Abstract
Very high-severity fires are a component of many fire-prone ecosystems, yet are often viewed as detrimental to vegetation. However, species in such systems are likely to have adapted to persist under a fire regime that includes high-severity fires. We examined how fire severity affects post-fire recruitment and residual seed banks of Acacia species and whether severity may affect plant responses to fire intervals. Nine sites of either high or low burn severity were identified after a large-scale mixed-severity fire in Warrumbungle National Park, south-eastern Australia. Transects were used to sample above-ground woody plant density. Seed bank size was surveyed by soil extraction from two depths and manual searching for seeds. Residual soil seed bank and recruitment were compared across the two burn severities. Acacia seedling density was higher in areas burnt at high severity, indicating that increased severity triggers increased germination from the seed bank. Size of residual seed bank was smaller after high-severity fire, but varied between species, with few Acacia cheelii seeds remaining despite high above-ground abundance. In contrast, A. penninervis retained a small residual seed bank. There was little evidence of negative effects on populations of Acacia species after high-severity burns. However, we found that high fire severity may impact on the ability of a species to persist in response to a subsequent short fire interval. Fire management for maintaining biodiversity needs to consider other key aspects of the fire regime, including severity and season, rather than focusing solely on fire frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To predict the response of plant populations and their ability to persist under altered fire regimes, we need to understand the mechanisms that determine the magnitude and spatial and temporal patterns of post-fire recovery (Whelan 1995; Bond and van Wilgen 1996). This requires consideration of many interacting factors, from key life-history traits to variation in fire conditions. For many species from fire-prone habitats, particularly obligate seeders (i.e., those that are killed by fire and depend on recovery from seeds), the recovery of plant populations depends on the pre-fire abundance and distribution of seeds in a seed bank, as well as the fire response and resilience of their seed bank. Seed bank dynamics are, therefore, a critical determinant of population persistence.
In fire-prone regions, seedling emergence is related to fire-cued germination and contributes to the magnitude of recruitment, while the number of seeds remaining dormant (a post-fire residual seed bank) can contribute to a bet-hedging capacity, ensuring seeds are retained in the event of a lost seedling cohort (Whelan 1995; Bond and van Wilgen 1996; Auld and Denham 2006). Seed dormancy is one mechanism that influences both these outcomes, as it assists in maintaining a seed bank for long periods of time (Auld et al. 2000; Fenner and Thompson 2005; Ooi et al. 2007). Seed mortality resulting from fire can affect both the above and below ground population, resulting in lower levels of recruitment and a reduced residual seed bank. High seed mortality could compromise the capacity of a population to effectively recruit or retain a residual seed bank large enough to produce a subsequent seedling flush if a fire occurs soon after the first (i.e., increased fire frequency) (Auld and Denham 2006; Auld et al. 2007).
Many obligate seeding species have seeds with physical dormancy (also known as hard-seeded species), a dominant dormancy type in fire-prone ecosystems (Merritt et al. 2007; Ooi 2007), which is controlled by an impermeable seed coat. Physical dormancy is usually broken by high, short duration temperatures experienced during the passage of fire. For successful recruitment to occur, the seed bank must be heated to temperatures high enough to break the dormancy of such species, but not so high as to cause excessive levels of seed mortality. The general pattern for species from many fire-prone regions around the world is that germination increases in response to increasing heat shock temperatures for physically dormant species (Keeley and Meyers 1985; Jeffery et al. 1988; Auld and O’Connell 1991; Herranz et al. 1998). In the field, this means that in areas where higher temperatures are generated in the soil during fire, a larger proportion of the seed bank would be exposed to dormancy-breaking thresholds (Bradstock and Auld 1995; Odion and Davis 2000; Ooi et al. 2014), resulting in a potentially higher abundance of seedlings and a greater diversity of species. However, once a high temperature threshold is exceeded, heat will cause seed mortality. Heat transfer into soil is dependent on the characteristics of the fire (e.g., energy release, temperature, and duration), composition of litter (Bradstock et al. 1992; Bradstock and Auld 1995) and thermal properties of the soil, which is influenced by its composition, density, and moisture content (Van Wijk 1963; Abu-Hamdeh and Reeder 2000; Stoof et al. 2011). Exposure of seeds to dormancy-breaking or mortality thresholds is also dependent on the depth that seeds occur in the soil, with soil temperatures attenuating steeply with soil depth (Bradstock et al. 1992; Bradstock and Auld 1995; Penman and Towerton 2008). Across regions with similar soil characteristics and litter composition, variation in fire intensity is likely to be a key determinant of recruitment response and seed mortality of physically dormant species.
Fire severity, a measure of the physical impact of fire on vegetation and, therefore, a correlate of intensity (Keeley 2009), is a key component of the fire regime. However, while several studies have reported on the vegetation response to varying fire severity, including survival, structure and magnitude of population recovery (e.g., Moreno and Oechel 1991; Morrison et al. 1992; Thaxton and Platt 2006), few have related this to the mechanisms driving these responses, such as temperature-related seed dormancy. Those that have are often focused on the potential negative effects of very cool fires on germination and recruitment (e.g., Bradstock et al. 1992; Bradstock and Auld 1995; Ooi et al. 2014). Very high-severity fires are an inherent component of many fire-prone ecosystems, yet are often considered detrimental to vegetation recovery and maintenance of biodiversity (e.g., Vivian et al. 2008; DellaSala et al. 2015). This is despite the fact that the majority of plant species in fire-prone vegetation are likely to have adapted to persist under a fire regime that includes high-severity fires. Quantification of the effects of very high fire severity on the persistence of plant populations is, therefore, required.
One basis for the perceived negative impact of extremely severe fires in naturally fire-prone systems, is the assumption that soil temperatures cause excessive seed mortality, to a much greater extent than during more moderate fire events (Bradstock and Auld 1995), potentially reducing both the magnitude of emergence and the size of the residual seed bank. Under this assumption, recruitment after extremely severe fire would depend on the resilience of seeds to high temperatures and/or the depth of burial of seeds. Soil offers highly effective insulation, and temperatures decrease considerably with increasing depth (Bradstock et al. 1992; Tozer 1998; Penman and Towerton 2008). During fire events, seeds that are closer to the surface, where soil heating is greatest, are much more likely to be exposed to dormancy-breaking temperatures, but may be at greater risk of mortality. The size of the residual soil seed bank, which can bet-hedge against a loss of a seedling cohort, would also be affected by increased seed mortality, potentially retaining fewer seeds and at greater depths (Auld and Denham 2006). Under extreme fire severity, it is, therefore, likely that a greater proportion of seeds will die, but seeds buried deeper will have less chance of suffering mortality. Thus, factors such as seed size, which can determine the ability to germinate successfully from depth (Bond et al. 1999; Auld and Denham 2005), and depth of burial will interact with fire severity to determine recruitment potential.
In this study, we investigated how fire severity impacts both recruitment and the residual soil seed bank of the dominant understorey species in a Eucalyptus-Callitris forest in south-eastern Australia. For the two key canopy species groups, high intensity, severe fire kills Callitris species which must then recruit from a canopy seed bank or from seeds dispersing into the burnt area, and causes above-ground mortality in Eucalyptus requiring resprouting from protected meristems below ground. However, these species are resilient to lower severity fires (Cohn et al. 2011; Denham et al. 2016). Extremely high-severity fires, therefore, retard the rate of recovery of these canopy species. The response to varying fire intensity of many of the understorey species is less well understood. Research identifying how fire regime shifts in temperate fire-prone regions may drive vegetation change has recently been highlighted as an important knowledge gap, and the impacts of extreme fire severity on critical life-history stages is still required (e.g., see Fairman et al. 2016; Gordon et al. 2017). One such stage is the seed bank. A key aim of our study is to, therefore, assess how extreme fire events affect recruitment and persistence of understorey species in this system, where the majority of plant diversity occurs.
Our study focused on the Warrumbungle National Park in south-eastern Australia, where an extensive high-severity wildfire occurred in January 2013. It was the most severe fire in the recorded history of the park and burnt 90% of its total area. Post-fire, there was a large flush of physically dormant heat-responsive species, dominated by those within the genus Acacia. Previous studies had investigated whether post-fire Acacia recovery contributed to increases in fuel hazard (Gordon et al. 2017), but had not investigated the soil seed bank. We considered two hypotheses; first, that higher fire severity will increase recruitment in Acacia species, and second, that higher fire severity would reduce the abundance of seeds in the residual soil seed bank. To test these hypotheses, we quantified plant recruitment above-ground and the density of the corresponding residual soil seed bank below ground in areas burnt by either extremely high or relatively low severity fire. More specifically, we addressed the following questions:
-
1.
Does fire severity impact recruitment of Acacia species from the seed bank and does the effect differ among species?
-
2.
Does fire severity affect the residual seed bank, and if so, is this related to seed burial depth?
-
3.
Is there evidence for negative effects of extremely high fire severity on the persistence of populations of fire-prone physically dormant species?
Methods
Study area
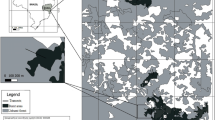
The Warrumbungle National Park (WNP) covers approximately 23 000 ha in the upper reaches of the Castlereagh River catchment, south-eastern Australia (31.29°S, 149.01°E). The region is topographically complex (elevation range 400–1200 m), with soils derived from basalts, trachyte and pyroclastic flows and from pre-volcanic sandstones. Summers are warm to hot (mean maximum temperature 23.7 °C), while winters are mild (mean minimum temperature 7.4 °C), with an average annual rainfall of 750 mm (mostly in summer, though soil remains moist in winter).
The vegetation consists mostly of open Eucalyptus-Callitris forest (North–west Slopes Dry Sclerophyll Woodlands and Western Slopes Dry Sclerophyll Forests) (Keith 2004), with an understorey of sclerophyllous shrubs in low soil fertility areas, while grasses and forbs dominate high soil fertility areas (Denham et al. 2016). Canopy dominants vary among habitats, but Callitris glaucophylla, Eucalyptus albens and E. crebra are the predominant tree species present in most communities (Hunter 2008), and are 20–30 m tall. Obligate seeder species with physically dormant seeds dominate the understorey of most vegetation communities in the region, with genera such as Acacia and Dodonaea being very common. While these understorey species re-establish rapidly after fire, Callitris populations may take over 10 years to recover from a severe fire event, as population regeneration depends on relatively slow growing seedlings (Denham et al. 2016).
The recorded fire history of the WNP shows that there have been several relatively small fires scattered over the past 70 years, but that a majority of the park has not been burned for 40 years (OEH unpublished data, Storey et al. 2016). The Wambelong Fire, which occurred in 2013, is the largest and most severe fire in the recorded history of the park and is the focus of this study. During extreme fire conditions in January 2013, the fire burnt over 39 000 ha of vegetation within 24 h. Following the initial conflagration, the fire continued to burn in low–moderate fire conditions for several weeks, burning another 17 000 ha before being extinguished on the 21 Feb. Overall, approximately 84% of WNP was burnt, with 13% burnt at low severity, indicated by scorched low shrubs but tree canopies remaining unburnt. Around 64% of the park was burnt at high or extremely high intensity, with severity class in these areas based on top-killed trees and consumption of all vegetation and canopy (Denham et al. 2016).
Site selection
Potential study areas were initially selected based on remote sensing classification of burn severity (see Storey et al. 2016), with severity subsequently confirmed by field assessment. Sites were categorised as being burnt at either low or high severity, which was a surrogate for fire intensity. While a number of woody species had recruited post-fire, Acacia species dominated the mid-storey within these sites and were, therefore, the target for this study (Table 2). Two species, Acacia cheelii and A. penninervis, occurred at each site and were, therefore, used as focal species in the study. Fire intensity was classed according to Chafer et al. (2004), who considered the amount of energy expended and type of burn. Classes were low–moderate (fire intensity < 500 kW/m, surface and low shrub fire) and high-extreme (500–70 000 kW/m, tall shrub fire with tree canopy scorch to total canopy consumption). Field assessment of severity was based on height of canopy scorch/consumption and the proportion of litter consumption. High-severity sites consisted of areas where all litter, shrubs, and most tree canopies (mainly E. crebra, E. albens, and C. glaucophylla canopies > 15 m) were consumed by fire, and only tree skeletons remained. At low severity sites, shrubs and litter were consumed, but tree canopies remained intact or partially burnt and Eucalyptus trees were observed resprouting from epicormic buds. Upon identification of suitable sites of high or low severity, a 20 × 20 m plot was established, within which surveys were conducted (see Table 1 for a description of sites).
Five sites burnt at low severity and four sites burnt at high severity were identified across different areas of the park, accounting for spatial variation of population distribution and fire characteristics. Acacia species within these sites were identified and targeted for the study (Table 2). We could not determine the pre-fire density of Acacias, because at many sites, no identifiable dead stems remained. Furthermore, it is reasonable to expect that above-ground populations of these species may decline with time-since-fire, persisting primarily in the soil as a seed bank. Topography was similar across all sites, being either flat or on gentle slopes (< 10% slope) and we, therefore, assumed that pre-fire densities of shrubs were not skewed towards either of our severity classes. The post-fire density of vegetation in the shrub layer varied among sites, ranging from scattered individuals to extremely dense (Table 1).
Recruitment response to fire severity
Field work to assess post-fire recruitment was conducted in August 2015, approximately two and a half years after the fire and recruited individuals had become woody but had not reached maturity. At each 20 m × 20 m plot within our five low- and four high-severity sites, we established three parallel 10 m long belt transects laid out equally spaced apart in an east–west orientation. Live stems of all shrub species in the plots, within 1 m either side of each transect, were identified and counted. This meant that in each transect we surveyed 20 m2 and the total area survey at each plot was 60 m2.
Densities (plants per m2) were calculated separately for each transect. As previously described, Acacia occurred throughout all sites, and two species, either A. cheelii, A. penninervis (or both co-occurring), dominated all plots surveyed. We, therefore, calculated density by dividing the total number of live stems by area for four subgroups: all woody species (species pooled), all Acacia species, A. cheelii and A. penninervis, in order to highlight the pattern of distribution between high and low severity fires for these groups. Note that these subgroups sometimes overlapped within sites.
Residual seed bank size
Post-fire recruits had not yet reached maturity, meaning that the residual seed bank could be surveyed (i.e., no new contributions to the seed bank prior to sampling). This allowed us to survey only the residual seed bank that persisted through the 2013 fire. Eight 30 × 30 cm seed bank plots were taken from each site. Locations of seed bank sample plots were stratified by the number of Acacia seedlings present. At each site, the eight 30 × 30 cm plots comprised of two each with 0, 1–2, 3–4, or 5+ seedlings. Once plots were located, seedlings were counted and removed. Soil from each plot was then removed to a depth of 5 cm, bagged and labelled. The soil was then dug to a depth of 10 cm (total volume of 9000 cm3/sample), ensuring soil from depth classes was kept separate. Soil was then taken to the laboratory, allowed to dry, and sieved to separate coarser particles to ease the sorting process. The remaining soil was then sorted by hand to search for seeds of all Acacia species, which were removed from the soil, identified and recorded. The mean number of seeds at each depth class was calculated for each severity class, using samples as replicates.
Data analysis
To compare the density of above-ground species among sites burnt at different severities, we used a Generalised Linear Mixed Model (GLMM) with Poisson distribution. Fire severity was included as a fixed factor, while the random factor included transects nested within sites. Separate models were fitted to compare the stem density of live woody species and the density of live Acacia stems (the dominant component of all sites) comparing high and low severity burn areas. Two further analyses explored the density of the two dominant species, A. cheelii and A. penninervis, using only the sites in which each target species occurred. Acacia cheelii was dominant in four sites, and Acacia penninervis dominant in three, with both species co-occurring in only two of the nine sites (see Table 1.)
To assess the effect of fire severity on residual seed bank density and whether this varied with depth, a two-factor Generalised Linear Model (GLM) with Poisson distribution was used, with the number of seeds recorded of all Acacia species as the dependent variable, and fire severity (high versus low) and depth class (0–5 cm and 5–10 cm) as the two factors. To examine the difference in residual seed bank density between A. cheelii and A. penninervis, depth classes were pooled and a two-factor GLM was used. To test whether seeds were abundant in areas that we did not sample, we used logistic regression to model the likelihood of seeds being present in the residual seed bank in relation with the number of seedlings present, fire severity, and species (A. cheelii or A. penninervis).
Results
Recruitment response to fire severity
The mean density of live woody stems (all species combined) in high-severity burn areas was 8.25/m2 compared to only 2.6/m2 in the low severity burn areas (Fig. 1, Table 1). All four comparisons with GLMMs of above-ground vegetation demonstrated that high-severity burn sites had a higher density of recruitment than low severity sites (although A. penninervis was only near significant; Table 3). This consistent relationship indicated that Acacia species, and in particular, A. cheelii and A. penninervis, drove the overall patterns of understorey shrub response. Acacia cheelii and A. penninervis were the dominant components in the studied sites, evident by their high density in both low- and high-severity burn areas when compared to other surveyed plant species (Fig. 2).
Density (mean ± standard error per m2) of live stems for woody plants recruiting post-fire. Data are for the four subgroups All woody species, All Acacia species, and the single species A. cheelii and A. penninervis in low (dark grey) and high (light grey) severity sites. Note that there is overlap among these groups within sites
Residual seed bank size
The number of seeds recovered from soil seed bank samples was very low across all sites (Fig. 3). Nevertheless, there was a significant effect of fire severity (df = 1, χ2 = 4.993, P = 0.025) on seed bank density, with higher densities of seeds in areas burnt at low severity (0–13 seeds/m2) than at high severity (0–7 seeds/m2). While there was no significant effect of depth (df = 1, χ2 = 1.625, P = 0.202), there was a trend for more seeds at greater depths at both fire severity classes.
Comparison of the residual seed banks of the two dominant Acacia species in relation to fire severity uncovered striking differences. Acacia penninervis seed density was much higher than for A. cheelii (Fig. 4). There was a significant interaction between species and severity (df = 1, χ2 = 8.033, P = 0.005), indicating that high-severity fire significantly reduced the size of the residual seed bank, but only for A. penninervis. Acacia cheelii seed density was negligible regardless of fire severity (Fig. 4).
Neither fire severity nor number of seedlings present significantly influenced the likelihood of finding a residual seed bank according to the logistic regression model. However, species was significant, such that the likelihood of finding seeds in the soil was approximately five times greater for A. penninervis than for A. cheelii (A. cheelii odds ratio 0.21, 95% CI 0.06–0.81). There was a greater proportion of samples that had seeds in samples with more seedlings of A. penninervis, but this trend was not significant (Fig. 5). The low proportion of samples with seeds where there were no seedlings (less than 20%) suggests that there are unlikely to be large numbers of seeds in areas where we did not sample.
Discussion
High-severity fire resulted in a greater recruitment response of the dominant physically dormant Acacia species than low severity fire, with Acacia species dominating the mid-storey vegetation in sites subjected to high-severity fire. The corollary of this response is that high-severity fire resulted in a greatly reduced residual seed bank. A combination of large seeds and a relatively large store of seeds in the soil, in conjunction with adequate soil heating from the fire, is likely to contribute to promoting the rapid establishment and growth of these species. The known heat response for breaking dormancy of Acacia seeds (Auld and O’Connell 1991; Ooi et al. 2014) provides a clear mechanism to explain the patterns of recruitment observed, with the dense recovery in high-severity areas indicating that more seeds were exposed to temperatures high enough to break dormancy. These findings also suggest that high-severity fire does not necessarily result in negative outcomes for species with physically dormant seeds, and that species are adapted to such events. Results from our assessment of residual seed banks demonstrate that some risk-spreading capacity can be maintained after high-severity fires. However, the interaction of fire severity and fire frequency is important when trying to determine appropriate fire intervals for the persistence of a species.
The above-ground recruitment recorded in our study is consistent with the hypothesis that higher severity fire will produce hotter soil temperatures and subsequently greater recruitment of physically dormant heat-responsive species (Ooi et al. 2014; Wright et al. 2016). Across regions with similar litter composition, it is clear that other factors, such as air temperature and soil moisture, contribute to fire intensity and, therefore, soil temperature (Whelan 1995; Stoof et al. 2011; Mondal and Sakumar 2014). Higher levels of recruitment 2.5 years post-fire in areas of high severity, compared to that in low severity areas, indicate that this is a key driver of the magnitude of recruitment of physically dormant species, as suggested by some other studies (e.g., Knox and Morrison 2005), including at other sites in relation to the same fire (Gordon et al. 2017). However, few studies have quantified this, or incorporated the dynamics and role of residual seed banks in determining persistence.
Extreme fire events, such as the one studied here, increase soil temperatures to very high levels, and, therefore, also increase the likelihood of greater amounts of seed mortality. However, despite this, high levels of above-ground recruitment were observed in higher severity burnt areas. This suggests that seed mortality alone, as a result of hotter fires, is unlikely to limit recruitment in these fire-adapted species. While lethal temperatures could cause greater seed mortality, this is likely to be restricted to the upper few centimetres due to the insulating effects of soil. Suitable dormancy-breaking temperatures would, therefore, shift further down the soil profile, and germination (rather than mortality) would be possible for depths beyond the first few centimetres. Seed size would subsequently be a critical determinant of recruitment success under increasingly severe fire, because smaller seeds are unable to emerge from greater depths (Bond et al. 1999). Acacia species have large seeds in comparison to most other species within the study region, and they would have some capacity to emerge from depths of at least 5 cm (Denham and Auld 2006; Liyanage and Ooi 2018). However, the fact that there is a range of seed sizes, even within our study group (9.2–72.8 mg, Table 2), means that there could still be relative differences in response. Understanding recruitment of different species to fire severity, therefore, requires knowledge of seed dormancy-breaking temperature and mortality thresholds (Ooi et al. 2014; Liyanage and Ooi 2015), along with their ability to emerge from depth (Bond et al. 1999; Hanley et al. 2003). Further work is required to estimate dormancy-breaking and mortality temperature thresholds for physically dormant species in fire-prone regions.
Fire severity had a clear effect on the size of the residual soil seed bank. For A. penninervis, there was more than three times the number of seeds remaining in the seed bank in the low severity compared to high-severity areas. In contrast, there was almost no evidence of a residual seed bank for A. cheelii or any other Acacia species at sites of either high or low severity, despite the high abundance of above-ground recruits. Above-ground recruitment of A. cheelii was comparable to A. penninervis, suggesting that there were either fewer A. cheelii seeds prior to the fire or that more were killed during the fire or died prior to emergence. Acacia cheelii seeds have a mass less than 25% that of A. penninervis, and would have a lower probability of successful emergence if dormancy was broken at depth. Individuals of larger seeded species, such as Acacia penninervis, would be able to emerge from greater depths where they are protected from the most extreme temperatures and have the resources to reach the surface.
We found no evidence that the size of the residual seed bank size is moderated by burial depth. In contrast, Auld and Denham (2006) showed that post-fire Acacia soil seed banks contained fewer seeds in the top 5 cm of soil than layers from 5 to 10 cm and 10–15 cm, indicating that heat shock cues declined with depth and caused more germination and mortality close to the surface. The lack of significance of burial depth in our study could be attributed to the extremely severe fire causing very high soil temperatures, even in low severity burn areas, a clear demonstration of the extreme nature of the event. These high soil temperatures were likely sufficient to produce seed mortality or promote germination down to a depth of at least 10 cm. The residual seed bank density (0–13 seeds/m2) was low in comparison to other post-fire studies. Auld et al. (2007) found approximately 21 seeds/m2 of Persoonia lanceolata (an obligate seeder) in heath and woodlands in coastal New South Wales, 9 years after fire. Another study found that several shrub species (including A. suaveolens) in open woodlands in south-eastern Australia maintained sizeable seed banks through low–moderate severity fires (Auld and Denham 2006). However, there has been little study regarding the size of residual soil seed banks following high-severity wild fires. We suggest that further studies are required after natural fires to gain a clearer understanding of the role that residual seed banks play.
Our estimation of fire effects is based on two components of genets of these species after fire—the above-ground recruits and the residual seed bank. We were unable to estimate the losses of genets due to excessive heating of seeds or early seedling mortality, nor were we able to assess pre-fire abundance of plants. There is some possibility, therefore, of an underlying difference in the landscape in which high and low severity sites occurred. Alternatively, there may be a relationship between severity and the abundance of Acacias, with these species establishing more successfully in sites subject to high-severity fire, or that the presence of Acacias predisposes these sites to high-severity fire. Although further elucidating this relationship is an important area for future research, our finding of a residual seed bank in low severity sites that was larger in absolute terms (Fig. 3) rather than in proportion to the total number of genets, demonstrates that any sampling bias did not influence the conclusions of this study.
Despite significant levels of above-ground recruitment of physically dormant species post-fire, the limited residual seed bank we observed suggests that a short interval before a subsequent fire event would threaten populations of many of the species in the study region. Frequent fire poses a risk of population decline and extinction for obligate seeding species, as individuals can be killed before they mature, and seed banks are not replenished (Keith 1996; Bowman et al. 2014a; Enright et al. 2015). Depending on the length of the juvenile period (from emergence to flowering), this can take many years (Keith 1996). While A. penninervis has some capacity to respond to a subsequent fire in the short term, due to a small residual seed bank, many other Acacia species are unlikely to have such a buffer. Here importantly, we have demonstrated that severity of the previous fire has important consequences for a species response to subsequent fire interval, similar to studies investigating obligate seeders in other regions (e.g., Bowman et al. 2014b in alpine habitat). This highlights that an understanding of key elements of the fire regime beyond only fire frequency, including severity and season (Ooi 2010; Mackenzie et al. 2016), need to be incorporated into implemented fire strategies when managing for biodiversity or the persistence of threatened species.
References
Abu-Hamdeh NH, Reeder RC (2000) Soil thermal conductivity effects of density, moisture, salt concentration, and organic matter. Soil Sci Soc Am J 64:1285
Auld TD, Denham AJ (2005) A technique to estimate the pre-fire depth of burial of Grevillea seeds using seedlings after fire. Aust J Bot 53:401–405
Auld TD, Denham AJ (2006) How much seed remains in the soil after a fire? Plant Ecol 187:15–24
Auld TD, O’Connell MA (1991) Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust J Ecol 16:53–70
Auld TD, Keith DA, Bradstock RA (2000) Patterns in longevity of soil seedbanks in fire-prone communities of south-eastern Australia. Aust J Bot 48:539–548
Auld TD, Denham AJ, Turner K (2007) Dispersal and recruitment dynamics in the fleshy-fruited Persoonia lanceolata (Proteaceae). J Veg Sci 18:903–910
Bond WJ, van Wilgen BW (1996) Fire and plants. Chapman and Hall, London
Bond WJ, Honig M, Maze KE (1999) Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120:132–136
Bowman DMJS, MacDermott HJ, Nichols SC, Murphy BP (2014a) A grass-fire cycle eliminates an obligate-seeding tree in a tropical savanna. Ecol Evol 4:4185–4194
Bowman DMJS, Murphy BP, Neyland DLJ, Williamson GJ, Prior LD (2014b) Abrupt fire regime change may cause landscape-wide loss of mature obligate seeder forests. Glob Change Biol 20:1008–1015
Bradstock RA, Auld TD (1995) Soil temperatures during experimental bushfires in relation to fire intensity: consequences for legume germination and fire management in south-eastern Australia. J Appl Ecol 32:76–84
Bradstock RA, Auld TD, Ellis MV, Cohn JS (1992) Soil temperatures during bushfires in semi-arid, mallee shrublands. Aust J Ecol 17:433–440
Chafer CJ, Noonan M, Macnaught E (2004) The post-fire measurement of fire severity and intensity in the Christmas 2001 Sydney wildfires. Int J Wildland Fire 13:227–240
Cohn JS, Lunt ID, Ross KA, Bradstock RA (2011) How do slow-growing, fire-sensitive conifers survive in flammable eucalypt woodlands? J Veg Sci 22:425–435
DECC (2002) NSW Flora fire response database Version 1.3. NSW Department of Environment and Climate Change, Hurstville
DellaSala DA, Lindenmayer DB, Hanson CT, Furnish J (2015) In the aftermath of fire: logging and related actions degrade mixed- and high-severity burn areas. In: DellaSala DA, Hanson CT (eds) The ecological importance of mixed-severity fires. Elsevier, Amsterdam, pp 313–347
Denham AJ, Vincent BE, Clarke PJ, Auld TD (2016) Responses of tree species to a severe fire indicate major structural change to Eucalyptus-Callitris forests. Plant Ecol 217:617–629
Enright NJ, Fontaine JB, Bowman DMJS, Bradstock RA, Williams RJ (2015) Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front Ecol Environ 13:265–272
Fairman TA, Nitschke CR, Bennett LT (2016) Too much, too soon? A review of the effects of increasing wildfire frequency on tree mortality and regeneration in temperate eucalypt forests. Int J Wildland Fire 25:831–848
Fenner M, Thompson K (2005) The ecology of seeds. CAB International, Wallingford
Gibson MR, Richardson DM, Marchante E et al (2011) Reproductive biology of Australian acacias: important mediator of invasiveness? Divers Distrib 17:911–933
Gordon CE, Price OF, Tasker EM, Denham AJ (2017) Acacia shrubs respond positively to high severity wildfire: implications for conservation and fuel hazard management. Sci Total Environ 575:858–868
Hanley ME, Unna JE, Darvill B (2003) Seed size and germination response: a relationship for fire-following plant species exposed to thermal shock. Oecologia 134:18–22
Herranz JM, Ferrandis P, Martinez-Sanchez JJ (1998) Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecol 136:95–103
Hunter JT (2008) Vegetation and floristics of Warrumbungle National Park. Report to NSW National Parks and Wildlife Service, Coonabarabran
Jeffery DJ, Holmes PM, Rebelo AG (1988) Effects of dry heat on seed germination in selected indigenous and alien legume species in South Africa. S Afr J Bot 54:28–34
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. Int J Wildland Fire 18:116–126
Keeley JE, Meyers A (1985) Effect of heat on seed germination of southwestern Yucca species. Southwestern Nat 30:303–304
Keith DA (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. P Linn Soc NSW 116:37–78
Keith DA (2004) Ocean shores to desert dunes: the native vegetation of New South Wales. Department of Environment and Conservation (NSW), Hurstville
Knox KJE, Morrison DA (2005) Effects of inter-fire intervals on the reproductive output of resprouters and obligate seeders in the Proteaceae. Austral Ecol 30:407–413
Liyanage GS, Ooi MKJ (2015) Intra-population level variation in thresholds for physical dormancy-breaking temperature. Ann Bot Lond 116:123–131
Liyanage GS, Ooi MKJ (2018) Seed size-mediated dormancy thresholds: a case for the selective pressure of fire on physically dormant species. Biol J Linn Soc 123:135–143
Mackenzie BDE, Auld TD, Keith DA, Hui FKC, Ooi MKJ (2016) The effect of seasonal ambient temperatures on fire-stimulated germination of species with physiological dormancy: a case study using Boronia (Rutaceae). PLoS ONE 11:e0156142
Merritt DJ, Turner SR, Clarke S, Dixon KW (2007) Seed dormancy and germination stimulation syndromes for Australian temperate species. Aust J Bot 55:336–344
Mondal NS, Sukumar R (2014) Fire and soil temperatures during controlled burns in seasonally dry tropical forests of southern India. Curr Sci India 107:1590–1594
Moreno JM, Oechel WC (1991) Fire intensity effects on germination of shrubs and herbs in southern California chaparral. Ecology 72:1993–2004
Morrison DA, Auld TD, Rish S, Porter C, McClay K (1992) Patterns of testa-imposed seed dormancy in native Australian legumes. Ann Bot Lond 70:157–163
Odion DC, Davis FW (2000) Fire, soil heating, and the formation of vegetation patterns in chaparral. Ecol Monogr 70:149–169
Ooi MJK (2007) Dormancy classification and potential dormancy-breaking cues for shrub species from fire-prone south-eastern Australia. In: Adkins SA, Ashmore SE, Navie SC (eds) Seeds: biology, development and ecology. CAB International, New York, pp 205–216
Ooi MKJ (2010) Delayed emergence and post-fire recruitment success: effects of seasonal germination, fire season and dormancy type. Aust J Bot 58:248–256
Ooi MKJ, Auld TD, Whelan RJ (2007) Distinguishing between persistence and dormancy in soil seed banks of three shrub species from fire-prone southeastern Australia. J Veg Sci 18:405–412
Ooi MKJ, Denham AJ, Santana VM, Auld TD (2014) Temperature thresholds of physically dormant seeds and plant functional response to fire: variation among species and relative impact of climate change. Ecol Evol 4:656–671
Penman TD, Towerton AL (2008) Soil temperatures during autumn prescribed burning: implications for the germination of fire responsive species? Int J Wildland Fire 17:572–578
Stoof CR, De Kort A, Bishop TFA, Moore D, Wesseling JG, Ritsema CJ (2011) How rock fragments and moisture affect soil temperatures during fire. Soil Sci Soc Am J 75:1133–1143
Storey M, Price O, Tasker E (2016) The role of weather, past fire and topography in crown fire occurrence in eastern Australia. Int J Wildland Fire 25:1048–1060
Thaxton JM, Platt WJ (2006) Small-scale fuel variation alters fire intensity and shrub abundance in a pine savanna. Ecology 87:1331–1337
Tozer MG (1998) Distribution of the soil seedbank and influence of fire on seedling emergence in Acacia saligna growing on the central coast of New South Wales. Aust J Bot 46:743–755
Van Wijk WR (1963) Physics of plant environment. North-Holland Publishing Company, Amsterdam
Vivian LM, Cary GJ, Bradstock RA, Gill AM (2008) Influence of fire severity on the regeneration, recruitment and distribution of eucalypts in the Cotter River Catchment, Australian Capital Territory. Austral Ecol 33:55–67
Whelan RJ (1995) The ecology of fire. Cambridge University Press, Cambridge
Wright BR, Latz PK, Zuur AF (2016) Fire severity mediates seedling recruitment patterns in slender mulga (Acacia aptaneura), a fire-sensitive Australian desert shrub with heat-stimulated germination. Plant Ecol 217:789–800
Acknowledgements
We thank Martin Henery and Justin Collette for field assistance, Lisa Metcalfe for laboratory assistance, Craig Wall and the National Parks and Wildlife Service Coonabarabran office for permission to work in Warrumbungle NP and Jessica Meade for assistance with drafting figures. The project was supported by funding from the NSW Office of Environment and Heritage, and as part of the Australian Government’s National Environmental Science Programme (NESP), Threatened Species Recovery Hub (1.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephen Brewer.
Rights and permissions
About this article
Cite this article
Palmer, H.D., Denham, A.J. & Ooi, M.K.J. Fire severity drives variation in post-fire recruitment and residual seed bank size of Acacia species. Plant Ecol 219, 527–537 (2018). https://doi.org/10.1007/s11258-018-0815-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-018-0815-5