Abstract
Plant species occupy distinct zones on coastal dunes, but the mechanisms limiting their distributions have not been fully explained. We combined field surveys of plant distributions and abiotic conditions with controlled germination experiments to assess the contribution of germination requirements to plant zonation. Species presence and abiotic conditions were measured in ten transects across the barrier dune at Waquoit Bay, Massachusetts. Germinating seeds of six species were exposed to four fully crossed treatments: pre-treatment (soaked in fresh water, soaked in salt water, or not soaked), temperature (low, moderate, or high), soil salinity (none, moderate, or high), and light (full light, shade, dark). Species distributions in the field were affected by both distance from the shore and presence of dominant shrubs. Germination tolerance of soil salinity reflected species zonation: species found on the front slope of the dune tolerated salinity, while germination of other species was limited by salinity alone or by salinity in combination with high temperature. Shrubs reduced soil surface temperature and decreased light, but these conditions had limited effects on germination. These results indicate that limitations to germination can contribute to explaining species distributions on coastal dunes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant communities on coastal sand dunes exhibit striking zonation patterns which have intrigued ecologists since Cowles (1899) first described the vegetation of Lake Michigan dunes. Despite a long history of investigations (Cowles 1899; Oosting and Billings 1942; van der Valk 1974; Pemadasa and Lovell 1975; Sykes and Wilson 1983; Ishikawa et al. 1995), the processes that lead to vegetation zonation on dunes are still not fully understood (Maun and Perumal 1999; Wilson and Sykes 1999). Understanding the limitations on species distributions within the dune system is becoming more urgent as dunes are subjected to changes in temperature and storm regimes due to climate change (Seabloom et al. 2013; Prisco et al. 2013; Mendoza-González et al. 2013), invasion by non-native species (Magnoli et al. 2013; Novoa and González 2014; Vecchio et al. 2015), and human disturbances related to recreational use (Santoro et al. 2012; Ciccarelli 2014).
Plants that germinate, establish and grow on coastal dunes experience a unique set of stresses, including resource limitation, high substrate temperature, exposure to salinity, and burial by shifting sand (Oosting and Billings 1942). These stresses vary with distance from the shoreline, geography of the dune, and presence of foundation species. Conditions are harshest on the ocean-facing front slope of the dune, where levels of salt spray and soil salinity are highest (Rajaniemi and Allison 2009) and the movement of sand is greatest (Rajaniemi and Allison unpublished data). Stresses are ameliorated under shrubs, where soil temperatures and salt spray are reduced (Shumway 2000; Rajaniemi and Allison 2009). Previous research has focused on understanding how tolerance of seedlings and adult plants to abiotic stress correlates with observed distributions, while the role of regeneration from seed has received less attention.
Various studies have found that growth of seedlings and of established plants on coastal dunes may be limited by salt spray (Barbour and DeJong 1977; Sykes and Wilson 1989) and burial (Maun and Lapierre 1986; Sykes and Wilson 1990; Brown 1997). In addition, growth is enhanced under shrubs due to increased nutrients and decreased water stress (Shumway 2000). Growth responses to these physical stresses can contribute to patterns of vegetation zonation (Oosting and Billings 1942; Barbour and DeJong 1977; Maun and Perumal 1999; Wilson and Sykes 1999), but do not fully explain species distributions (Maun and Perumal 1999; Wilson and Sykes 1999).
Germination requirements may also shape species distributions across the dune environment. Like growth rates, germination rates of dune species are influenced by salinity (Mariko et al. 1992; Martinez et al. 1992) and burial (Pemadasa and Lovell 1975; Maun and Lapierre 1986). Light and temperature, which are reduced under shrubs, also affect germination of dune species (Pemadasa and Lovell 1975; Martinez et al. 1992). Previous studies have either investigated germination requirements of species from similar micro-environments within the dune (Pemadasa and Lovell 1975; Maun and Lapierre 1986), or compared dune species to species from other habitats (Mariko et al. 1992; Martinez et al. 1992). The contribution of germination to zonation of species within a dune system has not been assessed. Germination requirements may help to further explain species distributions if germination is more senstive than growth to environmental stress, or if germination is limited by different stressors than growth.
Here, we first summarize the results of a four-year survey to measure abiotic conditions and establish the positions occupied by species on the dune system at Waquoit Bay Estuarine Research Reserve. We then describe an experiment testing the germination tolerances of six species. We hypothesize that the realized distributions of species are limited by the ability of their seeds to germinate under physical stress. Specifically, we predict that (1) species found at the dune front are able to germinate when exposed to high temperatures and soil salinity, and require light to germinate and (2) that species found in association with shrubs are less tolerant of high temperature and salinity, and are able to germinate in shade.
Methods
Study site and species
Field work was performed at Waquoit Bay National Research Reserve in Falmouth, MA, 41°34′53.24″N, 70°31′30.22″W. This system consists of a barrier beach separating Waquoit Bay and salt ponds east of the bay from the Atlantic Ocean at Vineyard Sound. The barrier beach is widest (175 m) at its western end, where it ends at Waquoit Bay, and narrowest (65 m) at its eastern end, where it transitions to salt ponds. A single, low dune crest, generally less than 2 m high, extends along the southern edge of the beach.
Ammophila breviligulata Fernald (American beachgrass) dominates the front slope of the dune, facing the ocean. Rosa rugosa Thunb. (rugosa rose) is abundant on the back slope of the dune. The flat expanse behind the dune is sparsely vegetated except for scattered shrubs including Rosa rugosa and Morella pensylvanica (Mirb.) Kartesz (northern bayberry).
Germination was studied for six dune species. Two species were annual forbs: Cakile edentula (Bigelow) Hook. (American searocket) and Arenaria serpyllifolia L. (thyme-leaf sandwort, non-native). Three species were biennial or perennial forbs: Solidago sempervirens L. (seaside goldenrod, perennial), Oenothera parviflora L. (northern evening primrose, biennial), and Artemisia campestris L. (field sagewort, biennial or perennial). The final species was Rosa rugosa, a non-native shrub.
Nomenclature follows the USDA Plants Database (USDA, NRCS 2018). We refer to species henceforth by genus.
Field survey
In 2005, ten transects were established at 200-m intervals across the beach, perpendicular to the shoreline. Each transect had its starting point within one meter of the dune crest. Plots measuring 1 m × 1 m were placed along each transect every 10 m, in both directions. Plots were added towards the shore until the transect reached the high tide line and no vegetation was present (never more than one additional plot per transect), and away from the shore until the transect reached the salt pond or the bay, or reached a maximum of 200 m from the starting point. Each plot was visited once in June or July in 2006, 2007, 2008, and 2009. Percent cover of each plant species in each plot was estimated visually.
To determine species distributions, plots were categorized as belonging to one of three zones: the dune front (at the dune crest, or on the beach side of the dune), the dune back (1–21 m from the crest, on the salt pond/bay side of the dune), or the dune flat (29 m or more from the crest). Each plot was further designated as having high or low vegetation cover (greater than or less than 30% cover of Ammophila, Rosa, or Morella).
Soil salinity, soil temperature, and light in transect plots were measured in July 2007, with each variable measured on a single day to avoid variation due to weather conditions. A single soil core measuring 1.7 cm in diameter and 30 cm deep was collected from the center of each plot. Soil salinity was quantified by adding 100 ml of deionized water to 20 mg of field-moist soil, shaking for 30 min, and measuring conductivity of the solution after allowing the soil to settle (Rowell 1994). Soil temperature was measured at the soil surface and with a 30 cm probe within 2 h of solar noon. Photosynthetically active radiation was measured with a Decagon Sunfleck Ceptometer (Decagon, Pullman, Washington, USA), which integrates PAR at 1-cm intervals along a 40 cm wand. PAR was measured within two hours of solar noon. Measurements were taken just above the soil surface and again above all vegetation, and the percentage of light reaching the soil surface was calculated.
To gauge the potential for burial of seeds, sand movement was quantified by driving a bamboo pole into the soil in each plot. The length of the exposed portion of the pole was measured in September 2006 and again in September 2007. A decrease in length indicated sand accumulation, while an increase indicated sand removal. The absolute value of the change was calculated as a measure of sand movement.
Germination experiment
Mature seeds were collected in late October 2009 and October and November 2010 at Waquoit Bay and stored at 4 °C for at least three months. All seeds were surface-sterilized in 8% bleach solution for 10 min and sown in 2 oz, lidded dishes containing 15 mL of sterilized sand. In each dish, ten seeds of a single species were pressed into the sand till just buried. Sets of 10 dishes of the same species were placed in plastic zipper-sealed bags to reduce evaporation. Each bag was assigned to a combination of pre-treatment, soil salinity, temperature, and light treatments.
Before planting, one third of the seeds were soaked in artificial seawater (Instant Ocean, Spectrum Brands, Blacksburg, VA, USA) for 18 h. Another third of the seeds were soaked in fresh water for 18 h. After 18 h, seeds were rinsed with de-ionized water and immediately planted in dishes. Soaking was intended to mimic the effect of inundation of the soil with either ocean water or rainwater during storms. The remaining seeds were not soaked and acted as a control.
Soil salinity treatments were applied at the time of planting by adding 5 ml of deionized water (no salinity), 5 ml of artificial sea water (high salinity), or 2.5 ml of artificial sea water mixed with 2.5 ml of deionized water (moderate salinity) to each dish. Soil salinity, when measured by the method applied to field samples, was 0.79 ppt in the moderate salinity treatment and 1.58 ppt in the high salinity treatment. The artificial seawater (Instant Ocean) contained no nitrogen or phosphorus and only low concentrations of potassium and calcium (400 and 420 ppm respectively) and so did not supply additional macronutrients to the dishes. Dishes remained moist and were not watered again for the duration of the experiment.
Dishes were maintained at one of three temperatures: a low temperature of 10 °C, a moderate temperature of 20–26 °C and a high temperature of 32 °C. The low and high temperatures were achieved by incubating dishes in growth chambers. Because space in growth chambers was limited, the moderate temperature treatment was accomplished by placing bagged dishes in a germination room at ambient temperature. The room temperature was constantly monitored, and use of the room was discontinued once the temperature rose above the target range.
Finally, dishes were exposed to one of three light treatments. All dishes were placed approximately 25 cm from fluorescent lighting. Dishes in the full light treatment were exposed to light on a 12/12 h light/dark cycle. Dishes in the shade treatment were covered with 40% shadecloth to mimic the light conditions under shrubs in the field. Dishes in the dark treatment were wrapped in aluminum foil to simulate the effects of seed burial.
Dishes were incubated for 15 days. Dishes were checked every other day for germination, which was defined as the emergence of a radicle, hypocotyl, or cotyledons. Germinated seeds were counted and removed. All bags were rotated approximately every four days.
Treatments were applied in a fully crossed design with two bags of ten dishes per treatment combination per species, for a total of 1620 dishes per species, except that Arenaria was not grown at the low temperature because there were not enough seeds available. Due to limited incubator space, it was necessary to perform the experiment in batches. Oenothera was planted in January 2011, Arenaria and Cakile in March, and Solidago in May. Artemisia in the low temperature treatment was planted in March, and in the moderate and high temperatures in August. At that time, the temperature of the germination room exceeded 26°, so the seeds in the moderate temperature treatment were kept in incubators at 23 °C.
Data analysis
All statistical analyses were conducted using R statistical software, version 3.4.3 (R Core Team 2017).
The objective for use of the cover data was to determine which microenvironments each species is able to establish and grow in. Therefore, species distributions were characterized based on presence or absence over all four survey years. A species was considered present in a plot if it was observed in the plot at least once. Frequency of presence of each species in each zone, with high and low vegetation cover, was calculated. For species with sufficient data, logistic regression was used to test whether presence/absence was dependent on zone or vegetation cover. No dune front plots had high cover, so interactions were excluded from the model. For Rosa, which made up the vegetation cover on the dune back, only zone was tested.
Two-way ANOVA was used to test for effects of zone and vegetation cover on each of the abiotic variables. Tukey post hoc tests were applied when the effect of zone was significant. Soil salinity, soil temperature, and sand movement were natural-log transformed to improve normality and homogeneity of variance, and percent light at the soil surface was arcsine-square root transformed. Mean values of abiotic conditions in the three dune zones were compared to treatment levels in the germination experiment.
In the analysis of the germination experiment, individual dishes, rather than bags of dishes, were treated as replicates, despite not being fully randomized. Conditions within the bags were expected to be identical, and we saw no evidence of differences such as drying or fungal growth affecting individual bags. Per-seed germination rates were low. Of the dishes with germination, 58% had only one germinating seed and only 6% had four or more germinating seeds. We therefore consider whether germination was observed in a dish of ten seeds rather than analyzing germination percentage within a dish.
The effects of treatments and their two-way interactions were tested with logistic regression, with the binary outcome of germination or no germination for each dish. Each species was analyzed separately. Treatment levels were excluded from analysis if two or fewer dishes (of 360 for Arenaria or 540 for other species) had germinating seeds.
Our interpretation of results focuses on observing the treatments or treatment combinations under which each species is able to germinate. Therefore, we categorize treatments as resulting in no germination, minimal germination (two or fewer dishes, of 360 for Arenaria or 540 for other species, had germinating seeds), or some germination. Treatment levels with no germination or minimal germination are discussed but were excluded from regression models.
Results
Field survey
Transects included a total of 100 plots. Cakile did not occur in transect plots, but was observed occasionally near the high tide line, in plots that were otherwise unvegetated. The placement of plots along our transects usually missed sampling in the narrow zone where Cakile occurred. Solidago was seen in at least one year in a total of 42 plots, in all three zones (Fig. 1). It occurred most frequently at the dune front (effect of zone on presence/absence, \(\chi_{2}^{2}\) = 11.93, P = 0.003), and was found more often in plots with high shrub cover (effect of vegetation, \(\chi_{1}^{2}\) = 3.88, P = 0.049). Artemisia was found in 71 plots and in all three zones (Fig. 1). It was least common at the dune front (\(\chi_{2}^{2}\) = 44.54, P < 0.001) and was found more often in plots with low shrub cover (\(\chi_{1}^{2}\) = 29.64, P = < 0.001). Rosa occurred in 17 plots in all three zones (\(\chi_{2}^{2}\) = 0.47, P = 0.790; Fig. 1). Oenothera and Arenaria were found only in the dune flat (Fig. 1), and both occurred infrequently (10 and 4 plots, respectively). Both occurred in plots with low and high shrub cover.
Frequency of occurrence of species in zones. Transect plots were categorized into zones based on position relative to the dune crest, and dune back and dune flat plots were further characterized as having low (< 30%) or high (≥ 30%) cover of the dominant shrub. A species was considered present in a plot if it was observed at least once in four years. See methods for details
Soil salinity was greater in dune front plots than in dune back or flat (F2,96 = 17.74, P < 0.001; Fig. 2a) and was not affected by vegetation cover (F1,96 = 0.42, P = 0.508). The moderate salinity treatment in the germination experiment was close to the mean value for dune front soil, while the high salinity treatment was about 0.5 standard deviations above the maximum observed salinity (Fig. 2a).
Abiotic conditions in field plots (mean + SEM) and in experimental treatments (dashed lines). a Soil salinity differed significantly among dune zones. Stars in A indicate maximum observed values in the field. Upper and lower dashed lines are values for high and moderate salinity treatments in the germination experiment. b Soil temperature differed significantly between plots with low and high shrub cover. Upper and lower dashed lines are values for high and moderate temperature treatments in the germination experiment. c Light at the soil surface differed significantly between plots with low and high shrub cover. The dashed line is the value for the shade treatment. d Sand movement differed significantly among dune zones
Soil temperature was reduced in plots with high vegetation cover (for surface temperature F1,97 = 36.44, P < 0.001; for temperature to 30 cm depth, F1,97 = 19.41, P < 0.001; Fig. 2b) but did not differ significantly among zones (F2,97 = 0.47, P = 0.627 and F1,97 = 0.01, P = 0.987). In the germination experiment, the high temperature treatment of 32 °C was similar to mean surface temperature in plots with high vegetation cover, while moderate temperature treatment of 20–26 °C was typical of soil temperature below the surface (Fig. 2b).
Light at the soil surface was reduced by high vegetation cover (F1,97 = 111.57, P < 0.001; Fig. 2c). The effect of zone on light was also significant (F2,97 = 3.23, P = 0.0413), but was due to variation in plant cover among zones. Zone was not significant when total cover was added to the model. The shaded treatment of 40% shade cloth in the germination experiment was typical of the level of shade found under vegetation in the field (Fig. 2c).
Sand movement was greater at the dune front than in the dune back or flat (F2,97 = 22.53, P < 0.001; Fig. 2d) and was not affected by vegetation cover (F1,97 = 1.88, P = 0.174).
Germination experiment
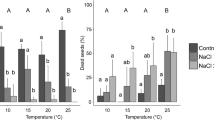
Cakile germination occurred in all three soil salinity treatments, although germination was reduced by moderate salinity and further reduced by high salinity (Fig. 2). Only one seed germinated at low temperature, so this treatment was not included in analysis. Germination was best at moderate temperature, and reduced at high temperature, particularly in combination with soil salinity (salinity × temperature interaction, \(\chi_{2}^{2}\) = 7.72, P = 0.021). Germination was light-dependent, with only one seed germinating in the dark (treatment not analyzed). Shade reduced germination when combined with soil salinity (salinity × light interaction, \(\chi_{2}^{2}\) = 12.38, P = 0.002). Pre-treatment by soaking seeds in fresh water or salt water had no significant effects (pre-treatment effect and interactions, P > 0.05).
Solidago had only two germinants at high salinity and one at low temperature (Fig. 2); these treatment levels were not included in the analysis. Germination was reduced in moderate salinity and at high temperature (temperature effect, \(\chi_{1}^{2}\) = 32.24, P < 0.001). Germination was light-dependent, with only one seed germinating in the dark (not included in analysis). Shade had no effect in the absence of soil salinity, but improved germination with moderate salinity (salinity × light interaction, \(\chi_{1}^{2}\) = 12.14, P < 0.001). Pre-treatment had no effects on germination (P > 0.05).
Rosa never germinated with high soil salinity and rarely germinated with moderate soil salinity (2 dishes, 1 seed each; Fig. 3). The effect of salinity was therefore not analyzed. Rosa germination was best at low temperature and reduced at moderate and high temperature, especially after pre-treatment by soaking in salt water (temperature × pre-treatment interaction, \(\chi_{4}^{2}\) = 29.91, P < 0.001). Germination was not affected by light level (P > 0.05).
Artemisia had only one seed germinate at high salinity and one at low temperature (Fig. 3; treatment levels not analyzed). The effects of salinity, temperature, and light were all dependent on pre-treatment. Germination at moderate salinity was reduced, especially after soaking in salt water (salinity × pre-treatment interaction, χ2 = 28.80, df = 2, P < 0.001). Seeds germinated at high temperature only after soaking in either fresh water or salt water (temperature × pre-treatment interaction, \(\chi_{2}^{2}\) = 25.69, P < 0.001). Seeds that were not soaked had reduced germination in the dark, while seeds soaked in fresh water had reduced germination in full sun or shade (light × pre-treatment interaction, \(\chi_{4}^{2}\) = 23.42, P < 0.001).
Oenothera germination was rare with any amount of soil salinity (1 seed; Fig. 3), so the effect of salinity was not analyzed. Its germination was very low at low temperature and reduced at high temperature, especially following pre-treatment by soaking in salt water (temperature × pre-treatment interaction, \(\chi_{4}^{2}\) = 11.33, P = 0.023). Germination was reduced in the dark, but the effect was not significant (P > 0.05).
Arenaria was not tested at low temperature and did not germinate with high soil salinity (Fig. 3; treatment levels not analyzed). It germinated with moderate soil salinity only at moderate temperature (salinity × temperature interaction, \(\chi_{1}^{2}\) = 7.02, P = 0.008) and with full light (salinity × light interaction, \(\chi_{2}^{2}\) = 29.78, P < 0.001). Without soil salinity, temperature and light had minimal effects on germination, although pre-treated seeds did not germinate in the combination of high temperature and dark (\(\chi_{2}^{2}\) = 9.09, P = 0.011).
Discussion
Consistent with our first prediction, the species that occur at the dune front, Cakile and Solidago, are able to germinate when exposed to high levels of soil salinity, while the combined stresses of soaking in salt water, soil salinity, and high temperatures exclude other species from the dune front. We found less support for our second prediction. The conditions associated with shrubs did not have differential effects on germination.
Salinity
Seeds at the dune front are exposed to high soil salinity and may experience inundation with salt water during storms. Species found at the dune front were expected to germinate with soil salinity and after soaking in salt water, while exposure to salinity was expected to exclude other species from that area.
Two species, Rosa and Oenothera, were unable to germinate with soil salinity typical of the dune front (moderate salinity treatment). These species did not occur at the dune front. Artemisia, which was found infrequently on the dune front, and Arenaria, which was not observed on the dune front, also germinated in moderate soil salinity, but these species were unable to tolerate the combination of soaking in salt water and soil salinity. The dune front species, Cakile and Solidago, germinated with soil salinity and were unaffected by soaking in saltwater. Cakile also germinated at low rates in the high soil salinity treatment, which was higher than salinity observed in field plots. Therefore, germination responses to salinity appear to allow Cakile and Solidago to inhabit the dune front and limit Artemisia and Arenaria in that environment, while excluding Rosa and Oenothera.
Previous studies have also shown that germination of Cakile and Solidago is tolerant of salinity (Barbour 1970; Orava and Drake 1997; Debez et al. 2004). In a comparison of species from a range of habitats from salt marshes and dunes to inland sites, germination tolerance to salinity correlated with salinity of the habitat where a species occurred (Mariko et al. 1992).
The ability of seedlings to tolerate salinity is also important in determining species distributions within coastal dunes. Growth of Cakile is unaffected by salt spray and soil salinity (Barbour 1970), and may be enhanced by low levels of soil salinity (Debez et al. 2004). Seedlings of Solidago are also tolerant of salt spray (van der Valk 1974). In one study of 12 dune species, tolerance of seedlings to both inundation of the soil and aboveground salt spray was correlated with species’ typical position on the dune (Barbour and DeJong 1977). In another study of 29 species, responses of shoot growth to aboveground salt spray did not correlate with root growth responses to soil salinity, and seedling salinity tolerance only partially explained species distributions (Sykes and Wilson 1988).
Temperature
All species were able to germinate at the high temperatures found at the soil surface in unshaded areas, although with reduced success. However, the combination of high temperature and salinity greatly reduced germination of Cakile and Solidago and prevented germination of the remaining species. High temperature following soaking in salt water also reduced germination in Oenothera and Rosa. Soil temperature may further contribute to the exclusion of the less salt-tolerant species from the dune front, and even tolerant species may rely on some amelioration of stress, such as shading of the soil by vegetation, cooler spring temperatures, or cooling on a cloudy day, to germinate.
Rosa germinated most successfully at the low temperature, and was the only species to germinate well at that temperature. This species may be able to germinate earlier in spring than the other species.
Temperature effects on dune species have not been extensively studied. A set of eight dune winter annuals was tested for the ability to germinate at temperatures ranging from 5 to 25 °C. These species germinated in the fall in the field. All the species germinated at all temperatures, although germination for some species was delayed by high temperatures (Pemadasa and Lovell 1975). In another study, four out of ten dune species required diurnal temperature fluctuations to germinate (Martinez et al. 1992).
Light
We expected germination of the dune front species to be light-sensitive. These species are exposed to shifting sands and are likely to experience burial. In our experiment, neither Cakile nor Solidago germinated in the dark. Previous studies, on the other hand, show that Cakile is able to germinate following burial and even that light inhibits germination (Barbour 1970). Cakile seeds planted at depths up to 12 cm successfully germinated and emerged (Maun and Lapierre 1986). The average emergence depth of Cakile in the field was 3.84 cm, and a few seedlings were observed to have emerged from depths greater than 7 cm (Maun and Lapierre 1986). Germination may have been delayed in the dark, as was observed for other dune annuals (Pemadasa and Lovell 1975), such that our experiment was not continued long enough to observe germination.
Germination was not light-dependent for the other species. Germination was reduced in the dark for Oenothera, enhanced for Arenaria, and unaffected for Rosa and Artemisia. Lack of light may not be a sufficient cue to signal burial and prevent germination following burial at the dune front. Low oxygen or reduced gas exchange may be important for inhibiting germination at depth (Benvenuti and Macchia 2006, 2010). Alternatively, seedlings may germinate at depth but fail to emerge (van der Valk 1974).
Variation in tolerance of seedlings to burial is an important contributor to zonation on lacustrine dunes (Maun and Perumal 1999), although its importance on coastal dunes varies (Sykes and Wilson 1990; Wilson and Sykes 1999). Van der Valk (1974) concluded that burial was an important limitation to both germination and seedling survival among six dune annuals, including Solidago.
Most of the species tested can be found growing under shrubs (Fig. 1), and their germination was shade-tolerant. Seedlings of Solidago and Ammophila growing beneath Northern bayberry shrubs grew larger and produced more flowers, due to the combination of shade, which lowers temperature and reduces water stress, and increased nutrient availability (Shumway 2000). We expected that Rosa might require full light to germinate, to avoid germination of seeds under already-established adults, but Rosa seeds were also tolerant of shade. However, our shadecloth treatment did not produce a shift in red:far-red ratio, which is an important signal for germination in low light (Pons 2000). Seeds of Arenaria have been shown to germinate in the dark but not under the shade of green leaves (King 1975).
Soaking of seeds
Soaking the seeds before germination did not have strong main effects, but sometimes helped or hindered germination when other stresses were present. For example, soaking in salt water prevented germination at moderate salinity in Artemisia. This species appeared to have limited tolerance for osmotic stress, which was exceeded by the combination of the two treatments.
Surprisingly, soaking in either seawater or fresh water allowed Artemisia to germinate at high temperature, but reduced germination at high temperature for Rosa and Oenothera. These combinations of conditions are unlikely to be relevant in the field, as saturated soils would generally be cooler. Overall, exposure of seeds to substrates saturated either by sea water or by rain probably has little direct effect on germination. One study of overwash due to storms found the effect on the plant community varied with timing of the storm and life history of the plants affected. Three annuals and the perennial Solidago were able to produce seeds following an August storm and germinate in spring, whereas no spring germination was observed for two of the annuals following an October storm (Cheplick 2017). In that study, storms did not directly interfere with germination but disrupted seed production and buried or removed seeds.
Conclusion
The distribution of species across the dune gradient may be partially determined by germination responses to physical conditions. In particular, high salinity in combination with high temperature and burial by shifting sand limits which species are able to germinate at the dune front and which are restricted to the dune back and flat. Previous studies indicate that these same conditions also generally determine growth and survival of any seedlings that do germinate. Growth and survival of Cakile and Solidago have been shown to tolerate salt spray (Barbour 1970; Cartica and Quinn 1980; Orava and Drake 1997; Lonard et al. 2015). Several of our own experiments have shown that high levels of salt spray greatly reduce survival of Rosa seedlings and survival and growth of Oenothera seedlings, but that survival and growth of Artemisia seedlings are surprisingly unaffected (unpublished data). Given that Artemisia seeds are abundant in the seedbank at the dune front (Messina and Rajaniemi 2011), germination requirements may be an important limitation for the distribution of this species.
Germination responses to burial and salinity may become more important limitations as climate change produces stronger storms, greater overwashing by waves, and more sand deposition (Schlacher et al. 2008; Frosini et al. 2012; Seabloom et al. 2013; Cheplick 2017).
The presence of shrubs, which alter physical conditions, had modest effects on relative frequencies of some species. We found no evidence that the conditions associated with shrubs had differential effects on germination, although our experiment did not consider cues such as red:far-red ratio. Shrubs acting as nurse plants facilitative effects on growth and survival in stressful environments (Filazzola and Lortie 2014), including coastal dunes (Shumway 2000; de Castanho and Prado 2014). Dune shrubs also support a different soil microbial community than is found in open areas of the dune (Rajaniemi and Allison 2009), which may influence plant growth and establishment.
Invasive species similarly have the potential to alter environmental conditions in ways that might enhance or hinder germination. For example, the succulent Carpobrotus edulis is a frequent invader of Mediterranean dunes, where it alters soil pH and organic matter content and negatively affects germination, growth, and survival of native species (Conser and Connor 2009; Novoa et al. 2013; Novoa and González 2014). The influence of dominant vegetation, both native and non-native, on the distribution of herbaceous dune species deserves further investigation.
References
Barbour MG (1970) Germination and early growth of the strand plant Cakile maritima. Bull Torrey Bot Club 97:13–22. https://doi.org/10.2307/2483986
Barbour MG, DeJong TM (1977) Response of west coast beach taxa to salt spray, seawater inundation, and soil salinity. Bull Torrey Bot Club 104:29–34. https://doi.org/10.2307/2484662
Benvenuti S, Macchia M (2006) Effect of hypoxia on buried weed seed germination. Weed Res 35:343–351. https://doi.org/10.1111/j.1365-3180.1995.tb01629.x
Benvenuti S, Macchia M (2010) Phytochrome-mediated germination control of Datura stramonium L. seeds after seed burial. Weed Res 38:199–205. https://doi.org/10.1046/j.1365-3180.1998.00086.x
Brown JF (1997) Effects of experimental burial on survival, growth, and resource allocation of three species of dune plants. J Ecol 85:151–158. https://doi.org/10.2307/2960647
Cartica RJ, Quinn JA (1980) Responses of populations of Solidago sempervirens (Compositae) to salt spray across a barrier beach. Am J Bot 67:1236–1242. https://doi.org/10.1002/j.1537-2197.1980.tb07756.x
Cheplick GP (2017) Responses of native plant populations on an unprotected beach to disturbance by storm-induced overwash events. Plant Ecol 218:105–118. https://doi.org/10.1007/s11258-016-0670-1
Ciccarelli D (2014) Mediterranean coastal sand dune vegetation: influence of natural and anthropogenic factors. Environ Manage 54:194–204. https://doi.org/10.1007/s00267-014-0290-2
Conser C, Connor EF (2009) Assessing the residual effects of Carpobrotus edulis invasion, implications for restoration. Biol Invasions 11:349–358. https://doi.org/10.1007/s10530-008-9252-z
Cowles HC (1899) The ecological relations of the vegetation on the sand dunes of Lake Michigan. Bot Gaz 27:95–117. https://doi.org/10.1086/327796
de Castanho C, Prado PI (2014) Benefit of shading by nurse plant does not change along a stress gradient in a coastal dune. PLoS ONE 9:e105082. https://doi.org/10.1371/journal.pone.0105082
Debez A, Hamed KB, Grignon C, Abdelly C (2004) Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 262:179–189. https://doi.org/10.1023/B:PLSO.0000037034.47247.67
Filazzola A, Lortie CJ (2014) A systematic review and conceptual framework for the mechanistic pathways of nurse plants. Glob Ecol Biogeogr 23:1335–1345. https://doi.org/10.1111/geb.12202
Frosini S, Lardicci C, Balestri E (2012) Global change and response of coastal dune plants to the combined effects of increased sand accretion (burial) and nutrient availability. PLoS ONE 7:e47561. https://doi.org/10.1371/journal.pone.0047561
Ishikawa S-I, Furukawa A, Oikawa T (1995) Zonal plant distribution and edaphic and micrometeorological conditions on a coastal sand dune. Ecol Res 10:259–266. https://doi.org/10.1007/BF02347851
King TJ (1975) Inhibition of seed germination under leaf canopies in Arenaria serpyllifolia, Veronica arvensis and Cerastum holosteoides. New Phytol 75:87–90. https://doi.org/10.1111/j.1469-8137.1975.tb01374.x
Lonard RI, Judd FW, Stalter R (2015) The biological flora of coastal dunes and wetlands: Solidago sempervirens L. and Solidago sempervirens L. subsp. mexicana (L.) Semple. J Coast Res. https://doi.org/10.2112/jcoastres-d-14-00261.1
Magnoli SM, Kleinhesselink AR, Cushman JH (2013) Responses to invasion and invader removal differ between native and exotic plant groups in a coastal dune. Oecologia 173:1521–1530. https://doi.org/10.1007/s00442-013-2725-5
Mariko S, Kachi N, Ishikawa S, Furukawa A (1992) Germination ecology of coastal plants in relation to salt environment. Ecol Res 7:225–233. https://doi.org/10.1007/BF02347091
Martinez ML, Valverde T, Moreno-Casasola P (1992) Germination response to temperature, salinity, light and depth of sowing of ten tropical dune species. Oecologia 92:343–353. https://doi.org/10.1007/BF00317460
Maun MA, Lapierre J (1986) Effects of burial by sand on seed germination and seedling emergence of four dune species. Am J Bot 73:450–455. https://doi.org/10.1002/j.1537-2197.1986.tb12058.x
Maun MA, Perumal J (1999) Zonation of vegetation on lacustrine coastal dunes: effects of burial by sand. Ecol Lett 2:14–18. https://doi.org/10.1046/j.1461-0248.1999.21048.x
Mendoza-González G, Martínez ML, Rojas-Soto OR, Vázquez G, Gallego-Fernández JB (2013) Ecological niche modeling of coastal dune plants and future potential distribution in response to climate change and sea level rise. Glob Change Biol 19:2524–2535. https://doi.org/10.1111/gcb.12236
Messina DS, Rajaniemi TK (2011) Does the seed bank reflect plant distributions in a coastal dune? Northeast Nat 18:107–114. https://doi.org/10.1656/045.018.0110
Novoa A, González L (2014) Impacts of Carpobrotus edulis (L.) N.E.Br. on the germination, establishment and survival of native plants: a clue for assessing its competitive strength. PLoS ONE 9:e107557. https://doi.org/10.1371/journal.pone.0107557
Novoa A, González L, Moravcová L, Pyšek P (2013) Constraints to native plant species establishment in coastal dune communities invaded by Carpobrotus edulis: implications for restoration. Biol Conserv 164:1–9. https://doi.org/10.1016/j.biocon.2013.04.008
Oosting HJ, Billings WD (1942) Factors effecting vegetational zonation on coastal dunes. Ecology 23:131–142. https://doi.org/10.2307/1931081
Orava C, Drake DR (1997) Effects of salinity on germination and growth of Solidago sempervirens var. mexicana (L.) Fern. Castanea 62:272–277
Pemadasa MA, Lovell PH (1975) Factors controlling germination of some dune annuals. J Ecol 63:41–59. https://doi.org/10.2307/2258840
Pons TL (2000) Seed responses to light. In: Seeds: the ecology of regeneration in plant communities. pp 237–260
Prisco I, Carboni M, Acosta ATR (2013) The fate of threatened coastal dune habitats in italy under climate change scenarios. PLoS ONE 8:e68850. https://doi.org/10.1371/journal.pone.0068850
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajaniemi TK, Allison VJ (2009) Abiotic conditions and plant cover differentially affect microbial biomass and community composition on dune gradients. Soil Biol Biochem 41:102–109. https://doi.org/10.1016/j.soilbio.2008.10.001
Rowell DL (1994) Soil science: methods and applications. Wiley, New York
Santoro R, Jucker T, Prisco I, Carboni M, Battisti C, Acosta ATR (2012) Effects of trampling limitation on coastal dune plant communities. Environ Manage 49:534–542. https://doi.org/10.1007/s00267-012-9809-6
Schlacher TA, Schoeman DS, Dugan J, Lastra M, Jones A, Scapini F, McLachlan A (2008) Sandy beach ecosystems: key features, sampling issues, management challenges and climate change impacts. Mar Ecol 29:70–90. https://doi.org/10.1111/j.1439-0485.2007.00204.x
Seabloom EW, Ruggiero P, Hacker SD, Mull J, Zarnetske P (2013) Invasive grasses, climate change, and exposure to storm-wave overtopping in coastal dune ecosystems. Glob Change Biol 19:824–832. https://doi.org/10.1111/gcb.12078
Shumway SW (2000) Facilitative effects of a sand dune shrub on species growing beneath the shrub canopy. Oecologia 124:138–148. https://doi.org/10.1007/s004420050033
Sykes MT, Wilson JB (1983) Sand and salt effects on some New Zealand sand dune species. Pac Sci Congr Proc 15:231
Sykes MT, Wilson JB (1988) An experimental investigation into the response of some New Zealand sand dune species to salt spray. Ann Bot 62:159–166. https://doi.org/10.1093/oxfordjournals.aob.a087646
Sykes MT, Wilson JB (1989) The effect of salinity on the growth of some New Zealand sand dune species. Acta Bot Neerlandica 38:173–182. https://doi.org/10.1111/j.1438-8677.1989.tb02040.x
Sykes MT, Wilson JB (1990) An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand. Acta Bot Neerlandica 39:171–181. https://doi.org/10.1111/j.1438-8677.1990.tb01485.x
USDA, NRCS (2018) The PLANTS Database (http://plants.usda.gov/). National Plant Data Team, Greensboro, NC 27401-4901 USA
van der Valk AG (1974) Environmental factors controlling the distribution of forbs on coastal foredunes in Cape Hatteras National Seashore. Can J Bot 52:1057–1073. https://doi.org/10.1139/b74-135
Vecchio SD, Pizzo L, Buffa G (2015) The response of plant community diversity to alien invasion: evidence from a sand dune time series. Biodivers Conserv 24:371–392. https://doi.org/10.1007/s10531-014-0814-3
Wilson JB, Sykes MT (1999) Is zonation on coastal sand dunes determined primarily by sand burial or by salt spray? A test in New Zealand dunes. Ecol Lett 2:233–236. https://doi.org/10.1046/j.1461-0248.1999.00084.x
Acknowledgements
The authors thank Chuck Talley, Andrew Poyant, David Messina, and Jonathan Breton for field assistance, and Chris Weideman at Waquoit Bay National Estuarine Research Reserve for advice and access to the field site.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Julie C. Zinnert.
Rights and permissions
About this article
Cite this article
Rajaniemi, T.K., Barrett, D.T. Germination responses to abiotic stress shape species distributions on coastal dunes. Plant Ecol 219, 1271–1282 (2018). https://doi.org/10.1007/s11258-018-0877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-018-0877-4