Abstract
Land clearing of habitat into smaller, isolated remnants is a major driver of plant and animal extinctions globally. In southeastern Australia, once widespread temperate savannas have been subjected to extensive land clearing since European settlement. Some small fragments have persisted, but declines in the dominant trees have been reported, with anecdotal observations of widespread recruitment bottlenecks and seed set failure. To test the hypothesis that populations of Banksia marginata are experiencing widespread recruitment bottlenecks, we examined tree size-class distribution and production of infructescences (cones) of 15 populations on the western plains of Victoria, Australia. We found no evidence of widespread recruitment bottlenecks or a failure to set seeds; most populations were recruiting, though we did find evidence of declining recruitment with population size, suggesting evidence of an Allee effect. The proportion of trees without cones varied between populations; three populations had large numbers of trees (> 40%) lacking mature fertile cones. Managers should focus on minimising threats to seedling survival and augment populations below 100 individuals to improve recruitment and maintain stand persistence in the landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragmentation is a global problem with around 1.5 billion hectares of habitat converted to farmland (IPBES 2019). In temperate southeast Australia, extensive land clearing since European settlement (Bennett 1993; Bradshaw 2012; Chesterfield 1986; Gordon et al. 2003) has resulted in highly fragmented ecosystems, particularly temperate grasslands (Kirkpatrick et al. 1995) and woodlands (Fisher and Harris 1999; Yates and Hobbs 1997). Fragmentation can have many detrimental effects on species in the remnant ecosystems. The slow decline of remnant tree populations in fragments represents an extinction debt (Tilman et al. 1994), and has been reported elsewhere in woodlands and savannas (Abrams 2003; Mogoutnov and Venning 2014; Tolera et al. 2013). For instance, smaller population sizes inhabiting uncleared fragments may be vulnerable to the effects of inbreeding or stochastic events that further reduce population sizes (Morgan et al. 2013; Westemeier et al. 1998). However, in the short term, indirect effects of fragmentation on animal-plant interactions also have the potential to drive changes to plant populations (Kolb 2008). The loss of insectivorous birds in forest fragments has, for example, been linked to increased insect herbivory (Elzinga et al. 2005; Levey et al. 2016; Peter et al. 2015). Habitat fragmentation can also lead to changes in pollinator foraging behaviour (Elliott et al. 2012; Goverde et al. 2002; Montgomery et al. 2003) or changes in the composition of the pollinator community (Brosi et al. 2007; Elliott et al. 2012; Hatfield and LeBuhn 2007). With such changes in animal-plant interactions, there is potential for small populations to be subject to Allee effects (Allee 1931; Lamont et al. 1993) that impact plant fitness or recruitment.
Size- and age-class distributions can provide insights about plant populations that can be used to make inferences about the demographic trajectory (Hazard and Parsons 1977; Tolera et al. 2013) or reproductive biology of a species (Ashton 1976; Zammit and Westoby 1987). This makes age- and size-class data particularly useful when investigating long-term declines in recruitment, particularly for species that recruit continually and should exhibit the reverse j-curve type distribution (Glenn-Lewin et al. 1992; Lykke 1998). A demographic bottleneck can be defined as a decline in population size in one or more size- or age-classes (Beck 1995; Holdo et al. 2014). Demographic bottlenecks can be caused by disturbance such as a fire that removes the reproductively mature plants from a population (Enright and Lamont 1989) or herbivores that may browse young recruits (Di Stefano 2003). A demographic bottleneck may not be a problem in the short term if the cause is fleeting; however, long-term demographic bottlenecks may eventually lead to population decline (population bottleneck). For example, short fire intervals may reduce population sizes in resprouters (Fairman et al. 2017), while long-term herbivory has led to population declines in many species (MacDougall et al. 2010; Salk et al. 2011; Tolera et al. 2013).
The savannas of western Victoria were once dominated by tree species such as Banksia marginata (Proteaceae), Allocasuarina verticillata (Casuarinaceae), Acacia melanoxylon (Fabaceae) and Bursaria spinosa (Pittosporaceae) (Sinclair and Atchison 2012). Early reports and surveyors’ maps show that these trees were once widespread, but scattered in these savanna-like ecosystems (Nicholson 1855; Webster 1858); more recent interpretations of vegetation change confirm that these landscapes were once common across western Victoria (Hateley 2010; Sinclair and Atchison 2012). However, they are now either locally extinct or verging on extinction due to extensive land clearing and habitat fragmentation (Sinclair and Atchison 2012). As such, trees like B. marginata may be vulnerable to the effects of small population size, altered biotic interactions and disturbance regimes. There are concerns among land managers that trees are experiencing widespread decline, demographic bottlenecks and low fecundity. In non-serotinous species, such as B. marginata, where seed release is not cued by fire (Lamont and Enright 2000), ongoing recruitment might be expected if viable seeds are produced continually and ‘safe sites’ (sensu Harper et al. 1965) are maintained.
Fragmented stands of B. marginata are reported to be in decline by land managers, with reports of widespread failure to set seed and a lack of recruitment, although empirical data are lacking to support these assertions. There have been several attempts to grow savanna trees in isolated stands and expand the remnant populations, create additional insurance populations, and create seed orchards. Such efforts have been led by community groups across western Victoria, reflecting widespread community concern (Liber 2004; Swan 2017). In this study, we test the hypotheses that B. marginata are suffering widespread failure to produce seed and that there are widespread demographic recruitment bottlenecks across western Victoria. However, studies in other Banksia species suggest some are self-incompatible (Ramsey and Vaughton 1991) and an alternative hypothesis is that small populations are vulnerable to small population Allee effects (Lamont et al. 1993).
Methods
Study area
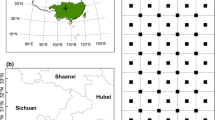
This study aimed to sample remnant Banksia marginata populations across western Victoria and encompassed an area between the towns of Hamilton (37° 44′ 31.6″ S, 142° 01′ 43.8″ E) in the west to Ballarat (37° 36′ 46.0″ S, 144° 14′ 11.7″ E) in the east (Fig. 1). The climate is Mediterranean, with winter-dominant rainfall and summer drought. Mean annual rainfall ranges between 558 and 702 mm, and mean temperatures range from 7.1–19.2 °C (Australian Bureau of Meteorology 2018). The study area covers a range of geologies, including the Volcanic Plains with basalt flows from the late Tertiary to late Quaternary, and adjoining sediments of the Dundas Tablelands and Central Victorian Uplands.
Study species
Banksia marginata is a shrub or small tree endemic to southeastern Australia (Collins et al. 2008; Taylor 1988). The species is highly variable, but to date it has defied attempts for any subspecific or variant recognition (George 1998), though Christopoulos (1988) found extensive clonality in the shrub while the tree-form was not clonal. Our study system is dominated by native C4 and C3 grasses (Stuwe and Parsons 1977) and B. marginata grows as the non-serotinous tree-form. It is possible that there has been selection for arborescence and the loss of serotiny in favour of frequent seed release, which may have occurred in response to the emergence of grassy biomes in southeast Australia. This is analogous to the emergence of savanna during the Miocene with taller forms and the loss of serotiny in Protea (Lamont et al. 2013).
Small individual trees can be reproductive, and in our study, flowers and infructescences were observed on young trees (> 58 mm DBH). It is capable of resprouting after fire and we observed postfire resprouting of saplings and young trees from lignotubers and epicormic buds after fires in some populations.
Demographic surveys
Fifteen populations, representing 30% of populations distributed across the western plains’ savanna, were selected to encompass populations of varying sizes and assessed for stand structure. Most populations are relatively small (< 500 trees); hence entire populations were censused (except the largest stand at Minhamite where a subset of the stand was censused). Diameter at breast height (DBH, at 1.3 m) was measured for each tree. For multi-stemmed trees, all main stems over 20 mm diameter were measured and combined by calculating the square root from the sum of all the squared DBH measurements to obtain an equivalent DBH (MacDicken et al. 1991; Stewart and Salazar 1992). If a fork occurred at 1.3 m, then the stem was measured below the fork. All living trees under 20 mm DBH were classed as saplings and assigned as the smallest size-class.
The presence of cones, which support few to many fruits (follicles) in Banksia, was noted by inspection of the entire crown. Anecdotal observations by land managers suggested that a failure to produce infructescences was happening over multiple years. Therefore, the assessment of cones included both new (developed from previous year’s flowers) and old (older than previous year’s crop) cones. We aimed to determine if an absence of cone set occurred at the population level over multiple years.
Statistical analyses
While many studies use histograms to make inferences about recruitment and stand dynamics (Considine et al. 2013; Erdos et al. 2015; Hulme 1996), there is some emerging evidence that histograms can be misinterpreted (Boels et al. 2019; Lem et al. 2013). To overcome this, we regressed the number of individuals in each of 15 size classes (y-axis) against the size class mid-point (x-axis) and used the slope of the regression line as a measure of recruitment success (Condit et al. 1998).
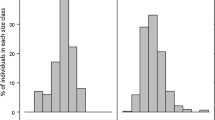
We used ten DBH size classes (after Condit et al. 1998): 10–19, 20–29, 30–39, 40–49, 50–99, 100–199, 200–299, 300–399, 400–499, 500–999 mm. The number of stems (ni) within each class was calculated by dividing the number of stems by the width of the size class (see Condit et al. 1998) to obtain an adjusted total number of stems for each class while the mid-point of the size class (di) was used. Both ni and di were then Log10 transformed. We then calculated a regression Log10 (di) as the independent variable and Log10 (ni + 1) as the dependent variable. As per Lykke (1998), where a size-class had ni = 0, we chose to retain the 0 values. The removal of 0 values at the smaller size-classes produces regressions with slopes that have a smaller negative value, while the removal of 0 values at larger size-classes produces a smaller positive values and therefore creates an impression that a population is experiencing more or less recruitment than it is. The slopes of these regressions were then used to define stand type; all slopes are henceforth referred to as ‘Condit slopes’. Figure 2 is a simple schematic of these outcomes, which can broadly be described as:
Type 1: Recruitment not limited: large numbers of juveniles, saplings and young trees relative to older trees; slopes are steeper with large negative values (< − 1.0).
Type 2: Recruitment moderately limited: fewer sapling and juvenile trees relative to older trees; slopes are flatter, with values falling between 0 and − 1.0.
Type 3: Recruitment severely limited: few to no observable trees in the smaller size classes relative to older trees; the slopes are flat with positive values (> 0).
It was assumed that all stems represent individual trees; however, in some shrub forms of B. marginata, some plants may have arisen from root suckers (Specht and Rayson 1957) rather than genetically distinct individual trees. However, Christopoulos (1988) found no clonality in one population of tree form B. marginata while in the same study area, recent work by Miller et al. (2020) found clones were at low frequency (between 0 and 6 clones). Where true clonal species are involved, care needs to be taken when interpreting population dynamics using this method.
To determine if an absence of fertile cones was widespread, we tested the differences in the proportion of trees with and without cones across 10 of the 15 sampled populations. Given juvenile plants are not likely to produce cones and we were able to determine that plants < 58 mm DBH were not reproductive, these were removed from the analyses. The proportion of trees (with > 58 mm DBH) with and without cones was analysed using χ2 test for homogeneity and pairwise comparisons were undertaken using Fisher’s exact test. Regressions were then computed to determine if there was a relationship between population size and proportion of trees without cones. A non-linear regression was also computed to determine if there was a relationship between population size and the value of the Condit slopes for each population.
With the exception of the calculations outlined in Condit et al. (1998), all analyses and plotting were performed using R version 3.5. (R Core Team 2018) and RStudio version 1.1.453 (RStudio Team 2016). Plots were produced using Lattice (Sarkar 2008) and ggplot2 (Wickham 2016).
Results
Stand dynamics encompassed all Condit slope types (Fig. 3). Seven populations had a stand Type 1, with high negative Condit slopes (− 1.024 to − 1.8158), indicating recruitment was evident. Five populations exhibited Type 2 dynamics, with Condit slopes between − 0.1776 and − 0.7998. Three populations had Type 3 stand dynamics (Condit slopes between 0.0649 and 0.1276), indicating recruitment bottlenecks. While this might not suggest evidence of widespread demographic bottlenecks, we did find evidence of a non-linear relationship (Fig. 5a; F (1,13) = 28.38, p < 0.001, r2 = 0.69) between recruitment and population size, which suggest an Allee effect in smaller populations.
Condit slope outputs for 15 analysed populations. Regressions are arranged from highest negative value (recruitment not limited) through to the highest positive value (substantial recruitment limitation). The x-axis represents the mid-point of the size class and has been Log10 transformed. The y-axis is the number of individuals and has been adjusted using the method outlined in Condit (1998). This was then Log10 + 1 transformed to ensure 0 values were retained
The failure of mature trees to produce fertile cones was widespread across the populations and the proportion varied between populations; three populations did have substantial cone set failure (> 40% of cones were barren). The results from the pairwise comparison using Fisher’s exact test to compare the proportion of trees with and without fertile cones showed that 20 pairs of Banksia populations out of a total of 36 comparisons were significant (p < 0.05); three populations (Moutajup, Trawalla and Caramut) stood out, with the most comparisons that were significant and these populations were also found to have high proportions of mature trees without cones (Fig. 4). The results of the linear regression suggest no significant relationship between population size and the percentage of trees without fertile cones (Fig. 5b; F (1,8) = 1.42, p = 0.27, r2 = 0.15) and a Pearson test only found a moderate negative correlation between B. marginata population size and the percentage of trees that were not producing fertile cones.
Discussion
Despite their fragmented nature, rural trees still provide many important ecosystem services (Manning et al. 2006) but their persistence may be transient (Tilman et al. 1994). Understanding if rural tree populations are in decline is an important task that informs policymakers and allows land managers to effectively manage small rural tree populations. Banksia marginata has been subjected to extensive habitat modification and land clearing since European settlement and we could expect to see evidence of decline, with reports from land managers suggesting this species is failing to recruit and set seed. While we did find that many populations had proportions of mature trees failing to produce fertile cones, only three populations had a substantially large proportion that failed to produce cones. Despite this, we did not observe widespread demographic bottlenecks in Banksia savannas in western Victoria, with only 3 of 15 populations found to have a demographic (recruitment) bottleneck. Most stands exhibited a Type 1 Condit slopes, signifying a recruiting stand, although Type 2 and Type 3 stands were also observed, the latter consistent with an absence of recruitment. Thus, despite the highly fragmented and isolated nature of some populations, recruitment is still occurring.
Nevertheless, long-term persistence of some of these populations is not assured given their isolated nature (Tilman et al. 1994) and the prospect of climate change accelerating declines as demonstrated in other Banksia species (Challis et al. 2016; Steel et al. 2019). We did observe that as populations increase in size, their Condit slope shift from positive (Type 3) to highly negative slopes (Type 1), suggesting that recruitment in this species declines rapidly when the population become small (< 100 individuals). This is suggestive of a demographic Allee effect, whereby potential recruitment declines as the population becomes smaller and fewer reproductive trees are present in a population. Alternatively, herbivory - both direct and indirect (granivory or florivory) - may be driving this. However, the mechanisms that underpin this relationship needs to be resolved if (very) small populations are to be maintained into the coming decades.
The proportion of trees without cones was not consistent across populations. Three populations (Trawalla, Moutajup and Caramut), in particular, had few cones, but there was no relationship between population size and the proportion of trees producing cones. Other factors, such as landscape context (isolation, distance to nearest native vegetation) or habitat quality, may affect seed set through their effects on pollinator movements (Llorens et al. 2012; Thavornkanlapachai et al. 2018). Banksias are often vertebrate (mammals and birds) pollinated (Cunningham 1991; Hackett and Goldingay 2001; Hooper 1980; Krauss et al. 2009) and factors that affect pollinator movements in fragmented landscapes will inevitably affect cone set. This has been demonstrated for many fragmented plants (Berry and Calvo 1991; Nayak and Davidar 2010; Pellegrino 2014), including other members of the Proteaceae (Holmes et al. 2008; Lamont et al. 1993).
All populations are likely to be experiencing recruitment limitations to differing degrees and factors limiting successful recruitment of individuals into populations are likely to include biotic (e.g. competition, herbivory) and abiotic (e.g. soil moisture availability) drivers. In savannas, recruitment-limitation may be driven by seasonally limited resources, such as competition for moisture with native (and increasingly) non-native grasses (D'Onofrio et al. 2015; Holdo and Brocato 2015; Sankaran et al. 2005; Vadigi and Ward 2013). Grasses both suppress tree growth (February et al. 2013; Riginos 2009) and limit the survival of trees (Morrison et al. 2018; Scholes and Archer 1997), particularly the more vulnerable smaller size classes, where the root zones of trees and grasses are more likely to interact (Holdo and Brocato 2015). Grasses also have the capacity to impact fire regimes, with concomitant effects on tree sapling survival (Haverkamp et al. 2018; Peterson and Reich 2001). However, we observed recruitment at sites where grasses, like Themeda triandra, were present and dominant in the understorey and given this, it seems unlikely that grass competition alone is the main cause of the recruitment limitation observed in some Banksia stands.
Another powerful biotic agent that affects recruitment is consumer-driven limitation (Bird et al. 2012; MacDougall et al. 2010; Price and Morgan 2003). The effects of herbivores may affect plants at multiple life stages, either through the direct consumption of seedlings and saplings or by more indirect effects (florivory, seed predation) that affects the pool of plant propagules in a population (Ferreira et al. 2011; Kurkjian et al. 2017). The preferential browsing of some palatable species by herbivores can lead to recruitment failure, a demographic bottleneck and, ultimately, changes in vegetation composition over time (Bradshaw and Waller 2016; Dexter et al. 2013; Neave and Tanton 1989; Salk et al. 2011). Exotic herbivores, such as the European rabbits, as well as ungulates, may also be involved. Bird et al. (2012) suggests that, even at low densities, rabbits may inhibit recruitment of Allocasuarina verticillata in an Australian coastal shrubland community. In North America, introduced European rabbits led to increased mortality of Quercus garryana (MacDougall et al. 2010). If B. marginata is palatable to herbivores, then this could explain recruitment limitation, yet a study on the role of herbivory was inconclusive and found that summer drought may be a more immediate cause of seedling mortality (unpublished data).
Initially we thought that the absence of cones may have been a result of pollination failure, particularly in smaller or isolated fragments where some pollinators might be missing. However, while many trees had no cones, we did observe discarded and damaged B. marginata cones under the crowns of many reproductive trees across many of the populations. This is consistent with observations by landholders and land managers of Calyptorhynchus funereus (Yellow-tailed Black cockatoos) visiting populations to consume seeds; damage to the cones appeared to be consistent with damage by cockatoo foraging described elsewhere (Scott and Black 1981). Pre-dispersal granivory by cockatoos in Banksia species has previously been documented in Western Australia (Johnston et al. 2016; Lamont et al. 2007; Scott and Black 1981; Valentine et al. 2014; Witkowski et al. 1991) and studies in South and Central America have also found parrots to be important pre-dispersal seed predators, contributing to significant loss of seed (Coates-estrada et al. 1993; Francisco et al. 2008; Villaseñor-Sánchez et al. 2010). In contrast, there has been little work to investigate the role of seed predation by parrots on recruitment limitation in eastern states of Australia but recent work found that cone removal by C. funereus was exceptionally high, with between 50 and 100% of cones removed (Heyes et al. 2019).
This study has demonstrated that there are limits to recruitment under current conditions in several populations. The reasons for these limits remains unclear and are yet to be explored, but probable causes could relate to fragmentation and small population size (Lamont et al. 1993; Morgan 1995), exotic plants (Hoffmann and Haridasan 2008), herbivory (MacDougall et al. 2010) or too much/little fire (Gent and Morgan 2007; Henzler et al. 2018). As the climate continues to warm and dry, this may lead to changes in microsite suitability that further limits tree recruitment. Under these conditions, recruitment in some populations may decline from higher rates of recruitment (Type 1 slopes), becoming increasingly recruitment-limited (Type 2 or Type 3 slopes) and this may be tested with revisitation studies (Gent and Morgan 2007; Naccarella et al. 2019).
Conclusion
We quantified recruitment in fifteen populations using the Condit method (Condit et al. 1998; Lykke 1998) and showed that most stands are recruiting. Only three stands were found to be experiencing recruitment failure and indicates that anecdotal observations of widespread recruitment bottlenecks in highly fragmented Banksia savanna populations are exaggerated. Severe recruitment limitations in these savannas are most likely governed by several local factors rather than a single widespread phenomenon. While there still is some recruitment at these sites, the question remains: is this type of recruitment enough to replace mortality of larger size-classes in small fragments or will these fragments experience further declines in recruitment?
Condit values approaching zero or positive are indicative of little to no recruitment and the relationship between population size and the Condit slopes seems to indicate that once populations fall below 100, their Condit scores also change to positive values. Therefore, as well as managing causes of recruitment limitations, conservation efforts should also focus on maintaining larger population sizes, above 100 individuals, to prevent further population decline and extinction. Further research is needed to understand the causes of recruitment-limitation in populations of B. marginata, particularly in fragmented populations such as those in our study area. Understanding the causes of limited seed availability might be of particular importance with focus on seed predation. Given the role of grasses in tree recruitment in other grassy ecosystems, further understanding of grass competition in these savannas is likely to be a valuable contribution.
References
Abrams MD (2003) Where has all the White Oak gone? Bioscience 53(10):927–939. https://doi.org/10.1641/0006-3568(2003)053[0927:WHATWO]2.0.CO2
Allee WC (1931) Animal aggregations, a study in general sociology. The University of Chicago Press, Chicago
Ashton DH (1976) The development of even-aged stands of Eucalyptus regnans F. Muell. in central Victoria. Aust J Bot 24(3):397–414. https://doi.org/10.1071/BT9760397
Beck MW (1995) Size-specific shelter limitation in Stone Crabs: a test of the demographic bottleneck hypothesis. Ecology 76(3):968–980. https://doi.org/10.2307/1939360
Bennett AF (1993) Fauna conservation in box and ironbark forests: a landscape approach. Vic Nat 110(1):15–23
Berry P, Calvo R (1991) Pollinator limitation and position dependent fruit set in the high Andean orchid Myrosmodes cochleare (Orchidaceae). Entwicklungsgeschichte und Systematik der Pflanzen 174(1):93–101. https://doi.org/10.1007/BF00937697
Bird P, Mutze G, Peacock D, Jennings S (2012) Damage caused by low-density exotic herbivore populations: the impact of introduced European rabbits on marsupial herbivores and Allocasuarina and Bursaria seedling survival in Australian coastal shrubland. Biol Invas 14(3):743–755. https://doi.org/10.1007/s10530-011-0114-8
Boels L, Bakker A, Van Dooren W, Drijvers P (2019) Conceptual difficulties when interpreting histograms: a review. Educ Res Rev 28:100291. https://doi.org/10.1016/j.edurev.2019.100291
Bradshaw CJA (2012) Little left to lose: deforestation and forest degradation in Australia since European colonization. J Plant Ecol 5(1):109–120. https://doi.org/10.1093/jpe/rtr038
Bradshaw L, Waller DM (2016) Impacts of white-tailed deer on regional patterns of forest tree recruitment. For Ecol Manag 375:1–11. https://doi.org/10.1016/j.foreco.2016.05.019
Brosi BJ, Daily GC, Ehrlich PR (2007) Bee community shifts with landscape context in a tropical countryside. Ecol Appl 17(2):418–430. https://doi.org/10.1890/06-0029
Challis A, Stevens J, McGrath G, Miller B (2016) Plant and environmental factors associated with drought-induced mortality in two facultative phreatophytic trees. Int J Plant-Soil Relat 404(1–2):157–172. https://doi.org/10.1007/s11104-016-2793-5
Chesterfield EA (1986) Changes in the vegetation of the river red gum forest at Barmah, Victoria. Aust For 49(1):4–15. https://doi.org/10.1080/00049158.1986.10674458
Christopoulos A (1988) Variation in Banksia marginata Cav. (Honours Thesis), University of Melbourne, Melbourne
Coates-estrada R, Estrada A, Meritt D (1993) Foraging by Parrots (Amazona autumnalis) on Fruits of Stemmadenia Donnell-Smithii (Apocynaceae) in the Tropical Rain Forest of Los Tuxtlas, Mexico. J Trop Ecol 9:121–124
Collins K, Collins K, George AS (2008) Banksias, Paperback edn. Bloomings Books, Richmond
Condit R, Sukumar R, Hubbell SP, Foster RB (1998) Predicting population trends from size distributions: a direct test in a tropical tree community. Am Nat 152(4):495–509
Considine CD, Groninger JW, Ruffner CM, Therrell MD, Baer SG (2013) Fire history and stand structure of high quality Black Oak (Quercus velutina) Sand Savannas. Nat Areas J 33(1):10–20. https://doi.org/10.3375/043.033.0102
Cunningham S (1991) Experimental evidence for pollination of Banksia spp. by non-flying mammals. Oecologia 87(1):86–90. https://doi.org/10.1007/BF00323784
D'Onofrio D, Baudena M, D'Andrea F, Rietkerk M, Provenzale A (2015) Tree-grass competition for soil water in arid and semiarid savannas: the role of rainfall intermittency. Water Resour Res 51(1):169–181. https://doi.org/10.1002/2014WR015515
Dexter N, Hudson M, James S, MacGregor C, Lindenmayer DB (2013) Unintended consequences of invasive predator control in an Australian forest: overabundant Wallabies and vegetation change. PLoS ONE 8(8):e69087. https://doi.org/10.1371/journal.pone.0069087
Di Stefano J (2003) Mammalian browsing in the Mt Cole State Forest: defining a critical browsing level and assessing the effect of multiple browsing events. Aust For 66(4):287–293. https://doi.org/10.1080/00049158.2003.10674923
Elliott C, Lindenmayer D, Cunningham S, Young A (2012) Landscape context affects honeyeater communities and their foraging behaviour in Australia: implications for plant pollination. Landsc Ecol 27(3):393–404. https://doi.org/10.1007/s10980-011-9697-9
Elzinga J, Turin H, Damme J, Biere A (2005) Plant population size and isolation affect herbivory of Silene latifolia by the specialist herbivore Hadena bicruris and parasitism of the herbivore by parasitoids. Oecologia 144(3):416–426. https://doi.org/10.1007/s00442-005-0096-2
Enright N, Lamont B (1989) Seed banks, fire season, safe sites and seedling recruitment in five co-occurring Banksia species. J Ecol 77(4):1111. https://doi.org/10.2307/2260826
Erdos L, Tolgyesi C, Cseh V, Tolnay D, Cserhalmi D, Kormoczi L, Gelleny K, Batori Z (2015) Vegetation history, recent dynamics and future prospects of a Hungarian sandy forest-steppe reserve: forest-grassland relations, tree species composition and size-class distribution. Commun Ecol 16(1):11. https://doi.org/10.1556/168.2015.16.1.11
Fairman TA, Bennett L, Tupper S, Nitschke CR (2017) Frequent wildfires erode tree persistence and alter stand structure and initial composition of a fire-tolerant sub-alpine forest. J Veg Sci 28(6):1151–1165. https://doi.org/10.1111/jvs.12575
February EC, Higgins SI, Bond WJ, Swemmeri L (2013) Influence of competition and rainfall manipulation on the growth responses of savanna trees and grasses. Ecology 94(5):1155–1164. https://doi.org/10.1890/12-0540.1
Ferreira AV, Bruna EM, Vasconcelos HL (2011) Seed predators limit plant recruitment in Neotropical savannas. Oikos 120(7):1013. https://doi.org/10.1111/j.1600-0706.2010.19052.x
Fisher AM, Harris SJ (1999) The dynamics of tree cover change in a rural Australian landscape. Landsc Urban Plan 45(4):193–207. https://doi.org/10.1016/S0169-2046(99)00054-7
Francisco MR, Lunardi VO, Guimarães PR, Galetti M (2008) Factors affecting seed predation of Eriotheca gracilipes (Bombacaceae) by parakeets in a cerrado fragment. Acta Oecol 33(2):240–245. https://doi.org/10.1016/j.actao.2007.11.002
Gent ML, Morgan JW (2007) Changes in the stand structure (1975–2000) of coastal Banksia forest in the long absence of fire. Austral Ecol 32:239–244
George AS (1998) Proteus in Australia. An overview of the current state of taxonomy of the Australian Proteaceae. Aust Syst Bot 11(4):257–266. https://doi.org/10.1071/SB98024
Glenn-Lewin DC, Peet RK, Veblen TT (1992) Plant succession : theory and prediction, 1st edn. Chapman & Hall, London
Gordon L, Dunlop M, Foran B (2003) Land cover change and water vapour flows: learning from Australia. Philos Trans 358(1440):1973–1984
Goverde M, Schweizer K, Baur B, Erhardt A (2002) Small-scale habitat fragmentation effects on pollinator behaviour: experimental evidence from the bumblebee Bombus veteranus on calcareous grasslands. Biol Conserv 104(3):293–299. https://doi.org/10.1016/S0006-3207(01)00194-X
Hackett DJ, Goldingay RL (2001) Pollination of Banksia spp. by non-flying mammals in north-eastern New South Wales. Aust J Bot 49(5):637–644. https://doi.org/10.1071/BT00004
Harper J, Williams J, Sagar G (1965) The behaviour of seeds in soil. I. The heterogeneity of soil surfaces and its role in determining the establishment of plants from seed. J Ecol 53(2):273–286. https://doi.org/10.2307/2257975
Hateley RF (2010) The Victorian bush: its ‘original and natural’ condition, South Melbourne, Vic., South Melbourne, Vic. : Polybractea Press
Hatfield RG, LeBuhn G (2007) Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biol Conserv 139(1):150–158. https://doi.org/10.1016/j.biocon.2007.06.019
Haverkamp C, Rann K, Prior L (2018) Differential demographic filtering by surface fires: how fuel type and fuel load affect sapling mortality of an obligate seeder Savanna tree. J Ecol 106(3):1010–1022. https://doi.org/10.1111/1365-2745.12819
Hazard J, Parsons RF (1977) Size-class analysis of coastal scrub and woodland, Western Port, southem Australia. Aust J Ecol 2(2):187–197. https://doi.org/10.1111/j.1442-9993.1977.tb01136.x
Henzler J, Weise H, Enright NJ, Zander S, Tietjen B (2018) A squeeze in the suitable fire interval: simulating the persistence of fire-killed plants in a Mediterranean-type ecosystem under drier conditions. Ecol Model 389:41–49. https://doi.org/10.1016/j.ecolmodel.2018.10.010
Heyes SD, Morgan JW, Sinclair SJ, Hoebee SE (2019) Effects of pre-dispersal seed predation on fruit crop and seed fitness in a highly fragmented savanna tree. Unpublished manuscript
Heyes SD, Sinclair SJ, Hoebee SE, Morgan JW (Unpublished raw data) Role of herbivory and grass competition on Banksia marginata seedling survival in a temperate Australian savanna
Hoffmann WA, Haridasan M (2008) The invasive grass, Melinis minutiflora, inhibits tree regeneration in a Neotropical savanna. Aust Ecol 33(1):29–36. https://doi.org/10.1111/j.1442-9993.2007.01787.x
Holdo R, Anderson T, Morrison T (2014) Precipitation, fire and demographic bottleneck dynamics in Serengeti tree populations. Landsc Ecol 29(9):1613–1623. https://doi.org/10.1007/s10980-014-0087-y
Holdo R, Brocato E (2015) Tree–grass competition varies across select savanna tree species: a potential role for rooting depth. Int J 216(4):577–588. https://doi.org/10.1007/s11258-015-0460-1
Holmes GD, James EA, Hoffmann AA (2008) Limitations to reproductive output and genetic rescue in populations of the rare Shrub Grevillea repens (Proteaceae). Ann Bot 102(6):1031–1041. https://doi.org/10.1093/aob/mcn195
Hooper SD (1980) Bird and Mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Aust J Bot 28(1):61–75. https://doi.org/10.1071/BT9800061
Hulme P (1996) Natural regeneration of yew (Taxus baccata L.): microsite, seed or herbivore limitation? J Ecol 84(6):853–861. https://doi.org/10.2307/2960557
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Retrieved from IPBES secretariat, Bonn, Germany
Johnston TR, Stock WD, Mawson PR (2016) Foraging by Carnaby's Black-Cockatoo in Banksia woodland on the Swan Coastal Plain, Western Australia. EMU Aust Ornithol 116(3):284–293. https://doi.org/10.1071/MU15080
Kirkpatrick JB, McDougall K, Hyde M (1995) Australia's most threatened ecosystem: the southeastern lowland native grasslands. Chipping Norton, N.S.W: Chipping Norton, N.S.W : Published by Surrey Beatty & Sons in association with the World Wide Fund for Nature Australia
Kolb A (2008) Habitat fragmentation reduces plant fitness by disturbing pollination and modifying response to herbivory. Biol Cons 141(10):2540–2549. https://doi.org/10.1016/j.biocon.2008.07.015
Krauss S, He T, Barrett L, Lamont B, Enright N, Miller B, Hanley M (2009) Contrasting impacts of pollen and seed dispersal on spatial genetic structure in the bird-pollinated Banksia hookeriana. Heredity 102(3):274–285. https://doi.org/10.1038/hdy.2008.118
Kurkjian HM, Carothers SK, Jules ES, Nunez M (2017) Seed predation has the potential to drive a rare plant to extinction. J Appl Ecol 54(3):862. https://doi.org/10.1111/1365-2664.12808
Lamont B, Enright N, Witkowski E, Groeneveld J (2007) Conservation biology of banksias: insights from natural history to simulation modelling. Aust J Bot 55(3):280–292. https://doi.org/10.1071/BT06024
Lamont B, Klinkhamer P, Witkowski E (1993) Population fragmentation may reduce fertility to zero in Banksia goodii - a demonstration of the Allee effect. Oecologia 94(3):446–450. https://doi.org/10.1007/BF00317122
Lamont BB, Enright NJ (2000) Adaptive advantages of aerial seed banks. Plant Species Biol 15(2):157–166. https://doi.org/10.1046/j.1442-1984.2000.00036.x
Lamont BB, He T, Downes KS (2013) Adaptive responses to directional trait selection in the Miocene enabled Cape proteas to colonize the savanna grasslands. Evol Ecol 27:1099–1115
Lem S, Onghena P, Verschaffel L, Van Dooren W (2013) On the misinterpretation of histograms and box plots. Educ Psychol 33(2):155–174. https://doi.org/10.1080/01443410.2012.674006
Levey DJ, Brudvig TTCLA, Haddad NM, Damschen EI, Tewksbury JJ, Evans DM (2016) Disentangling fragmentation effects on herbivory in understory plants of longleaf pine savanna. Ecology 97(9):2248–2258. https://doi.org/10.1002/ecy.1466
Liber C (2004) Landcare and Lone Silver Banksias. Assoc Soc Grow Aust Plants 9(1):2–3
Llorens TM, Byrne M, Yates CJ, Nistelberger HM, Coates DJ (2012) Evaluating the influence of different aspects of habitat fragmentation on mating patterns and pollen dispersal in the bird-pollinated Banksia sphaerocarpa var caesia. Mol Ecol 21(2):314–328. https://doi.org/10.1111/j.1365-294X.2011.05396.x
Lykke AM (1998) Assessment of species composition change in savanna vegetation by means of woody plants' size class distributions and local information. Biodivers Conserv 7:1261–1275
MacDicken KG, Wolf GV, Briscoe CB (1991) Standard research methods for multipurpose trees and shrubs. Arlington, Virginia: Winrock International Institute for Agricultural Development, Forestry/Fuelwood Research and Development Project (F/FRED)
MacDougall AS, Duwyn A, Jones NT (2010) Consumer-based limitations drive oak recruitment failure. Ecology 91(7):2092–2099. https://doi.org/10.1890/09-0204.1
Manning AD, Fischer J, Lindenmayer DB (2006) Scattered trees are keystone structures: implications for conservation. Biol Cons 132(3):311–321. https://doi.org/10.1016/j.biocon.2006.04.023
Miller AD, Nitschke C, Weeks AR, Weatherly WL, Heyes SD, Sinclair SJ, Holland OJ, Stevenson A, Broadhurst LM, Hoebee SE, Sherman CDH, Morgan JW (2020) Genetic data and climate niche suitability models highlight the vulnerability of a functionally important plant species from south-eastern Australia. Evolut Appl. https://doi.org/10.1111/eva.12958
Mogoutnov A, Venning J (2014) Remnant tree decline in agricultural regions of South Australia. Pac Consev Biol 20(4):366–375. https://doi.org/10.1071/PC140366
Montgomery BR, Kelly D, Robertson AW, Ladley JJ (2003) Pollinator behaviour, not increased resources, boosts seed set on forest edges in a New Zealand Loranthaceous mistletoe. N Z J Bot 41(2):277–286. https://doi.org/10.1080/0028825X.2003.9512846
Morgan JW (1995) Ecological studies of the endangered Rutidosis leptorrhynchoides. I. Seed production, soil seed bank dynamics, population density and their effects on recruitment. Aust J Bot 43(1):1–11
Morgan JW, Meyer MJ, Young AG (2013) Severe habitat fragmentation leads to declines in genetic variation, mate availability, and reproductive success in small populations of a once-common Australian grassland daisy. Int J Plant Sci 174(9):1209–1218. https://doi.org/10.1086/673242
Morrison TA, Holdo RM, Rugemalila DM, Nzunda M, Anderson TM (2018) Grass competition overwhelms effects of herbivores and precipitation on early tree establishment in Serengeti. J Ecol 107(1):216–228. https://doi.org/10.1111/1365-2745.13010
Naccarella A, Morgan JW, Cutler SC, Venn SE (2019) Alpine treeline ecotone stasis in the face of recent climate change and disturbance by fire. PLoS ONE 15(4):E0231339
Nayak KG, Davidar P (2010) Pollinator limitation and the effect of breeding systems on plant reproduction in forest fragments. Acta Oecol 36(2):191–196. https://doi.org/10.1016/j.actao.2009.12.004
Neave HM, Tanton MT (1989) The effects of grazing by kangaroos and rabbits on the vegetation and the habitat of other fauna in the Tidbinbilla Nature Reserve, Australian Capital Territory. Wildl Res 16(3):337–351. https://doi.org/10.1071/WR9890337
Nicholson M (1855) Township, suburban & country allotments at Framlingham at McWilliam's Inn, Hopkins River
Pellegrino G (2014) Pollinator limitation on reproductive success in Iris tuberosa. AoB Plants 7:1–9. https://doi.org/10.1093/aobpla/plu089
Peter F, Berens DG, Grieve GR, Farwig N (2015) Forest fragmentation drives the loss of insectivorous birds and an associated increase in herbivory. Biotropica 47(5):626–635. https://doi.org/10.1111/btp.12239
Peterson D, Reich P (2001) Prescribed fire in oak savanna: fire frequency effects on stand structure and dynamics. Ecol Appl 11(3):914–927. https://doi.org/10.1890/1051-0761(2001)011[0914:PFIOSF]2.0.CO2
Price JN, Morgan JW (2003) Mechanisms controlling establishment of the non-bradysporous Banksia integrifolia (Coast Banksia) in an unburnt coastal woodland. Aust Ecol 28(1):82–92
R Core Team (2018) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
Ramsey M, Vaughton G (1991) Self-incompatibility, protandry, pollen production and pollen longevity in Banksia menziesii. Aust J Bot 39(5):497–504. https://doi.org/10.1071/BT9910497
Riginos C (2009) Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90(2):335–340. https://doi.org/10.1890/08-0462.1
RStudio Team (2016) RStudio: integrated development for R. Boston, MA: RStudio, Inc. https://www.rstudio.com/
Salk TT, Frelich LE, Sugita S, Calcote R, Ferrari JB, Montgomery RA (2011) Poor recruitment is changing the structure and species composition of an old-growth hemlock-hardwood forest. For Ecol Manag 261(11):1998–2001
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS et al (2005) Determinants of woody cover in African savannas. Nature 438(7069):846–849. https://doi.org/10.1038/nature04070
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer, New York
Scholes RJ, Archer SR (1997) Tree-grass interactions in savannas. Annu Rev Ecol Syst 28:517–544
Scott JK, Black R (1981) Selective predation by White-tailed Black-cockatoos on fruit of Banksia Attenuata containing the seed-eating weevil Alphitopis Nivea. Wildl Res 8(2):421–430. https://doi.org/10.1071/WR9810421
Sinclair SJ, Atchison K (2012) The pre-colonial distribution of grasslands, woodlands and forests on the Werribee plains Victoria. Cunninghamia 12(3):213–227
Specht RL, Rayson P (1957) Dark Island heath (Ninety-mile Plain, South Australia). III. The root systems. Aust J Bot 5(1):103–114. https://doi.org/10.1071/BT9570103
Steel EJ, Fontaine JB, Ruthrof KX, Burgess TI, Hardy GESJ (2019) Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave. Aust Ecol 44(8):1438–1450. https://doi.org/10.1111/aec.12818
Stewart J, Salazar R (1992) A review of measurement options for multipurpose trees. Int J Incorp Agrofor Forum 19(2):173–183. https://doi.org/10.1007/BF00138507
Stuwe J, Parsons RF (1977) Themeda australis grasslands on the Basalt Plains, Victoria: floristics and management effects. Aust J Ecol 2(4):467–476. https://doi.org/10.1111/j.1442-9993.1977.tb01162.x
Swan E (2017) A bright future for Silver Banksias. https://victorianvolcanicplainscmn.wordpress.com/2017/02/27/a-bright-future-for-silver-banksias/
Taylor A (1988) The Banksia Atlas. Australian Government Publishing Service, Canberra
Thavornkanlapachai R, Ladd PG, Byrne M (2018) Population density and size influence pollen dispersal pattern and mating system of the predominantly outcrossed Banksia nivea (Proteaceae) in a threatened ecological community. Biol J Lin Soc 124(3):492–503. https://doi.org/10.1093/biolinnean/bly050
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371(6492):65–66. https://doi.org/10.1038/371065a0
Tolera M, Sass-Klaassen U, Eshete A, Bongers F, Sterck FJ (2013) Frankincense tree recruitment failed over the past half century. For Ecol Manag 304:65–72. https://doi.org/10.1016/j.foreco.2013.04.036
Vadigi S, Ward D (2013) Shade, nutrients, and grass competition are important for tree sapling establishment in a humid savanna. Ecosphere 4(11):1–27. https://doi.org/10.1890/ES13-00239.1
Valentine LE, Fisher R, Wilson BA, Sonneman T, Stock WD, Fleming PA, Hobbs RJ (2014) Time since fire influences food resources for an endangered species, Carnaby’s cockatoo, in a fire-prone landscape. Biol Conserv 175:1–9. https://doi.org/10.1016/j.biocon.2014.04.006
Villaseñor-Sánchez EI, Dirzo R, Renton K (2010) Importance of the lilac-crowned parrot in pre-dispersal seed predation of Astronium graveolens in a Mexican tropical dry forest. J Trop Ecol 26(2):227–236. https://doi.org/10.1017/S0266467409990447
Webster C (1858) Country lands in the parishes of Livingstone and Brewster, County of Ripon
Westemeier RL, Brawn JD, Simpson SA, Esker TL, Jansen RW, Walk JW, Kershner EL, Bouzat JL, Paige KN (1998) Tracking the long-term decline and recovery of an isolated population. Science 282(5394):1695–1698
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Witkowski ETF, Lamont BB, Connell SJ (1991) Seed bank dynamics of three co-occurring Banksias in south coastal Western Australia: the role of plant age, cockatoos, senescence and interfire establishment. Aust J Bot 39(4):385–397. https://doi.org/10.1071/BT9910385
Yates CJ, Hobbs RJ (1997) Temperate Eucalypt woodlands: a review of their status, processes threatening their persistence and techniques for restoration. Aust J Bot 45(6):949–973. https://doi.org/10.1071/BT96091
Zammit C, Westoby M (1987) Population structure and reproductive status of two Banksia shrubs at various times after fire. Vegetatio 70(1):11–20. https://doi.org/10.1007/BF00040753
Acknowledgements
This study was funded by the Hamilton branch of the Australian Native Plants Society (APS) and supported by the Friends of the Forgotten Woodlands and Glenelg Hopkins Catchment Management Authority. We thank Dan Frost (Ballarat Region Seed Bank) for his time and to the volunteers who helped us in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Neal J. Enright.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heyes, S.D., Sinclair, S.J., Hoebee, S.E. et al. How widespread are recruitment bottlenecks in fragmented populations of the savanna tree Banksia marginata (Proteaceae)?. Plant Ecol 221, 545–557 (2020). https://doi.org/10.1007/s11258-020-01033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01033-0