Abstract
Seed traits play an important role in seed dispersal and possibly in eventual plant community structure and dynamics. Though seed traits have been shown to influence seed dispersal of one given tree species, it is not clear if and how seed dispersal and seed survival of one tree species are affected by neighboring tree species at inter-specific level. In the present study, we investigated acorn dispersal of two oak species Q. variabilis (bearing large acorn) and Q. serrata var. brevipetiolata (bearing small acorn) in the presence of sympatric Q. aliena acorns (medium size), to test the ‘context-dependent’ hypothesis, which states that seed dispersal patterns of one tree species can be affected by sympatric tree species at inter-specific level. Our results showed that the probability of acorn removal of Q. variabilis was higher in the presence of Q. aliena acorns. However, the presence of Q. aliena acorns significantly decreased acorn removal rates of Q. serrata var. brevipetiolata. Acorns of Q. serrata var. brevipetiolata were less likely to be cached by small rodents in the presence of Q. aliena acorns, whereas, acorns of Q. variabilis tended to be scatter-hoarded in the presence of Q. aliena acorns. The presence of Q. aliena acorns promoted Q. variabilis acorns to establish seedlings, but arrested seed-seedling transition in Q. serrata var. brevipetiolata. Our study verifies the interspecific interactions of seed dispersal systems of sympatric oak species, showing the ‘context-dependent’ hypothesis that seed dispersal of one given tree species can be regulated by another or more sympatric tree species bearing seeds with contrasting traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a great number of tree species bearing large-sized seeds, seed dispersal and caching by hoarding animals play an important role in the successful seed-seedling transition and tree regeneration (Schupp and Fuentes 1995; Terborgh et al. 2008). Small rodents, birds and large mammals have been recognized as very important seed dispersers and predators in various ecosystems (Gómez 2003; Vander Wall and Longland 2004; Meng et al. 2012; Matsuda et al. 2013; O’farrill et al. 2013). The probability of seed survival and seedling establishment is influenced by their foraging behavior in response to different seed traits (Moles et al. 2003; Vander Wall 2003; Xiao et al. 2004; Zhang and Zhang 2008; Wang and Chen 2009; Xiao et al. 2009, 2010; Lai et al. 2014). Seed traits (e.g., seed size, tannin concentration, seed coat thickness, and germination schedule) directly affect the eating and caching preferences of food hoarding animals during seed dispersal processes (Xiao et al. 2004, 2013a, b; Muňoz and Bonal 2008; Zhang and Zhang 2008; Wang and Chen 2009), and these preferences could ultimately determine seed fates and thereby tree recruitment patterns (Brewer 2001; Vander Wall 2001; Chauvet et al. 2004; Jansen et al. 2004; Zhang and Zhang 2008; Zhang et al. 2008; Wang and Chen 2008, 2011; Perea et al. 2012a, b).
Seed traits, in terms of seed size/mass, nutrient content, physical and chemical defenses, germination schedules and so on, vary greatly in different plant species (Moles et al. 2003; Chang et al. 2009; Zhang and Zhang 2008; Chen et al. 2012; Wang et al. 2014). Among seed traits, seed size has usually been regarded as one of the main factors affecting seed fates and removal rates (Moore et al. 2007; Zhang et al. 2008; Wang and Chen 2009; Lai et al. 2014). Larger seeds are expected to suffer heavy predation rates than smaller seeds, since larger food items offer more energetic rewards for foraging efforts of animals (Brewer 2001; Gómez 2004). However, Jansen et al. (2004) and Gómez et al. (2008) have found that seed size shows a positive effect on dispersal because larger seeds have an increase in the probability of being successfully cached and dispersed further away from the parent trees (Vander Wall 2003; Xiao et al. 2006; Chang et al. 2009). Preference for larger seeds still remains to be a controversial trend (Brewer 2001; Theimer 2003; Xiao et al. 2004; Kennedy 2005; Zhang et al. 2008). Seed selection by dispersers can also be regulated strongly by physical trait and seed nutrition status (Zhang and Zhang 2008; Lei et al. 2012). Besides, secondary metabolites (e.g., tannin) and fiber in seeds act as important antifeedant to animals (Xiao et al. 2008; Wang and Chen 2008, 2009; Chen et al. 2012; Wang et al. 2012). Moreover, a squirrel’s decision whether to cache an acorn is simply based on the seed’s germination schedule, reflecting the role of germination schedule in determining seed dispersal and survival (Smallwood et al. 2001; Chang et al. 2009, 2012; Xiao et al. 2009, 2010). Although a large number of studies have improved our understanding of the interaction between seeds and animals in the last three decades (Smallwood and Peters 1986; Briones-Salas et al. 2006; Muňoz and Bonal 2008; Zhang et al. 2008); how seed traits affect seed selection and caching by food hoarding animals still remains controversial (Moles et al. 2003; Vander Wall 2003; Xiao et al. 2004, 2006; Wang and Chen 2009), possibly due to a mixed effect of different seed traits on seed fate (Meng et al. 2012; Lai et al. 2014; Wang et al. 2014).
Sympatric tree species bearing seeds with differing traits are usually synchronized in seed masting (Kelly 1994; Kelly and Sork 2002; Jansen et al. 2004). The masting of sympatric tree species has been proposed to improve their own seed dispersal (Vander Wall 2002; Jansen et al. 2004; Li and Zhang 2007). However, we lack information of the effect of seed masting of one species on other tree species. Moreover, animals may selectively consume seeds with different traits and show differentiated effects on seed fates of sympatric trees (Zhang and Zhang 2008; Yi et al. 2014; Chang et al. 2012). Although an increasing body of literature has documented the effects of different seed traits on seed dispersal of sympatric tree species (Brewer 2001; Shimada 2001; Jansen et al. 2002; Heredia and Detrain 2005), few studies have investigated how seed dispersal and seed survival of one tree species are affected by other neighboring tree species through manipulation by animals (Morales et al. 2012; Lichti et al. 2014). Moreover, those investigations usually consider the effects of seed traits on food selection of animals (Carlo 2005; Chang et al. 2012; Wang et al. 2012), but neglect the influence of seed abundance on seed selection of food hoarding animals (Yi et al. 2011; Lichti et al. 2014). Therefore, we still have little knowledge of how seed dispersal processes of different plant species interact with each other and then influence their dispersal successes. Seed dispersal patterns of one tree species can be benefited from other sympatric species at inter-specific level; however, the competitive advantages of such a mechanism remain to be explored (Lichti et al. 2014).
Q. variabilis, Q. aliena and Q. serrata var. brevipetiolata occur together in the Tianchishan Mountain National Forest Park, in central China. Acorns of these three Quercus species fall synchronously from late August to early October, and differ greatly in seed traits, especially seed size and seed coat thickness. Q. variabilis acorns are characterized by the largest seed size and thickest seed coat, while acorns of Q. serrata var. brevipetiolata have the smallest seed size and thinnest seed coat. As to acorns of Q. aliena, medium values exhibit (Table 1). In the present study, we investigated seed dispersal of two oak species Q. variabilis and Q. serrata var. brevipetiolata in the presence of Q. aliena acorns, to test the ‘context-dependent’ hypothesis stating that seed dispersal and seed survival of one tree species can be affected by other sympatric tree species at the inter-specific level. Our study is believed to be one of the few to investigate the interspecific interaction of sympatric tree species in the process of seed dispersal and seedling establishment. Because seeds with small size and thin hull are more likely to be eaten rather than dispersed (Zhang and Zhang 2008), we predicted that acorns of Q. serrata var. brevipetiolata with smaller size and thinner seed coat are less likely to be scatter-hoarded in the presence of Q. aliena acorns. On the contrary, seed caching of Q. variabilis bearing large acorns can be benefited from the presence of acorns of Q. aliena. Consequently, the presence of Q. aliena acorns will promote Q. variabilis acorns to establish seedlings, but reduce seed-seedling transition in Q. serrata var. brevipetiolata.
Methods
Study site
The study was conducted in the Tianchishan Mountain (1,400 m a.s.l., 33°45′–33°85′N, 111°75′–112°45′E), located in a transition belt between the north subtropical and warm zones in central China. Annual mean temperature is 17 °C, and annual mean rainfall is 812 mm. Vegetation is dominated by deciduous Q. variabilis, Q. aliena and Q. serrata var. brevipetiolata, mixed with other broad-leaved tree species, e.g., Castanea mollissima, C. seguinii, and Diospyros kaki. The shrubby understory is diverse and rich in endemic taxa (Corylus sp). Apodemus peninsulae, Niviventer confucianus and Sciurus davidianu are the main seed dispersers, while, Cansumys canus is the main seed predator in the forests. A. peninsulae (body mass: 20–35 g), N. confucianus (70–100 g) and Cansumys canus (40–110 g) are nocturnal and have a large population, while the diurnal S. davidianu with the largest body mass (230–330 g) are relatively rare in this region.
Experimental design and data collection
We carried out our experiments in three consecutive years (2010, 2011, and 2012) with contrasting acorn crop sizes of the three oak species (Table 2). In mid-September 2010, we collected acorns under >10 trees of each oak species to encompass intraspecific variation in seed traits (e.g., seed size) at the peak period of seed fall of Q. variabilis, Q. aliena and Q. serrata var. brevipetiolata. A subsample of 30 sound acorns was randomly selected for seed trait measurements in each year. Then, 1,000 sound acorns were randomly selected for each oak species for seed dispersal experiments. A tiny hole of 0.3 mm in diameter was drilled at the basal end of each acorn with a portable electrical drill. Acorns were tied with a small, light plastic-tag (length × width, 3.5 cm × 2.5 cm, <0.3 g) through the hole using a thin steel wire of 10 cm long, and each tag was coded with a serial number in order to identify every acorn (see Yi et al. 2011). When small rodents bury the tagged seeds in the soil, the tags are often left on the ground surface, making them easy to be re-located. Tagging has been shown to have a negligible effect on seed removal and caching by rodents (Xiao et al. 2006). Then, 10 seed stations spaced at 50 m apart each other were established on each of four transects (e.g., I, II, III, and IV) (Fig. 1). Because acorns of Q. aliena exhibit medium seed size and coat thickness, we paired Q. aliena with Q. variabilis and Q. serrata var. brevipetiolata, respectively, to explore whether the presence of Q. aliena acorns show different influences on seed dispersal and survival of Q. variabilis and Q. serrata var. brevipetiolata bearing large and small acorns. Then, we released 50 acorns of Q. variabilis (i.e., paired Q. variabilis) together with 50 acorns of Q. aliena (i.e., paired Q. aliena) in each seed station on transect I. For comparison, 50 acorns of Q. variabilis (i.e., single Q. variabilis) were placed in each seed station on transect II. Similarly, we placed 50 paired acorns of Q. serrata var. brevipetiolata (i.e., paired Q. serrata var. brevipetiolata) and Q. aliena (i.e., paired Q. aliena) in each seed station on transect III. On transect IV, 50 acorns of Q. serrata var. brevipetiolata (i.e., single Q. serrata var. brevipetiolata) were released in each seed station for comparison (Fig. 1). After release, we checked the tagged acorns every day until all acorns were removed by rodents. At the same time, we searched the area around each station (radius, <30 m) through daily visit with equal efforts of 20 min for two people each visit, looking for the tagged acorns dispersed by small rodents. Seed removal rate was defined as the decrease rate of remaining acorns at the seed stations. Seed fates of the released acorns in each station were measured as: intact in situ (IIS), eaten in situ (EIS); eaten after removal (EAR), intact after removal (on surface) (IAR); scatter-hoarded after removal (excluding IAR) (SH); missing (in burrow or not seen) (M). Seedling establishment from scatterhoards was surveyed in June next year. Seedlings were identified by checking the numbers on the plastic tags attached to the remnant cotyledons of oak seedlings. We repeated the same procedures in 2011 and 2012 with different acorn crop sizes.
Data analyses
One-way ANOVA was used to detect differences in seed traits of acorns of the three oak species (length, width, dry mass, pericarp thickness). Cox regression (Wald Chi-square) was used to compare the differences in seed removal rates between acorns of Q. variabilis, Q. aliena and Q. serrata var. brevipetiolata, with seed removal rates as dependent variable and survey date and the presence of Q. aliena acorns as explanatory variables. Three-way ANOVA was used to evaluate the effects of releasing years, oak species and the presence of Q. aliena acorns on the probability of acorn scatter-hoarding of Q. variabilis and Q. serrata var. brevipetiolata, as only the scatter-hoarded acorns contribute to the final seedling establishment. One-way ANOVA was used to test the difference in acorn removal rates of Q. aliena in the presence of Q. variabilis and Q. serrata var. brevipetiolata. General linear model (multiple comparisons) was used to see the differences in seedling establishment from acorns of Q. aliena, Q. variabilis, and Q. serrata var. brevipetiolata in the three years.
Results
Acorn removal
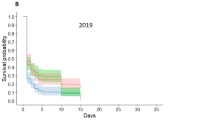
Cox regression analyses showed that acorn dispersal rate of Q. variabilis was not influenced in the presence of Q. aliena acorns in 2010 with lean crops (Wald = 0.985, df = 2, P = 0.611). The same pattern was found in acorns of Q. serrata var. brevipetiolata (Wald = 3.913, df = 2, P = 0.141) (Fig. 2A, D). However, acorns of both Q. variabilis and Q. serrata var. brevipetiolata tended to be dispersed much more slowly in the presence of Q. aliena acorns in 2011 (Wald = 12.820, df = 2, P = 0.002; Wald = 6.459, df = 2, P = 0.040) (Fig. 2B, E). Similar patterns were observed in 2012 (Wald = 14.718, df = 2, P = 0.001; Wald = 20.474, df = 2, P < 0.001) (Fig. 2C, F). Overall, our results showed that acorns of Q. variabilis (large size but thick coat) and Q. serrata var. brevipetiolata (thin coat but small size) tended to be dispersed slower in the presence of acorns of Q. aliena, and this trend became much more evident with increasing seed crop in the three years.
Acorn removal rates of Q. variabilis and Q. serrata var. brevipetiolata in the presence of Q. aliena acorns. Shown are Q. variabilis acorns in 2010 (A), 2011 (B), and 2012 (C), and Q. serrata var. brevipetiolata acorns in 2010 (D), 2011 (E), and 2012 (F). Each row of two sub-figures represents one year
Post-removal seed fates
Three-way ANOVA analyses showed that the number of acorns scatter-hoarded by small rodents varied significantly among the three years with contrasting acorn crops (F = 72.960, df = 2, P < 0.001), with more acorns being cached in 2012 than in 2010 (multiple comparisons: P = 0.001) (Table 3; Fig. 3). Acorns of Q. variabilis were more likely to be scatter-hoarded than those of Q. serrata var. brevipetiolata (F = 883.696, df = 1, P < 0.001). The presence of Q. aliena acorns showed significant effects on scatter-hoarding probability of acorns of Q. variabilis and Q. serrata var. brevipetiolata (F = 11.119, df = 1, P = 0.001) (Table 3; Fig. 3). Moreover, we found a correlated effect of oak species and the presence of Q. aliena on acorn caching by small rodents (F = 81.186, df = 1, P < 0.001). Despite variations in acorn crops of the three oak species across the three consecutive years, much more acorns of Q. variabilis were scatter-hoarded in the presence of Q. aliena acorns, while acorns of Q. serrata var. brevipetiolata were less likely to be scatter-hoarded in the presence of Q. aliena acorns (Table 3; Fig. 3). The number of scatter-hoarded acorns of Q. aliena varied significantly among the three years (F = 42.247, df = 2, P < 0.001), more acorns were scatter-hoarded in years with large seed crops (2010 < 2011 < 2012, all P < 0.001) (Fig. 3). Moreover, acorns of Q. aliena were less likely to be scatter-hoarded in the presence of Q. variabilis than in the presence of Q. serrata var. brevipetiolata (F = 16.280, df = 1, P < 0.001) (Table 3; Fig. 3).
Seed fates of Q. variabilis and Q. serrata var. brevipetiolata acorns in the presence of Q. aliena acorns. Shown are Q. variabilis acorns in 2010 (A), 2011 (B), and 2012 (C), and Q. serrata var. brevipetiolata acorns in 2010 (D), 2011 (E), and 2012 (F). EIS, EAR, IAR, SH, and M stand for acorns eaten in situ, eaten after removal, intact after removal, scatter-hoarded after removal, and missing, respectively. Each row of two sub-figures represents one year. Data are expressed as mean ± SE
Seedling establishment
Filed survey in spring showed that an average of 18.0 ± 4.7 seedlings of paired Q. variabilis were located among the three years, much higher than the seedlings germinated from acorns of single Q. variabilis (8.3 ± 3.0) (Table 4). However, the presence of Q. aliena acorns showed no significant effect on seedling establishment of paired Q. serrata var. brevipetiolata (Table 4). Seedling establishment of paired Q. aliena acorns seemed not to be significantly affected by the presence of either Q. variabilis or Q. serrata var. brevipetiolata acorns (Table 4).
Discussion
Although the presence of Q. aliena decreased acorn removal rates of Q. variabilis and Q. serrata var. brevipetiolata, acorns with large size were consistently removed by animals faster than small-sized acorns (Q. variabilis > Q. aliena > Q. serrata var. brevipetiolata). These observations are well in agreement with previous studies showing that large-sized seeds are more likely to be removed than those with small size (Vander Wall 1990; Steele et al. 1996; Brewer 2001; Gómez 2004; Jansen et al. 2004; Caccia et al. 2006; Gómez et al. 2008; Muňoz and Bonal 2008; Zhang et al. 2008). However, our results were somewhat inconsistent with other studies showing that seed size does not exert any significant effects (Xiao et al. 2004; Kennedy 2005) or seed selection by animals is influenced by other species-specific seed traits rather than seed size (Pons and Pausas 2007a, Pons and Pausas 2007b). This contradiction can be attributed to contrasting difference in other seed traits in those studies (e.g., seed coat thickness or chemical defense) (Zhang and Zhang 2008). The other acorn traits were not measured in this study; however, they may also exert influence on seed dispersal and seed fate both at intra- and interspecific levels. The fact that the presence of Q. aliena decreased acorn removal rates of the other two sympatric oak species suggests that seed dispersal of one given tree species can be regulated by another or more sympatric tree spescies bearing seeds with contrasting traits (Lichti et al. 2014).
Our study showed that more acorns were scatter-hoarded in 2012 (high masting year) than in 2010 (low masting year), supporting the previous results that small rodents increase seed caching when large seed crops are produced (Vander Wall 2002; Jansen et al. 2004; Li and Zhang 2007). The increase in the proportion of acorn scatter-hoarding can be explained by the ‘predator satiation effect’ stating that food hoarding animals tend to eat less but bury more seeds when food is sufficient (Xiao et al. 2013c). This prediction can be partly supported by the decrease in acorn removal rate and the probability of acorn consumption in 2012 with large acorn crop. However, the interference effects of Q. aliena acorns on seed dispersal and seed fate of Q. variabilis and Q. serrata var. brevipetiolata remain consistent in the three consecutive years in regardless of acorn crops. The interspecific effect of seed traits on seed dispersal appears to be independent of seed abundance.
The effect of seed size on seed fate is context-dependent (Hulme and Hunt 1999; Shimada 2001; Hoshizaki and Hulme 2002). Although few studies show that large seeds have higher seed predation probability and lower survivorship than small seeds (e.g., Moles et al. 2003), much more observations show an opposite pattern that large seeds are more likely to be dispersed and hoarded (Zhang et al. 2008). In our study, Q. variabilis acorns with large size were more likely to be dispersed while Q. serrata var. brevipetiolata acorns with small size were more likely to be eaten in situ in the presence of Q. aliena acorns. Eating large acorns in situ not only increases the energy expenditure and time consumption but also increases their predation risk (Xiao et al. 2004; Zhang and Zhang 2008). This can be supported by the fact that the presence of Q. aliena acorns benefited dispersal of Q. variabilis acorns, whereas, acorns of Q. serrata var. brevipetiolata were less likely to be dispersed in this scenario. Although the other seed traits (nutrition status and chemical defense) were not measured, seed size is expected to be one of important factors determining seed fate in our study. Alternatively, different seed handling ability of small rodents in response to may account for this; however, seed dispersal interaction mediated by seed traits is expected to better explain differences in seed fates of sympatric tree species at inter-specific level (Yi et al. 2011; Klinger and Rejmánek 2009). Consistent with acorn scatter-hoarding probability, more seedlings of Q. variabilis were found in the presence of Q. aliena acorns. However, acorns of Q. serrata var. brevipetiolata were less likely to establish seedlings in the presence of Q. aliena acorns, reflecting different influences of Q. aliena acorns on seed dispersal fitness of Q. variabilis and Q. serrata var. brevipetiolata differing in seed traits. Our results verify the ‘context-dependent’ hypothesis that seed dispersal and seed survival of one tree species can be affected by other sympatric tree species at inter-specific level. In these interactions, seed traits (e.g., seed size and seed coat thickness) appear to play an important role and attribute to differences in seed dispersal and seed survival patterns of sympatric oak species.
References
Brewer SW (2001) Predation and dispersal of large and small seeds of a tropical palm. Oikos 92:245–255
Briones-Salas M, Sanchez-Cordero V, Sanchez-Rojas G (2006) Multi-species fruit and seed removal in a tropical deciduous forest in Mexico. Can J Bot 84:433–442
Caccia FD, Chaneton EJ, Kitzberger T (2006) Trophic and non-trophic pathways mediate apparent competition through post-dispersal seed predation in a Patagonian mixed forest. Oikos 113:469–480
Carlo TA (2005) Interspecific neighbors change seed dispersal pattern of an avian-dispersed plant. Ecology 86:2440–2449
Chang G, Xiao Z, Zhang Z (2009) Hoarding decisions by Edward’s long-tailed rats (Leopoldamys edwardsi) and South China field mice (Apodemus draco): the responses to seed size and germination schedule in acorns. Behav Proc 82:7–11
Chang G, Jin T, Pei J, Chen X, Zhang B, Shi Z (2012) Seed dispersal of three sympatric oak species by forest rodents in the Qinling Mountains, Central China. Plant Ecol 213:1633–1642
Chauvet S, Feer F, Forget PM (2004) Seed fates of two Sapotaceae species in a Guianan rain forest in the context of escape and satiation hypotheses. J Trop Ecol 20:1–9
Chen X, Cannon CH, Conklin-Brittan NL (2012) Evidence for a trade-off strategy in stone oak (Lithocarpus) seeds between physical and chemical defense highlights fiber as an important antifeedant. PLoS One 7(3):e32890
Gómez JM (2003) Spatial patterns in long-distance of Quercus ilex acorns by jays in a heterogeneus landscape. Ecography 26:573–584
Gómez JM (2004) Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution 58:71–80
Gómez JM, Puerta-Piñero C, Schupp EW (2008) Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537
Heredia A, Detrain C (2005) Influence of seed size and seed nature on recruitment in the polymorphic harvester ant Messor barbarus. Behav Proc 70:289–300
Hoshizaki K, Hulme PE (2002) Mast seeding and predatormediated indirect interactions in a forest community: evidence from post-dispersal fate of rodent-generated caches. In: Levey D, Silva WR, Galetti M (eds) Seed dispersal, frugivory: ecology, evolution, conservation. CABI Publishing, Wallingford, pp 227–239
Hulme PE, Hunt MK (1999) Rodent post-dispersal seed predation in deciduous woodland: predator response to absolute and relative abundance of prey. J Anim Ecol 68:417–428
Jansen PA, Bartholomeus M, Bongers F, Elzinga JA, Den Ouden J, Van Wieren SE (2002) The role of seed size in dispersal by a scatter-hoarding rodent. In: Levey D, Silva WR, Galetti M (eds) Seed dispersal, frugivory: ecology, evolution, conservation. CABI Publishing, Wallingford, pp 209–225
Jansen PA, Bongers F, Hemerik L (2004) Seed mass and mast seeding enhance dispersal by a noetropical scatter-hoarding rodent. Ecol Monogr 74:569–589
Kelly D (1994) The evolutionary ecology of mast seeding. Trends Ecol Evol 9:465–470
Kelly D, Sork VL (2002) Mast seeding in perennial plants: why, how, where? Ann Rev Ecol Syst 33:427–447
Kennedy PG (2005) Post-dispersal seed predation varies by habitat not acorn size for Quercus chrysolepis (Fagaceae) and Lithocarpus densiflora (Fagaceae) in central coastal California. Madronõ 52:30–34
Klinger R, Rejmánek M (2009) The numerical and functional responses of a granivorous rodent and the fate of Neotropical tree seeds. Ecology 90:1549–1563
Lai X, Xiao Z, Guo C (2014) Trait-mediated seed predation, dispersal and survival among frugivore-dispersed plants in a fragmented subtropical forest, Southwest China. Integr Zool 9:246–254
Lei J, Shen Z, Yi X (2012) Pericarp thickness and seed size determine acorn dispersal of five rodent-dispersed oak species. Acta Theriol Sin 32:83–89
Li H, Zhang Z (2007) Effects of mast seeding and rodent abundance on seed predation and dispersal by rodents in Prunus armeniaca (Rosaceae). Forest Ecol Manag 242:511–517
Lichti NI, Steele MA, Zhang H, Swihart RK (2014) Mast species composition alters seed fate in North American rodent-dispersed hardwoods. Ecology 95:1746–1758
Meng L, Gao X, Chen J, Martin K (2012) Spatial and temporal effects on seed dispersal and seed predation of Musa acuminata in southern Yunnan, China. Integr Zool 7:30–40
Moles AT, Warton D, Westoby M (2003) Do small-seeded species have higher survival through seed predation than large-seeded species? Ecology 84:3148–3161
Moore J, McEuen AB, Swihart RK, Contreras TA, Steele MA (2007) Determinants of seed removal distance by scatter-hoarding rodents in deciduous forests. Ecology 88:2529–2540
Morales JM, Rivarola MD, Amico G, Carlo TA (2012) Neighborhood effects on seed dispersal by frugivores: testing theory with a mistletoe–marsupial system in Patagonia. Ecology 93:741–748
Muňoz A, Bonal R (2008) Are you strong enough to carry that seed? Seed size/body size ratios influence seed choices by rodents. Anim Behav 76:709–715
Matsuda I, Higashi S, Otani Y, Tuuga A, Bernard H, Corlett RT (2013) A short note on seed dispersal by colobines: the case of the proboscis monkey. Integr Zool 8:395–399
O’Farrill G, Galetti M, Campos-Arceiz A (2013) Frugivory and seed dispersal by tapirs: an insight on their ecological role. Integr Zool 8:4–17
Perea R, Miguel AS, Martinez-Jauregui M, Valbuena-Carabana M, Gil L (2012a) Effects of seed quality and seed location on the removal of acorns and beechnuts. Europ J Forest Res 131:623–631
Perea R, Miguel AS, Lopez D (2012b) Incorporating insect infestation into rodent seed dispersal: better if the larva is still inside. Oecologia 170:723–733
Pons J, Pausas JG (2007a) Rodent acorns selection in a Mediterranean oak landscape. Ecol Res 22:535–541
Pons J, Pausas JG (2007b) Not only size matters: acorn selection by the European jay (Garrulus glandarius). Acta Oecol 31:353–360
Schupp EW, Fuentes M (1995) Spatial patterns of seed dispersal and the unification of plant population ecology. Ecoscience 2:267–275
Shimada T (2001) Hoarding behaviors of two wood mouse species: different preference for acorns of two Fagaceae species. Ecol Res 16:127–133
Smallwood PD, Peters WD (1986) Grey squirrel food preferences: the effect of tannin and fat concentration. Ecology 67:168–174
Smallwood PD, Steele MA, Faeth SH (2001) The ultimate basis of the caching and dispersers. In: Murray DR (ed) Seed dispersal. Academic Press, Sydney, pp 191–235
Steele MA, Hadj-Chikh LZ, Hazeltine J (1996) Caching and feeding decisions by Sciurus carolinensis: responses to weevil-infested acorns. J Mammal 77:305–314
Terborgh J, Nuñez-Ituri G, Pitman N, Cornejo F, Alvarez P, Pringle B, Swamy V, Paine T (2008) Tree recruitment in an ‘empty’ forest. Ecology 89:1757–1768
Theimer TC (2003) Intra-specific variation in seed size affects scatterhoarding behaviour of an Australian tropical rain forest rodent. J Trop Ecol 17:177–189
Vander Wall SB (1990) Food hoarding in animals. University of Chicago Press, Chicago
Vander Wall SB (2001) The evolutionary ecology of nut dispersal. Bot Rev 67:74–117
Vander Wall SB (2002) Masting in animal-dispersed pines facilitates seed dispersal. Ecology 83:3508–3516
Vander Wall SB (2003) Effects of seed size of wind-dispersed pines (Pinus) on secondary seed dispersal and the caching behavior of rodents. Oikos 100:25–34
Vander Wall SB, Longland WS (2004) Diplochory: are two seed dispersers better than one? Trends Ecol Evol 19:155–161
Wang B, Chen J (2008) Tannin concentration enhances seed caching by scatterhoarding rodents: an experiment using artificial ‘seeds’. Acta Oecol 34:379–385
Wang B, Chen J (2009) Seed size, more than nutrient or tannin content, affects seed caching behavior of a common genus of Old World rodents. Ecology 90:3023–3032
Wang B, Chen J (2011) Scatter-hoarding rodents prefer slightly astringent food. PLoS One 6:e26424
Wang B, Wang G, Chen J (2012) Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol 213:1329–1336
Wang Z, Cao L, Zhang Z (2014) Seed traits and taxonomic relationships determine the occurrence of mutualisms versus seed predation in a tropical forest rodent and seed dispersal system. Integr Zool 9:309–319
Xiao Z, Zhang Z, Wang Y (2004) Dispersal and germination of big and small nuts of Quercus serrata in subtropical evergreen broadleaved forest. Forest Ecol Manag 195:141–150
Xiao Z, Wang Y, Harris M, Zhang Z (2006) Spatial and temporal variation of seed predation and removal of sympatric large-seeded species in relation to innate seed traits in a subtropical forest, Southwest China. Forest Ecol Manage 222:46–54
Xiao Z, Chang G, Zhang Z (2008) Testing the high-tannin hypothesis with scatter-hoarding rodents: experimental and field evidence. Anim Behav 75:1235–1241
Xiao Z, Gao X, Jiang M, Zhang Z (2009) Behavioral adaptation of Pallas’s squirrels to germination schedule and tannins in acorns. Behav Ecol 20:1050–1055
Xiao Z, Gao X, Steele M, Zhang Z (2010) Frequency-dependent selection by tree squirrels: adaptive escape of nondormant white oaks. Behav Ecol 21:169–175
Xiao Z, Gao X, Zhang Z (2013a) Sensitivity to seed germination schedule by scatter-hoarding Pére David’s rock squirrels during mast and non-mast years. Ethology 119:472–479
Xiao Z, Gao X, Zhang Z (2013b) The combined effects of seed perishability and seed size on hoarding decisions by Pére David’s Rock squirrels. Behav Ecol Sociobiol 67:1067–1075
Xiao Z, Zhang Z, Krebs CJ (2013c) Long-term seed survival and dispersal dynamics in a rodent-dispersed tree: testing the predator satiation hypothesis and the predator dispersal hypothesis. J Ecol 101:1256–1264
Yi X, Yang Y, Zhang Z (2011) Intra- and inter-specific effects of mast seeding on seed fates of two sympatric Corylus species. Plant Ecol 212:785–793
Yi X, Wang Z, Liu C, Liu G (2014) Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures. iForest. doi: 10.3832/ifor1185-007
Zhang H, Zhang Z (2008) Endocarp thickness affects seed removal speed by small rodents in a warm-temperate broad-leafed deciduous forest, China. Acta Oecol 158:57–63
Zhang H, Chen Y, Zhang Z (2008) Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. Forest Ecol Manag 255:1243–1250
Acknowledgments
This study was financially supported by the National Natural Science Foundation (No. 31470113, 31172101) and the Program for New Century Excellent Talents in University (NCET-12-0693). The authors declare no conflict of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. M. Ramsay.
Rights and permissions
About this article
Cite this article
Yi, X., Wang, Z. Context-dependent seed dispersal determines acorn survival of sympatric oak species. Plant Ecol 216, 123–132 (2015). https://doi.org/10.1007/s11258-014-0421-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-014-0421-0