu and delta opioid receptor agonists into the nucleus accumbens (NAc) or ventral tegmental area (VTA) were able to induce a increase in food consumption in rats while the kappa receptor agonist had no influence on feeding behavior (Le Merrer et al. 2009). Based on the current study, the increased food intake induced with the ICV injection of U-50488H was not affected by co-injection of L-arginine and U-50488H or L-NAME and U-50488H in FD3 chickens (Figs. 8 and 9). Although the hyperphagic effect of U-50488H was not related to nitric oxide pathway in neonatal chicks, recently it was demonstrated that ICV administration of L-NAME reduced the U-50488H-induced hypothermia in rats, suggesting that NO production in the CNS plays an important role in the development of the hypothermic response (Benamar et al. 2002).
The involvement of nitric oxide in other actions produced by opioids, such as the antihyperalgesia induced by κ-opioid receptor agonist bremazocine was reduced when NO biosynthesis blockers were administered, has been also demonstrated (Amarante and Duarte 2002). In this regard, Barjavel and Bhargava revealed that NOS activity was inhibited by the κ-opioid receptor agonist U-50488H and was not affected by selective δ-opioid receptor agonist in rat cerebral cortex (Barjavel and Bhargava 1994). Previous investigations have shown a functional interaction between the κ-opioid receptors and nNOS in the same intracellular network of the rat periaqueductal gray appears to control the development of morphine tolerance and dependence (Herráez-Baranda et al. 2005).
The accurate mechanisms by which opioids induce variations in NO synthesis is not completely established. However, some studies have shown that, opioids have stimulatory as well as the inhibitory effects on neurotransmission which involve not only disinhibition of interneurons, but also direct stimulation via cAMP, phospholipase C and calcium (Smart and Lambert 1996). Regarding the complexity of interactions between nitric oxide and opioid systems (e.g. in analgesia or other effects as mentioned above), the present study provides no evidence about the role of nitrergic/opioidergic systems interactions on food intake in neonatal layer-type chicks through the δ or κ-opioid receptors.
Most research on feeding behavior regulatory mechanisms have done in rat models whereas considering few investigations done in birds. Actually, there was no similar research to compare our results on mediatory role of NO on κ and δ opioid release as poultry model. Presumably central appetite regulation mechanisms are somewhat different among animals. These controversial data might be the consequence of injection methods and species difference. Additionally, it seems genetic selection for meat or egg production has altered chicken brain neurological pathways associated with appetite regulation. Also, it seems more researches needs on other physiological systems to clarify physiology of food intake regulation in poultry.
Conclusion
In summary, based on our findings, it seems DAMGO-induced hypophagia is maybe modulated by nitrergic system through the μ-opioid receptor (not δ or κ-opioid receptors) in chicks. However, further investigation is required to elucidate the underlying cellular and molecular signaling pathways in the interconnection between nitrergic and opioidergic systems on feeding behavior in neonatal layer-type chicks.
References
Amarante LH, Duarte ID (2002) The kappa-opioid agonist (+/−)-bremazocine elicits peripheral antinociception by activation of the L-arginine/nitric oxide/cyclic GMP pathway. Eur J Pharmacol 454(1):19–23
Anand R, Gulati K, Ray A (2012) Pharmacological evidence for the role of nitric oxide in the modulation of stress-induced anxiety by morphine in rats. Eur J Pharmacol 676:71–74
Barjavel MJ, Bhargava HN (1994) Effect of opioid receptor agonists on nitric oxide synthase activity in rat cerebral cortex homogenate. Neurosci Lett 181(1–2):27–30
Benamar K, Geller EB, Adler MW (2002) Role of the nitric oxide pathway in κ-opioid-induced hypothermia in rats. J Pharmacol Exp Ther 303(1):375–378
Boswell T (2005) Regulation of energy balance in birds by the neuroendocrine hypothalamus. J Poult Sci 42(3):161–181
Bredt DS, Snyder SH (1992) Nitric oxide, a novel neuronal messenger. Neuron 8(1):3–11
Bungo T, Izumi T, Kawamura K, Takagi T, Ueda H, Furuse M (2003) Intracerebroventricular injection of muscimol, baclofen or nipecotic acid stimulates food intake in layer-type, but not meat-type, chicks. Brain Res 993(1–2):235–238
Bungo T, Kawamura K, Izumi T, Dodo K-I, Ueda H (2004) Feeding responses to μ, δ and κ-opioid receptor agonists in the meat-type chick. Pharmacol Biochem Behav 78(4):707–710
Bungo T, Dodo K-I, Kawamura K, Izumi T, Ueda H (2005) Effects of various μ- and δ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85(5):519–523
Bungo T, Dodo K-I, Izumi T (2007) Central injection of endomorphin-2, but not endomorphin-1, increases food intake in chicks via μ1-opioid receptors. J Poult Sci 44(2):205–208
Choi YH, Furuse M, Okumura J, Denbow DM (1994) Nitric oxide controls feeding behavior in the chicken. Brain Res 654:163–166
Choi YH, Furuse M, Okumura J, Denbow DM (1995) The interaction of clonidine and nitric oxide on feeding behavior in the chicken. Brain Res 699:161–164
Cuellar B, Fernandez AP, Lizasoain I, Moro MA, Lorenzo P, Bentura ML, Rodrigo J, Leza JC (2000) Up-regulation of neuronal NO synthase immunoreactivity in opiate dependence and withdrawal. Psychopharmacology 148:66–73
Czech DA, Kazel MR, Harris J (2003) A nitric oxide synthase inhibitor, N(G)-nitro-L-arginine methyl ester, attenuates lipoprivic feeding in mice. Physiol Behav 80(1):75–79
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
De Luca B, Monda M, Sullo A (1995) Changes in eating behavior and thermogenic activity following inhibition of nitric oxide formation. Am J Physiol 268:1533–1538
Dodo K-I, Izumi T, Ueda H, Bungo T (2005) Response of neuropeptide Y-induced feeding to μ-δ- and κ-opioid receptor antagonists in the neonatal chick. Neurosci Lett 373:85–88
Dortch-Carnes J, Russell K (2007) Morphine-stimulated nitric oxide release in rabbit aqueous humor. Exp Eye Res 84(1):185–190
Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y (2012) Current research on opioid receptor function. Curr Drug Targets 13(2):230–246
Filizola M, Devi LA (2013) Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci 34(1):6–12
Furuse M (2002) Central regulation of food intake in the neonatal chick. Anim Sci J 73(2):83–94
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Gray AC, Coupar IM, White PJ (2006) Comparison of opioid receptor distributions in the rat central nervous system. Life Sci 79(7):674–685
Guix FX, Uribesalgo I, Coma M, Muñoz FJ (2005) The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76(2):126–152
Han C, Zhao Q, Lu B (2013) The role of nitric oxide signaling in food intake; insights from the inner mitochondrial membrane peptidase 2 mutant mice. Redox Biol 1(1):498–507
Herráez-Baranda LA, Carretero J, González-Sarmiento R, Laorden ML, Milanés MV, Rodríguez RE (2005) Evidence of involvement of the nNOS and the kappa-opioid receptor in the same intracellular network of the rat periaqueductal gray that controls morphine tolerance and dependence. Brain Res Mol Brain Res 137(1–2):166–173
Hervera A, Negrete R, Leánez S, Martín-Campos JM, Pol O (2011) Peripheral effects of morphine and expression of μ-opioid receptors in the dorsal root ganglia during neuropathic pain: nitric oxide signaling. Mol Pain. doi:10.1186/1744-8069-7-25
Homayoun H, Khavandgar S, Namiranian K, Gaskari SA, Dehpour AR (2002) The role of nitric oxide in anticonvulsant and proconvulsant effects of morphine in mice. Epilepsy Res 48:33–41
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198:827–832
Kamerman P, Mitchell D, Laburn H (2002) Circadian variation in the effects of nitric oxide synthase inhibitors on body temperature, feeding and activity in rats. Eur J Physiol 443:609–616
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-Involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Khan MSI, Dodo K-I, Yahata K, Nishimoto S, Ueda H, Taneike T, Kitazawa T, Hosaka Y, Bungo T (2006) Intracerebroventricular administration of growth hormone releasing peptide-6 (GHRP-6) inhibits food intake, but not food retention of crop and stomach in neonatal chicks. J Poult Sci 43(1):35–40
Khan MSI, Tachibana T, Hasebe Y, Masuda N, Ueda H (2007) Peripheral or central administration of nitric oxide synthase inhibitor affects feeding behavior in chicks. Comp Biochem Physiol A 148:458–462
Khan MSI, Nakano Y, Tachibana T, Ueda H (2008) Nitric oxide synthase inhibitor attenuates the anorexigenic effect of corticotropin-releasing hormone in neonatal chicks. Comp Biochem Physiol A Mol Integr Physiol 149(3):325–329
Khan MSI, Ohkubo T, Masuda N, Tachibana T, Ueda H (2009) Central administration of metastin increases food intake through opioid neurons in chicks. Comp Biochem Physiol A Mol Integr Physiol 153(2):209–212
Kiss JP, Vizi ES (2001) Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 24(4):211–215
Koch JE, Pasternak GW, Arjune D, Bodnar RJ (1992) Naloxone benzoylhydrazone, a κ3 opioid agonist, stimulates food intake in rats. Brain Res 581(2):311–314
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89(4):1379–1412
Llorente J, Santamarta MT, Henderson G, Pineda J (2012) Enhancement of μ-opioid receptor desensitization by nitric oxide in rat locus coeruleus neurons: involvement of reactive oxygen species. J Pharmacol Exp Ther 342(2):552–560
McCormack JF, Denbow DM (1988) Feeding, drinking and temperature responses to intracerebroventricular beta-endorphin in the domestic fowl. Peptides 9(4):709–715
McCormack JF, Denbow DM (1989) Ingestive responses to mu and delta opioid receptor agonists in the domestic fowl. Br Poult Sci 30(2):327–340
Morley JE, Flood JF (1991) Evidence that nitric oxide modulates food intake in mice. Life Sci 49(10):707–711
Morley JE, Farr SA, Sell RL, Hileman SM, Banks WA (2011) Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides 32:776–780
Olszewski PK, Levine AS (2004) Minireview: characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology 145(6):2627–2632
Ortiz MI, Castro-Olguin J, Pena-Samaniego N, Castaneda-Hernandez G (2005) Probable activation of the opioid receptor-nitric oxide-cyclic GMP-K+ channels pathway by codeine. Pharmacol Biochem Behav 82(4):695–703
Pacheco DF, Reis GML, Francischi JN, Castro MSA, Perez AC, Duarte IDG (2005) Opioid receptor agonist SNC80 elicits peripheral antinociception via δ1 and δ2 receptors and activation of the L-arginine/nitric oxide/cyclic GMP pathway. Life Sci 78:54–60
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res 260:131–138
Pu S, Horvath TL, Diano S, Naftolin F, Kalra PS, Kalra SP (1997) Evidence showing that β-endorphin regulates cyclic guanosine 3′,5′-monophosphate (cGMP) efflux: anatomical and functional support for an interaction between opiates and nitric oxide. Endocrinology 138:1537–1543
Sahraei H, Poorheidari G, Foadaddini M, Khoshbaten A, Asgari A, Noroozzadeh A, Ghoshooni H, Firoozabadi SH, Zarrindast MR (2004) Effects of nitric oxide on morphine self-administration in rat. Pharmacol Biochem Behav 77:111–116
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Savory CJ, Gentle MJ, Yeomans MR (1989) Opioid modulation of feeding and drinking in fowls. Br Poult Sci 30:379–392
Schneider GM, Lysle DT (1998) Role of central μ-opioid receptors in the modulation of nitric oxide production by splenocytes. J Neuroimmunol 89:150–159
Smart D, Lambert DG (1996) The stimulatory effects of opioids and their possible role in the development of tolerance. Trends Pharmacol Sci 17(7):264–269
Southam E, East SJ, Garthwaite J (1991) Excitatory amino acid receptors coupled to the nitric oxide/cyclic GMP pathway in rat cerebellum during development. J Neurochem 56:2072–2081
Toda N, Kishioka S, Hatano Y, Toda H (2009) Anesthesiology 110(1):166–181
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Wang C, Hou SS, Huang W, Xu TS, Rong GH, Xie M (2014) Arginine affects appetite via nitric oxide in ducks. Poult Sci 93(8):2048–2053
Yanagita K, Shiraishi J, Fujita M, Bungo T (2008) Effects of N-terminal fragments of β-endorphin on feeding in chicks. Neurosci Lett 442(2):140–142
Yang SJ, Denbow DM (2007) Interaction of leptin and nitric oxide on food intake in broilers and Leghorns. Physiol Behav 92(4):651–657
Yoo JH, Cho JH, Lee SY, Lee S, Loh HH, Ho IK, Jang CG (2006) Differential effects of morphine and cocaine-induced nNOS immunoreactivity in the dentate gyrus of hippocampus of mice lacking μ-opioid receptors. Neurosci Lett 395:98–102
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2012) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37(1):37–41
Zheng H, Patterson LM, Berthoud HR (2007) Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27(41):11075–11082
Zhu W, Ma Y, Bell A, Esch T, Guarna M, Bilfinger TV, Bianchi E, Stefano GB (2004) Presence of morphine in rat amygdala: evidence for the mu3 opiate receptor subtype via nitric oxide release in limbic structures. Med Sci Monit 10(12):433–439
Acknowledgments
This research was supported by a grant from the Research Council of the Faculty of Veterinary Medicine, University of Tehran, Iran.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alimohammadi, S., Zendehdel, M. & Babapour, V. Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39, 105–113 (2015). https://doi.org/10.1007/s11259-015-9631-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-015-9631-8