Abstract
Carnivorous plants inhabit nutrient-poor environments and supplement nutrient acquisition by capturing prey. Carnivorous adaptations have been hypothesized to be beneficial only in environments with high-light availability. We hypothesized that plant morphology would change in response to resource availability (light and prey capture). In a field experiment in Leon County, Texas, we examined the effects of feeding, shading, and their interaction on pitcher plant (Sarracenia alata) morphology. When light availability was reduced, plants produced pitchers that had smaller diameters. The sum of pitcher heights was significantly lower for unfed plants than fed plants. As the season progressed, competing vegetation reduced light availability to pitchers in all treatments. Plants in all treatments produced pitchers that were blade-like with a small, non-functional opening and a widened keel. This experiment provides support for the theoretical model that carnivorous structures are only beneficial under conditions of high-light availability. It also emphasizes the importance of periodic burns of carnivorous plant bogs to reduce competing vegetation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bog habitats support a unique assemblage of organisms (Renou-Wilson et al. 2019), but they are being decimated in the United States due to human encroachment, land-use conversion, and human abatement of fires (Dahl and Pywell 1989; Johnson and Hale 2002). This is especially true for carnivorous plant bogs (Folkerts 1977). As much as 95% of carnivorous plant habitat has been lost in the United States since colonial times (Folkerts 1982), and a number of carnivorous plant species are being placed on the endangered species list (Furches et al. 2013). Therefore, it is increasingly important that we understand the species interactions and environmental factors that influence the survival and conservation of these unique organisms.
The nature of carnivorous plants and their evolved specialization of prey capture have been studied at least since Darwin (1875). The soils of carnivorous plant bogs are typically deficient in nitrogen, phosphorus, and/or potassium (Juniper et al. 1989; Adamec 1997; Ellison 2006; Ellison and Adamec 2011). The leaves of carnivorous plants photosynthesize but are also adapted for nutrient acquisition through the attraction, capture, and digestion of prey (Juniper et al. 1989; Horner et al. 2018). Prey capture by carnivorous plants increases leaf tissue nutrient concentrations, photosynthetic rate, and growth (Farnsworth and Ellison 2008), and allows carnivorous plants to survive under these conditions. However, carnivorous adaptations are costly and come with trade-offs (Karagatzides and Ellison 2009): carnivorous plants have lower photosynthetic rates and photosynthetic nutrient-use efficiencies than non-carnivorous plants (Ellison 2006). Because these adaptations are costly, the relative allocation to photosynthesis versus prey capture may change in response to variations in the availability of resources.
The availability of resources such as light and nutrients can vary over time. The quantity and quality of light is affected by competing vegetation, and prey abundance may vary intrinsically or due to the action of other organisms. Plants, both carnivorous and non-carnivorous, can adjust to these variations through phenotypic plasticity (Via and Lande 1985; Adamec et al. 2021). Altering the relative allocation to, and morphology of, specific organs allows plants to balance the acquisition of carbon, water, and nutrients (Bloom et al. 1985). This is observed, for example, in the leaves of pitcher plants.
The leaves of pitcher plants form tubular or cup-shaped pitchers that attract, capture, and digest prey (usually insects; Horner et al. 2018). When competing with neighbors for light, the morphology of pitcher plants may change to increase light capture at the expense of prey capture (Brewer 2003, 2019; Adamec et al. 2021). In low-light environments pitchers grow taller with a smaller opening to maximize light capture, whereas in high-light conditions pitchers are shorter with wider openings to maximize prey capture (Brewer 1999, 2019; Adamec et al. 2021). Likewise, nutrient acquisition rates from prey capture can vary due to differences in natural prey abundance, herbivores that occlude the pitcher openings (Carmickle and Horner 2019), or kleptoparasitic spiders (Cresswell 1993). Variations in nutrient acquisition through prey capture (Farnsworth and Ellison 2008) or atmospheric nutrient deposition (Ellison and Gotelli 2002) can also influence carnivorous plant morphology. However, while both light availability and nutrient acquisition are known to affect pitcher plant morphology, the effects of the interaction between light availability and prey capture are not well understood. Responses to variations in light availability and prey capture may be either additive or non-additive.
The goal of this study was to examine the effects of light availability, prey capture, and their interaction on morphology of the carnivorous plant, Sarracenia alata Wood. We hypothesized that under conditions of low-light availability, pitchers would have greater height-to-diameter ratios (taller pitchers with smaller diameters) to maximize light capture. Under conditions of reduced prey capture, we hypothesized that pitcher plants would exhibit reduced number and height of pitchers. Finally, we hypothesized that the interaction of low-light and loss of prey capture would result in fewer pitchers with a greater height-to-diameter ratio as well as reduced growth due to reduced resource availability.
Methods
Study organism
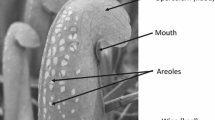
Sarracenia alata, the pale pitcher plant, is a rhizomatous carnivorous plant that is native to the southeastern United States from Alabama to eastern Texas (Schnell 1976). Many plants produce a single flower at the beginning of the growing season (Horner 2014) and subsequently produce funnel-shaped leaves called pitchers that are specialized for prey capture (Fig. 1a). Each genet produces pitchers in a rosette throughout the growing season of April through October. Pitchers open after they reach their maximum height (Horner et al. 2012). A rib or keel extends along one side of the pitcher. An extension of leaf tissue referred to as the hood covers but does not occlude the opening. Surrounding the opening is a slippery lip called the peristome. Nectar and volatiles attract prey to the peristome, where they slip and fall into the pitcher (Juniper et al. 1989; Horner et al. 2018). The lower portion of the pitcher interior is lined with downward-pointing hairs that prevent escape of the prey, and the bottom contains enzymes that digest the prey. Although several Sarracenia species (e.g., S. purpurea; Ellison and Gotelli 2002) produce leaf-like phyllodia (non-carnivorous leaves), S. alata does not (Schnell 1976; Farnsworth and Ellison 2008). However, under certain conditions (see below), they produce similar structures (which we call “pseudo-phyllodia;” Fig. 1b), blade-like pitchers with small (less than 2 mm diameter), non-functional openings and widened keels.
Field and laboratory methods
This study was conducted in a bog on private property in Leon County, TX, USA (31.54 N, − 95.91 W). We employed a two-factor, cross-classified design, with shading (two levels, shaded and unshaded) and prey capture (two levels, fed and unfed) as factors. Eighty plants that were at least one meter apart were chosen and randomly assigned to one of four treatments (20 plants in each): (1) unshaded and fed (control); (2) shaded and fed; (3) unshaded and unfed; and (4) shaded and unfed. The treatments were applied 14 April 2019. Plants in treatments receiving shade were covered by a 50% light reduction shade cloth suspended by an 80 cm × 80 cm × 80 cm cube constructed with 1.9-cm polyvinylchloride (PVC) pipe. The shade cloth extended 30 cm down the sides of the PVC structure to reduce lateral light while allowing insects access. Plants in the unshaded treatments had unmanipulated light availability. Plants in the treatments receiving feeding were surrounded at the base by 10.2-cm-tall, 10.2-cm-diameter PVC rings that served as herbivore-exclusion rings. The rings were coated with a sticky insect trap (Tanglefoot) to prevent larvae of the specialist noctuid moth, Exyra semicrocea, from crawling up the pitchers (Carmickle and Horner 2019). Exyra larvae can reduce or prevent prey capture by girdling the pitcher or spinning a web across the opening, both of which obstruct the pitcher openings (Carmickle and Horner 2019). The pitcher openings were plugged with cotton to prevent adult Exyra females from ovipositing. Loss of prey capture caused by plugging the opening was compensated for by supplementing dried, ground mealworms. The feeding occurred one time when the pitcher opened, and the quantity supplied was based on the estimated seasonal capture rates of pitchers of comparable size (Green and Horner 2007; Bhattarai and Horner 2009; Horner et al. 2012; Carmickle and Horner 2019). Pitchers with an opening less than 1 cm in diameter were provided with 0.104 ± 0.033 g; pitchers with a 1–2 cm diameter opening were provided with 0.210 ± 0.014 g; and pitchers with an opening greater than 2 cm diameter were provided with 0.442 ± 0.018 g. Plants in the unfed treatments were also plugged but were not fed the compensatory mealworms.
There was a prescribed fire at the study site on 18 February 2019. This burn removed much of the competing vegetation. As the season progressed, competing non-carnivorous vegetation returned. Measurements of photosynthetically active radiation (PAR) showed that this regrowth reduced light availability to pitchers in all treatments late in the growing season. Because of this unexpectedly quick regeneration of competing vegetation, we decided to separate the season into three periods: the Early Season (14-April-2019 to 29-May-2019,) which examined the effects of the field manipulation experiment; a transitional phase; and the Late Season (31-July-2019 to 20-October-2019), which examined the effects of rapid regrowth of vegetation.
We visited the site weekly from April until June, every 10 days from June through July, then every 2 weeks until the end of growing season in October. As new pitchers opened, they were tagged with wire clips, plugged, and pitchers in the appropriate treatments were fed. Two visits after being tagged, the height (ground to peristome) and diameter of the opening of each pitcher were measured. Keel width was measured once in Early Season and during every visit in Late Season. At each study genet, we recorded PAR at ground level, PAR at pitcher height (average 40 cm from ground), and temperature each month.
Specific leaf mass
At the end of the season, we collected 70 0.20-cm2 discs of pitcher and keel tissue from randomly selected study pitchers from all treatments to determine the specific leaf mass of pitchers and keels. We also collected 69 whole pitchers (maximum 2 per genet) from plants approximately evenly distributed among all treatments. The punches were placed in airtight plastic vials and whole pitchers were transported in plastic Ziploc bags with a tissue soaked in bog water to maintain humidity. The wet mass of discs and whole pitchers was measured the following day. Both discs and pitchers were then dried in an oven at 60 °C for 72 h, after which their dry mass was recorded. Specific leaf mass (mass/unit area) was determined for pitchers and keels for each treatment by dividing the dry mass of discs by the area (0.20 cm2).
Estimated pitcher mass
The surface area for each of the 69 collected pitchers was estimated by calculating the area of a cone [A = π*radius (radius + √ (height2 + radius2)] for pitcher area and the area of a cylinder (A = 2*π*radius*height) for the rib of pseudo-phyllodia. The surface area of keels was estimated as the area of half an ellipse [A = (π*(height/2)*radius)/2]. Pitcher mass was estimated by multiplying the calculated area of each pitcher and keel by the specific leaf mass for the tissue from that treatment. The estimated masses were regressed against the actual dry masses of the corresponding collected pitcher. The regressions yielded correction formulae for pitchers and pseudo-phyllodia: pitcher mass = 1.766x1.0706 (R2 = 0.89; x = estimated pitcher mass), pseudo-phyllodia mass = 1.7614x1.3809 (R2 = 0.65; x = estimated pseudo-phyllodium mass). As there was no significant difference among treatments in specific leaf mass (see below), the same regression equations for pitcher and pseudo-phyllodia were used regardless of treatment. The mass of each pitcher measured throughout the season was then estimated by calculating the surface area multiplied by specific leaf mass, then corrected with the regression equation. Because keel measurements were taken only once in the Early Season and because keel width was minimal (< 2 mm) in the Early Season, Early Season pitcher mass was estimated using the area of the cone; keel mass was not factored into estimated pitcher mass for pitchers in the Early Season.
Statistical analysis
Height-to-diameter ratio was calculated by log (height/diameter) (Brewer 2019), and diameter-to-keel ratio was calculated by diameter/keel. The mean pitcher heights per genet, mean diameters per genet, mean height-to-diameter ratios per genet, mean keel width per genet, mean diameter-to-keel ratios per genet, number of pitchers per genet, summed masses per genet, sum of pitcher heights per genet, and specific leaf mass were analyzed by separate two-factor analyses of variance (ANOVA), with shade and feeding as the factors with two levels each. We generated interaction plots to display differences among treatments and their interaction. Mean seasonal (Early versus Late) differences for diameters, keel width, and ground PAR were compared using a Students t-test assuming equal variance. Measurements of PAR were log-transformed to improve heteroscedasticity. Temperature and log-transformed PAR were also analyzed by two-factor ANOVA with shade and feeding as factors (each with two levels), and time of day as a covariate. Statistical significance was determined at α = 0.05 for all tests. Measures of variance displayed are ± 2 standard error unless otherwise noted. Analyses were performed in Minitab 18.
Results
Field manipulation: early season (14-april-2019 to 29-may-2019)
The shade structures reduced PAR in the shaded treatments at both ground level (F0.05(2),3,76 = 7.25, p = 0.009; Fig. 2) and at pitcher height (F0.05(2),3,76 = 25.55, p < 0.001, similar pattern to Fig. 2). The shade structures had no significant effect on temperature (p = 0.310).
The shade treatment affected pitcher morphology. Average pitcher diameter per genet was significantly smaller in plants in the shaded treatments (p = 0.037; Table 1; Fig. 3a). There was no significant effect of treatments on average height-to-diameter ratio per genet in the Early Season (p > 0.05; Table 1).
Interaction plots of the effects of feeding, shading, and their interaction in the Early Season on a diameter (cm) of pitchers per genet, b average height-to-diameter ratio of pitchers per genet, c average pitcher height (cm) per genet, d average number of pitchers per genet, e average sum of pitcher heights per genet (cm), and f sum of estimated leaf mass (g) per genet. Error bars are ± 2 SE
The average pitcher height per genet was not significantly affected by the feeding treatments (p = 0.161, Fig. 3c). Unfed plants had fewer pitchers (Table 1; Fig. 3d) and a lower sum of pitcher heights (Table 1; Fig. 3e), but these differences were not significant. There was a significant effect of the interaction between shading and feeding on estimated leaf mass per genet: shading had no effect on mass of unfed plants, but shading reduced the mass of fed plants (p < 0.001; Table 1; Fig. 3f).
Late season (31-july-2019 to 20-october-2019)
Late in the growing season, shade structures continued to significantly reduce PAR at ground level (F0.05(2),3,76 = 54.68, p < 0.001, Fig. 2) and at pitcher height (F0.05(2),3,76 = 144.47, p < 0.001, similar pattern to Fig. 2) compared to unshaded plants, but PAR decreased across all treatments (Fig. 2). Mean ground PAR across all treatments for the Late Season (247.99 ± 66.78 µmol/m2/s, n = 80) was significantly lower (t0.05(2)156 = 4.66, p < 0.001) than that in the Early Season (582 ± 23.74 µmol/m2/s, n = 78). Shade structures had no significant effect on temperature (p = 0.571).
The average pitcher diameter per genet across all treatments in the Late Season (0.59 ± 0.08 cm, n = 66) was significantly smaller (t0.05(2),144 = − 17.77, p < 0.001) than that in the Early Season (2.23 ± 0.06 cm, n = 80). In contrast to results in the Early Season, there was no significant effect of shading on the average diameter per genet in the Late Season (p = 0.750; Table 1; Fig. 4a). However, there was a significant interaction effect on height-to-diameter ratio per genet (p = 0.043; Table 1; Fig. 4b). Fed plants in the shade had a higher height-to-diameter ratio than those in full sun. Unfed plants in full sun had a greater height-to-diameter ratio than those in shade.
Interaction plots of the effects of feeding, shading, and their interaction in the Late Season on a diameter (cm) of pitchers per genet, b average height-to-diameter ratio of pitchers per genet, c average pitcher height (cm) per genet, d average number of pitchers per genet, e average sum of pitcher heights per genet (cm), and f sum of estimated leaf mass (g) per genet. Error bars are ± 2 SE
Treatments affected plant growth in the Late Season. There were significantly fewer pitchers produced per genet in the unfed and shaded treatments (p = 0.020 and p = 0.008 for feeding and shading, respectively; Table 1; Fig. 4d). The sum of pitcher heights per genet was significantly lower in the unfed treatments (p = 0.038; Table 1; Fig. 4e). There was no significant difference among treatments in specific leaf mass (p > 0.05). There was a significant interaction effect on estimated leaf mass per genet (p = 0.024; Table 1; Fig. 4f): the estimated leaf mass per genet was not significantly affected by shading in unfed plants, but shading reduced the leaf mass per genet of fed plants.
Pitcher keels across all treatments in the late season (9.88 ± 0.35 mm, n = 71) were significantly wider than those in the Early Season (4.19 ± 0.21 mm, n = 46; t0.05(2),115 = − 12.53, p < 0.001). The ratio of diameter-to-keel width changed significantly over the growing season; pitchers from the Late Season had wider keels and smaller diameters (thus a smaller ratio) than those in the Early Season (p = 0.001; Table 2; Fig. 5).
Discussion
Early season: field manipulation
We predicted that pitcher plants that experienced low-light availability and/or reduction in prey capture would acquire a morphology that would partially alleviate the associated resource limitations. We hypothesized that under conditions of low-light availability, pitcher plants would have greater height-to-diameter ratios (grow taller with smaller diameters) to maximize light capture. The pitchers of shaded plants had significantly smaller diameters. This could be interpreted as a reduced dependence on prey capture in conditions of low light, as capture rates correlate positively with opening size (Heard 1998; Green and Horner 2007; Bhattarai and Horner 2009). The smaller diameter may therefore be interpreted as a shift away from a carnivorous morphology and towards a photosynthetic morphology. However, we did not observe a significantly greater height-to-diameter ratio, although there was a strong trend (p = 0.078). This was in part due to the fact that pitcher heights were not statistically affected by the treatments. In contrast, Brewer (2003, 2019) observed increased height-to-diameter ratios in response to aboveground competition with herbaceous competitors. In the studies by Brewer (2003, 2019), focal plants experienced altered light from the sides but unaltered light from overhead. Our use of shade structures reduced light from the side as well as directly overhead, and our use of 50% shade cloth may have been a greater reduction in light than experienced by plants in experiments by Brewer (2003, 2019).
Under conditions of reduced prey capture, we hypothesized that pitcher plants would exhibit reduced growth rates (fewer pitchers and lower sum of pitcher heights). There was a trend (p < 0.10) toward fewer pitchers per genet and a lower sum of pitcher heights in unfed plants. Carnivorous machinery is an expensive investment (Karagatzides and Ellison 2009), and prey exclusion from pitchers likely reduced nutrient uptake. We hypothesize that differences in indices of growth accrue over the season, and that this may explain why significant differences in growth were only observed in the Late Season, as insufficient time had elapsed for the treatments to significantly impact growth in the Early Season.
We hypothesized that the interaction of low light and loss of prey capture would result in pitchers with a greater height-to-diameter ratio but would also exhibit reduced growth due to lessened resource availability. Estimated leaf mass per genet was significantly affected by the interaction between feeding and shading. Shade did not affect estimated leaf mass in unfed plants. This may be because unfed plants were nutrient limited and incapable of responding to differences in light availability. In contrast, shade had a significant effect on estimated leaf mass in fed plants. Growth in fed plants was reduced by shading.
Late season: rapid regrowth of competing vegetation
Due to the regrowth of competing vegetation and the shade it produced, light availability was reduced across all treatments in the Late Season (Fig. 2). This reduction in light across all genets diminished the relative impact of the shade treatment and altered the morphology of all pitchers in all treatments during the Late Season. One manifestation of this morphological shift was a reduction of average diameter of pitcher openings across all treatments. This may explain why the significant difference in average diameter of pitcher openings observed in shaded plants in the Early Season was not also observed in the Late Season.
In the Late Season, plant growth was affected by all treatments. Unfed plants produced fewer pitchers and had lower sum of pitcher heights than fed plants, and shaded plants produced fewer pitchers than unshaded plants. The interaction between light and prey capture affected estimated leaf mass in the same manner as in the Early Season. Because overwintering and the production of the flower and first pitcher each spring are dependent on reserves stored during the previous growing season (Butler et al. 2008), the reduction in resource acquisition and growth may negatively impact survival and reproduction.
Pitcher keels were significantly wider in the Late Season. Thus, the pitchers produced in the Late Season began to abandon a carnivorous morphology and produce pseudo-phyllodia. A similar morphological response has been observed in Sarracenia purpurea exposed to high nitrogen deposition (Ellison and Gotelli 2002). Higher levels of nitrogen diminished the value of prey capture and the need for carnivorous structures, and pitchers formed larger keels and smaller diameters. This study demonstrates that the abandonment of carnivorous structures is dependent on resource availability and can occur not only under conditions of high nutrient availability where prey capture is no longer advantageous (Ellison and Gotelli 2002), but also in low-light environments if light availability is insufficient to utilize the nutrients available.
Botanical carnivory has been hypothesized to be beneficial only under conditions of high availability of water and light (Givnish et al. 1984). The evolved specialty of prey capture in carnivorous plants comes at the cost of reduced photosynthetic rate and photosynthetic nutrient-use efficiency (Ellison 2006; Karagatzides and Ellison 2009). The Early Season field manipulation and the rapid regrowth of vegetation in the Late Season provide support for this. In the shade treatments in the Early Season and in response to competing vegetation in the Late Season, plants abandoned the carnivorous morphology. Therefore, the plants transitioned to a primarily photosynthetic morphology (wide keels and non-functional pitchers).
Conservation Implications
Carnivorous plant bogs in the United States are quickly vanishing, and some Sarracenia species are endangered (Furches et al. 2013). For species that are not currently endangered, they are at risk of becoming so due to shrinking habitats and fragmented populations (Folkerts 1977). In addition, fire management has affected these habitats. Natural fires typically occur every three to 4 years in the habitats occupied by carnivorous plants in the southeastern United States, but humans have suppressed fires until recent shifts in conservation ideologies (Johnson and Hale 2002). In carnivorous plant bogs of the southeastern United States, high-light conditions are maintained by periodic fires that reduce the abundance of invading competitive species (Brewer 2006). As a result, many carnivorous plants in the southeastern United States are dependent on periodic fire disturbances (Schnell 1976). Fire suppression can lead to woody vegetation invading carnivorous plant bogs and becoming major competitors for light. If shrubby vegetation persists and reduces light availability over numerous seasons, carnivorous plants would not be able to compete because of their low maximum photosynthetic rate (Karagatzides and Ellison 2009). The timing and intensity of fires can also affect the performance of pitcher plants in these habitats. For example, Brewer (1999) has suggested that winter burns are less effective at reducing competing vegetation. The winter burn at our bog was relatively cool and, although much of the competing vegetation was removed, the burn was relatively ineffective in that many shrubs remained and competing vegetation recovered quickly. Another factor that may have allowed for the quick recovery of the competing vegetation was the low-intensity of the burn, as a low-intensity fire does not penetrate into the bottom layer of vegetation or into the soil (Alcaniz et al. 2018). The impact that competing vegetation has on pitcher morphology can be observed in a single growing season and can be used as an index for land managers to assess the health of carnivorous plant inhabitants. Decreasing pitcher diameter, widening keels, or production of phyllodia/pseudo-phyllodia, depending on the species of Sarracenia present, can be used as indicators that the pitchers are becoming light limited and a prescribed burn is required. Therefore, landowners and public land agencies can monitor carnivorous plants for signs of morphological change and regularly perform prescribed burns to maintain the health of the bogs.
References
Adamec L (1997) Mineral nutrition of carnivorous plants: a review. Bot Rev 63:273–299
Adamec L, Matusikova I, Pavlovic A (2021) Recent ecophysiological, biochemical and evolutional insights into plant carnivory. Ann Bot 128:241–259
Alcaniz M, Outeiro L, Francos M, Ubeda X (2018) Effects of prescribed fires on soil properties: a review. Sci Total Environ 613:944–957
Bhattarai GP, Horner JD (2009) The importance of pitcher size in prey capture in the carnivorous plant, Sarracenia alata Wood (Sarraceniaceae). Am Midl Nat 161:264–272
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants - an economic analogy. Annu Rev Ecol Syst 16:363–392
Brewer JS (1999) Short-term effects of fire and competition on growth and plasticity of the yellow pitcher plant, Sarracenia alata (Sarraceniaceae). Am J Bot 86:1264–1271
Brewer JS (2003) Why don’t carnivorous pitcher plants compete with non-carnivorous plants for nutrients? Ecology 84:451–462
Brewer JS (2006) Resource competition, and fire-regulated nutrient demand in carnivorous plants of wet pine savannas. Appl Vegetation Sci 9:11–16
Brewer JS (2019) Inter- and intraspecific competition and shade avoidance in the carnivorous pale pitcher plant in a nutrient-poor savanna. Am J Bot 106:81–89
Butler JL, Gotelli NJ, Ellison AM (2008) Linking the brown and green: nutrient transformation and fate in the Sarracenia microecosystem. Ecology 89:898–904
Carmickle RN, Horner JD (2019) Impact of the specialist herbivore Exyra semicrocea on the carnivorous plant Sarracenia alata: a field experiment testing the effects of tissue loss and diminished prey capture on plant growth. Plant Ecol 220:553–561
Cresswell JE (1993) The morphological correlates of prey capture and resource parasitism in pitchers of the carnivorous plant Sarracenia purpurea. Am Midl Nat 129:35–41
Dahl TE , Pywell HR (1989) National status and trends study - estimating wetland resources in the 1980s. Proceedings of the symposium on wetlands: Concerns and successes. American Water Resources Association Symposium, Tampa, FL, pp. 25–31
Darwin C (1875) Insectivorous plants. John Murray, London
Ellison AM (2006) Nutrient limitation and stoichiometry of carnivorous plants. Plant Biol 8:740–747
Ellison AM, Adamec L (2011) Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same? Oikos 120:1721–1731
Ellison AM, Gotelli NJ (2002) Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proc Natl Acad Sci USA 99:4409–4412
Farnsworth EJ, Ellison AM (2008) Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. J Ecol 96:213–221
Folkerts GW (1977) Endangered and threatened carnivorous plants of North America. In: Prance GT, Elias TS (eds) Extinction is forever: Threatened and endangered species of plants in the Americas and their significance in ecosystems today and in the future. New York Botanical Garden, Bronx, NY, pp 301–313
Folkerts GW (1982) The gulf coast pitcher plant bogs. Am Sci 70:260–267
Furches MS, Small RL, Furches A (2013) Genetic diversity in three endangered pitcher plant species (Sarracenia; Sarraceniaceae) is lower than widespread congeners. Am J Bot 100:2092–2101
Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD (1984) Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am Nat 124:479–497
Green ML, Horner JD (2007) The relationship between prey capture and characteristics of the carnivorous pitcher plant, Sarracenia alata Wood. Am Midl Nat 158:424–431
Heard SB (1998) Capture rates of invertebrate prey by the pitcher plant, Sarracenia purpurea L. Am Midl Nat 139:79–89
Horner JD (2014) Phenology and pollinator-prey conflict in the carnivorous plant, Sarracenia alata. Am Midl Nat 171:153–156
Horner JD, Cross Steele J, Underwood CA, Lingamfelter D (2012) Age-related changes in characteristics and prey capture of seasonal cohorts of Sarracenia alata pitchers. Am Midl Nat 167:13–27
Horner JD, Plachno BJ, Bauer U, Di Giusto B (2018) Attraction of prey. In: Ellison AM, Adamec L (eds) Carnivorous plants: Physiology, ecology, and evolution. Oxford University Press, Oxford, pp 157–166
Johnson AS , Hale PE (2002) The historical foundations of prescribed burning for wildlife: a Southeastern perspective. In: Ford W. M., Russell K. R. and E M. C. (eds), Proceedings: the role of fire for nongame wildlife management and community restoration: traditional uses and new directions. . USDA National Research Station, General Technical Report pp. 11–23
Juniper BE, Robins RJ, Joel DM (1989) The carnivorous plants. Academic Press, New York
Karagatzides JD, Ellison AM (2009) Construction costs, payback times, and the leaf economics of carnivorous plants. Am J Bot 96:1612–1619
Renou-Wilson F, Moser G, Fallon D, Farrell CA, Mueller C, Wilson D (2019) Rewetting degraded peatlands for climate and biodiversity benefits: Results from two raised bogs. Ecol Eng 127:547–560
Schnell DE (1976) Carnivorous plants of the United States and Canada. John F. Blair, Winston-Salem, NC
Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39:505–522
Acknowledgements
This research was supported by grants from the Texas Christian University Research and Creative Activity Fund (TCU RCAF) and the TCU Science and Engineering Research Center (SERC). The Adkins Fund at TCU provided summer support for MCS. Karis Kang provided help in the field. Dr. Amanda Hale, Dr. Dean Williams, two anonymous reviewers and the handling editor made helpful comments on earlier drafts of this manuscript.
Funding
This work was supported by grants from the Texas Christian University (TCU) Research and Creative Activities Fund (RCAF) and the Science and Engineering Research Center (SERC). Summer support for MS was provided by an Adkins Grant from the Department of Biology, TCU.
Author information
Authors and Affiliations
Contributions
Authors contributed equally to the design, field work, statistical analysis, and writing of the project. MS performed the laboratory analyses.
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Additional information
Communicated by Christopher Anderson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Segala, M.C., Horner, J.D. The effects of light availability, prey capture, and their interaction on pitcher plant morphology. Plant Ecol 224, 539–548 (2023). https://doi.org/10.1007/s11258-023-01320-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-023-01320-6