Abstract
Mast seeding is a common phenomenon, and has important effects on seed dispersal and hoarding by animals. At population level, the predator satiation hypothesis proposes that the satiating effect of a large amount of seeds on a relatively small number of predators benefits seed survival in mast-seeding years. However, the effect of mast seeding on the scatter-hoarding of rodents at the individual level is largely unknown. In this study, we investigated the effects of seed abundance (by simulating mast seeding and non-mast seeding) on the removal, consumption and scatter-hoarding of seeds of Camellia oleifera (Theaceae) by Edward’s rat Leopoldamys edwardsi (Muridae) in seminatural enclosures in southwest China. We wanted to test the masting-enhanced hoarding hypothesis, which suggests that rodents tend to scatter-hoard more seeds in mast-seeding years in order to occupy more food resources. Our results indicate that L. edwardsi tended to disperse and scatter-hoard more seeds of C. oleifera per night with increasing seed abundance, and to eat less seeds per night when there was a high level of seed abundance in the enclosure experiments. These results support the masting-enhanced hoarding hypothesis. This capacity of rodents may be an evolutionary adaptation to the mast-seeding phenomenon. Our results suggest that mast seeding benefits forest regeneration not only through the predator satiation effect at the population level, but also through increased hoarding by animals at the individual level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast seeding, the phenomenon where a population of plants synchronously but intermittently productes a large amount of seeds, while exhibiting low seed production at other times, is common in perennial plants where the seeds are dispersed by scatter-hoarding animals (Silvertown 1982; Vander Wall 2002; Kelly and Sork 2002; Jansen et al. 2004; Xiao et al. 2005; Li and Zhang 2007). The predator satiation hypothesis proposes that producing massive crops of plants in mast years can satiate seed predators, which allows more seeds to be cached and so to escape predation by animals (Janzen 1970; Crawley and Long 1995; Sork 1993; Sork et al. 1993; Theimer 2001; Kelly et al. 2001; Schnurr et al. 2002; Kelly and Sork 2002; Li and Zhang 2007). However, some studies have failed to support this hypothesis (e.g., Herrera et al. 1994; Montiel and Montana 2000; Hoshizaki and Hulme 2002; Jansen 2003; Vander Wall 2002; Xiao et al. 2005). Many co-varying factors, such as intra- or interspecific competitors, may affect seed removal and hoarding by rodents under natural field conditions, and thus may affect conclusions drawn from field observations.
Previous studies of mast seeding have mostly focused on the predator satiation effect at the population level. The predator satiation hypothesis suggests that there is a satiating effect of producing a large amount of seeds in mast-seeding years on a relatively small number of predators. Some seeds may escape from predation and finally become seedlings due to the predator satiation effect induced by the large seed population and the small predator population. However, how individual animals respond to mast seeding is largely unknown. There are three possibilities: the seed hoarding of the animals may decrease, increase or not change in mast-seeding years. Jenkins and Peters (1992) suggest that animals tend to scatter-hoard seeds surrounding seed resources in order to occupy more resources (rapid sequestering hypothesis). Animals often face many intra- or interspecific competitors when searching for foods. Thus, we hypothesize that animals would increase their hoarding in mast-seeding years in order to occupy more food resources (the masting-enhanced hoarding hypothesis). In this study, the hoarding effect is defined as the number of seeds hoarded per night by an individual animal.
Camellia oleifera (Theaceae) grows widely in forests of south and southwest China, its native land (Lin and Li 1989; Zhang 1998). The capsules of C. oleifera, each containing one to eight seeds (mean ± S.D., 0.9 ± 0.3 g weight, 1.5 ± 0.2 cm length, 1.2 ± 0.3 cm width, n = 40), mature and fall during September to November in our study area, the Dujiangyan region, a subtropical area in southwestern China. After ripening, the fruit pulp of C. oleifera naturally dehisces and its seeds fall on the ground around the mother trees, or whole fruits sometimes fall on the ground directly. Seed production of C. oleifera varies greatly among years and stands (Xiao 2003). Because of their hard coats, seeds of C. oleifera are strictly rodent-dispersed (not bird-dispersed) in the study area (Xiao 2003; Xiao et al. 2003, 2004a; Wang et al. 2004; Xiao and Zhang 2004). Leopoldamys edwardsi (Muridae) is a dominant nocturnal rodent species in the Dujiangyan area, 250–300 mm in body length and 200–500 g in body weight. L. edwardsi eats and scatter-hoards a number of tree seeds (e.g., Quercus varialilis, Q. serrata, Castanopsis fargesii, Lithocarpus hardlandii, Cyclobalanopsis glauca and C. oleifera) and, as a seed disperser, has a positive effect on seeding regeneration for these tree species (Xiao et al. 2003, 2004a; Wang et al. 2004; Cheng et al. 2005a, 2005b). Because of their high nutrition (51.8% of crude fat and 29.6 KJ/g of caloric value), seeds of C. oleifera are more commonly selected by a number of small rodents (Xiao et al. 2003). L. edwardsi is the most important rodent species that scatter-hoards seeds of C. oleifera (Cheng et al. 2005a).
In this study, we simulated the seed abundance of C. oleifera in mast and non-mast seeding years by presenting different amount of tin-tagged seeds to L. edwardsi in seminatural enclosures. We wanted to know how mast seeding affects the hoarding behaviors of rodents (e.g., L. edwardsi) at the individual level, and to test the masting-enhanced hoarding hypothesis.
Materials and methods
Study area
The study was conducted in the middle of a subtropical area in the Dujiangyan region of Sichuan Province, southwest China (altitude 700–1,000 m, 31°4′N, 103°43′E), which has a mean annual temperature of 15.2 °C, 1,200–1,800 mm of annual precipitation, a mean of 800–1,000 h of sunshine annually, and a mean annual relative humidity of over 80% (Chen 2000; Xiao et al. 2004a). In the belt of subtropical evergreen broadleaved forest (altitude, 700–1,500) in this area, forests are isolated and fragmented by cultivation, and some trees are becoming very rare or extinct (Chen 2000). The common tree species are Castanopsis fargesii, Quercus variabilis, Pinus massoniana, Acer catalpifolium, C. oleifera, Q. serrata, Lithocarpus harlandii, Cyclobalanopsis glauca and Phoebe zhennan, etc. Trees and shrubs of C. oleifera are widely distributed in both primary and secondary stands (Xiao et al. 2004a, 2006a). In this area, at least ten small nocturnal rodent species (e.g., Niviventer fulvescens, L. edwardsi, Berylmys bowersi, N. confucianus, Rattus nitidu, R. norvegicus, Apodemus latronum) are responsible for the consumption of C. oleifera seeds. N. fulvescens, L. edwardsi, B. bowersi and N. confucianus are the dominant rodent species and mainly account for the seed loss of C. oleifera, but only L. edwardsi scatter-hoards C. oleifera seeds under soil or leaf-litter surfaces (Xiao et al. 2002, 2003, 2004a; Cheng et al. 2005a, 2005b).
Experimental animals
All of the experimental animals, L. edwardsi, were captured with live traps (12 cm × 12 cm × 25 cm, made of steel wire, baited with peanuts; 20–30 traps were placed about 5 m apart along a transect, and were open in one direction) in the study area (in both primary and secondary stands) during August to October 2003. All of the captured animals were fed in special plastic boxes (37 cm × 26 cm × 17 cm) individually at ambient temperature and photoperiod with abundant commercial mouse feed and water after being assessed, including for body mass, sex and reproductive status. A total of 12 healthy adults (five males and seven females with body weight 427.2 ± 46.1 g, mean ± S.D.) were used in the experiments. All experimental animals were fed at least one week before the tests.
Seed collection and marking
Intact seeds of C. oleifera were randomly collected on the ground in a primary stand during September to October 2003, when they had matured. All experimental seeds were marked with a tin-tag in order to relocate them after removal by experimental animals (Zhang and Wang 2001). A tiny hole (about 0.5 mm width in diameter) was drilled at the base of each seed; a small, light, uniquely coded tin-tag [5.0 cm × 1.0 cm, larger than that of Zhang and Wang (2001)] was tied to each seed with a fine steel wire [5 cm long, longer than that of Zhang and Wang (2001)] (see Xiao et al. 2004a; Zhang et al. 2004, 2008; Cheng et al. 2005a, 2005b; Zhang and Zhang 2006; Li and Zhang 2007). This tin- or plastic-tagged method has been shown to be effective at tracking seeds removed by small rodents (Xiao et al. 2006b; Gómez et al. 2008; Yi et al. 2008).
Enclosure design
Four separated seminatural enclosures (10 m × 10 m) were constructed in a flat area. To prevent the experimental animals from escaping from the enclosures, the brick walls of the enclosures were smoothed 1.5 m above and 0.3 m below the ground. To keep predators or other animals from entering the enclosures, each enclosure was covered with plastic sheets supported with steel frames. Some grasses and shrubs were artificially planted inside the enclosures to simulate the natural vegetation and coverage (about 20%) of the surrounding areas. A wooden nest box (40 cm × 18 cm × 18 cm) and a water plate were placed in one corner, and the experimental seeds were placed in the central point (seed station, about 0.5 m2 wide) of each enclosure. In order to relocated the removed seeds easily, each enclosure was divided into four quadrants, with the seed station in the center and the nest box in the corner (Cheng et al. 2005a, 2005b; Lu and Zhang 2005).
Experimental design
We defined three levels of seed abundances of C. oleifera in the study: low seed abundance (10 seeds/enclosure/night), medium seed abundance (20 seeds/enclosure/night) and high seed abundance (50 seeds/enclosure/night), based on the daily intake of the Edward’s rat. An individual L. edwardsi eats about ten seeds of C. oleifera per night in captivity (unpublished data).
The experiments were conducted during September to November in 2003. Seven days before carrying out the experiments, all of the experimental animals were fed with C. oleifera seeds in feeding boxes for adaptation. The coded tin-tagged seeds and experimental animals were placed into enclosures at 4:30 p.m.~5:30 p.m. Seeds were placed at different abundance levels at the seed stations of the enclosures; one randomly selected animal was introduced into each enclosure and allowed to move freely. The next morning, at 9:00 a.m.~10:00 a.m., the experimental animals were taken out of the enclosures. All seeds and fragments were relocated by searching the enclosure extensively, and they were collected for subsequent measurements of weight. Seed status and location were recorded. The enclosure was cleared by removing all remaining seeds and their fragments. A total of 12 animals were used in each of the three treatments: low-, medium- and high-abundance levels. Animals were used only once in each of the three treatments; but were reused in the other treatments. A total of 10, 20 and 50 intact seeds of C. oleifera were presented to each rat in each enclosure each night. In all three treatments, the rat tested in each enclosure was selected randomly.
Following our previous studies (e.g., Cheng et al. 2005a, 2005b; Zhang and Zhang 2006, 2007; Wang et al. 2007), the seed fate status was defined as either: (1) intact in situ (II): tagged seeds were intact at the seed station; (2) eaten (E): seeds were eaten fully or partially, with tin-tags and/or seed fragments left on the ground; (3) buried (B): seeds were scatter-buried in the soil with tin-tags on the ground; (4) intact after removal (IR): seeds were intact and lay on the ground; (5) seed removed (R): tagged seeds were removed from the seed station.
We used the average values for the amount and weight of seeds handled by each animal per night to evaluate the effects of seed abundance on seed consumption and seed scatter-hoarding of animals.
Statistics and analysis
SPSS for Windows (13.0) was used for data analyses. Repeated-measures ANOVA was used to test significant differences in seed status (except in the case of IR due to its small sample size) among the three levels of seed abundance, between levels of seed abundance, between males and females of experimental animals, and to test the interaction effects between seed abundance levels and the sex of the experimental animal.
Results
Difference in amount of seed
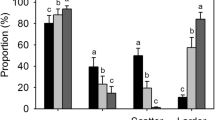
Repeated-measures ANOVA analysis demonstrates that the amounts of intact in situ (II), removed (R), eaten (E) and scatter-buried (B) seeds were all significantly different among the three levels of seed abundance (Table 1, Fig. 1a), but were not significantly different between male and female rats (Table 1), and there were no significant interactions between level of seed abundance and sex of the rat (Table 1). Amounts of R and B seed for medium-level seed abundance were significantly higher than those for low-level seed abundance; amounts of II, R and B seeds for high-level seed abundance were all significantly higher than those for low-level seed abundance, but the amount of E seed for high-level seed abundance was significantly lower than that for low-level seed abundance; amounts of II, R and B seeds for high-level seed abundance were all significantly higher than those for medium-level seed abundance, but the amount of E seed for high-level seed abundance was significantly lower than that for medium-level seed abundance (Table 2) (Fig. 1a). These results indicate that L. edwardsi tended to remove and scatter-hoard more seeds per night as the level of seed abundance (in terms of amount of seed) increased, while L. edwardsi also exhibited a tendency to eat fewer seeds per night when there was a high level of seed abundance.
Results of repeated-measures ANOVA for seed amount (a) and seed weight (b) (mean ± S.E.) for the different fates of Camellia oleifera seeds handled by the rats (Leopoldamys edwardsi) at the three levels of seed abundance. II, intact in situ; R, removal; E, eaten; B, buried; IR, intact after removal. The error bars are standard errors. *P < 0.05, **P < 0.01, ***P < 0.001
Difference in seed weight
Repeated-measures ANOVA analysis demonstrates that the weights of II, R, E and B seeds are all significantly different among the three levels of seed abundance (Table 1, Fig. 1b), but are not significantly different between male and female rats (Table 1), and that there are no significant interactions between the level of seed abundance and the sex of the rat (Table 1). Weights of II and R seeds for medium-level seed abundance were significantly higher than those for a low level of seed abundance; weights of II, R and B seeds for a high level of seed abundance were all significantly higher than those for a low level of seed abundance, but the weight of E seeds for a high level of seed abundance was significantly lower than that for a low level of seed abundance; the weights of II, R and B seeds for a high level of seed abundance were all significantly higher than those for a medium level of seed abundance, but the weight of E seeds for a high level of seed abundance was significantly lower than that for a medium level of seed abundance (Table 2, Fig. 1b). These results indicate that the L. edwardsi tended to remove and scatter-hoard more seeds per night as the level of seed abundance increased in terms of seed weight, but it tended to eat fewer seeds when there was a high level of seed abundance.
Discussion
Our results clearly indicate that with increasing seed abundance, L. edwardsi tends to disperse and scatter-hoard more seeds of C. oleifera in terms of both seed amount and seed weight per night. These results support our masting-enhanced hoarding hypothesis: that animals increase their hoarding efforts in order to occupy more food resources in mast-seeding years. This hoarding mechanism is very similar to previous observations that rodents prefer to hoard more and eat less valuable seeds (such as large or high nutritional seeds) (e.g., Smith and Reichman 1984; Vander Wall 1995; Forget et al. 1998; Jansen and Forget 2001; Jansen et al. 2004; Ulft 2004; Xiao et al. 2004b; Xiao et al. 2006a; Zhang et al. 2008). Because surface seeds are exposed to many intra- and interspecific seed predators in the field, scatter-hoarding is thought to be an evolutionary behavior that enables rodents to occupy more food resources through rapid sequestering, and subsequently transport them to nests (Jenkins and Peters, 1992). Though the rapid sequestering hypothesis indicates that scatter-hoarding is an evolutionary adaptation for occupying abundant resources, it does not establish the relation between scatter-hoard effort and seed abundance. Our masting-enhanced hoarding hypothesis proposes that the hoarding effort (measured as the average amount or weight of seed removed or cached by a single animal per night) is positively related to seed abundance. Thus it is different from the rapid sequestering hypothesis. Based on the results of this study, scatter-hoarding of L. edwardsi is obviously enhanced by seed abundance. This capacity of rodents may be another evolutionary adaptation to the mast-seeding phenomenon.
Our results also revealed that L. edwardsi tended to eat fewer seeds of C. oleifera per night in terms of both seed amount and seed weight when there was a high level of seed abundance. This was likely caused by the increased effort of the animals to scatter-hoard more seeds when they encounter high levels of seed abundance. Animals may be much too occupied with removing and caching seeds to have enough time to eat the seeds the same night. The reduction in seed consumption by rodents in mast-seeding years also benefits the seeding of reforestation.
Our results also imply that the mast seeding benefits forest regeneration not only through predator satiation at the population level, but also through the increased hoarding of animals at an individual level. Mast seeding also stimulates rodents to disperse seeds further away from seed resources (e.g., Stapanian and Smith 1978; Tamura et al. 1999; Jansen et al. 2004; Li and Zhang 2007; but Xiao et al. 2005; Moore et al. 2007). Thus, in general, greater seed abundance (e.g., mast seeding) stimulates rodents to act more as dispersers and less as predators of seeds at both population and individual levels. It is noticeable that the mechanisms of these two levels differ between the population level and the individual level.
Under field conditions, both seed abundance and granivore abundance account for seed consumption and hoarding in a given community (Ostfeld et al. 1996; Ostfeld and Keesing 2000; Hoshizaki and Hulme 2002; Li and Zhang 2007). Polyphagous seed predators, such as small rodents, are likely to respond not only to the absolute abundance of a given seed species, but also to its relative abundance in relation to other seed species (Greenwood 1985; Hulme and Hunt 1999; Hoshizaki and Hulme 2002). For example, seed predation on a particular plant species can decrease dramatically following an increase in the availability of a preferred species (Hoshizaki and Hulme 2002). Furthermore, great variations in seed abundance and granivores among habitats, seasons and years are also responsible for the inconsistent results obtained for seed removal, consumption and scatter-hoarding in many field observations (Kollmann et al. 1998; Ostfeld and Keesing 2000; Hoshizaki and Hulme 2002; Xiao et al. 2005; Li and Zhang 2007; Moore et al. 2007). Therefore, we should be very cautious in interpreting how seed abundance affects seed removal, hoarding and consumption by rodents in field studies. Our results regarding the effect of seed abundance on hoarding by rodents were achieved by using enclosures and controlling other factors (e.g., intra- or interspecific competitors, tree species, etc.), which may be more suitable for interpreting the effects of seed abundance on seed removal, consumption and hoarding by rodents at the individual level.
In general, our studies based on enclosure experiments demonstrate that a high seed abundance stimulates L. edwardsi to disperse and scatter-hoard more seeds of C. oleifera at the individual level, and also stimulates the rat to eat fewer seeds of C. oleifera. These observations support our masting-enhanced hoarding hypothesis: that animals tend to occupy more resources when they encounter a high abundance of food resources. The increased hoarding effect at the individual level, together with the predator satiation effect at the population level, may benefit the natural regeneration of trees in mast-seeding years.
References
Chen C (2000) The Dujiangyan Region—pivot sector of assemblage, differentiation and maintenance of biodiversity in northern part of Hengduan Mountain. Acta Ecol Sin 20:28–34 (In Chinese with English abstract)
Cheng JR, Xiao ZS, Zhang ZB (2005a) Seed consumption and caching on seeds of three sympatric tree species by four sympatric rodent species. For Ecol Manage 216:331–341
Cheng JR, Zhang ZB, Xiao ZS (2005b) Analysis of the effect of a conspecific competitor on the caching of oil tea seeds by Edward’s rats. Acta Theriol Sin 25(2):143–149 (in Chinese with English abstract)
Crawley MJ, Long CR (1995) Alternate bearing, predator satiation and seedling recruitment in Quercus robur. J Ecol 83:683–696
Forget PM, Milleron T, Feer F (1998) Patterns in post-dispersal seed removal by neotropical rodents and seed fate in relation to seed size. In: Newbery DM, Brown ND (eds) Dynamics of tropical communities. Blackwell Science, Oxford, pp 25–47
Gómez JM, Puerta-Piñero C, Schupp EW (2008) Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537
Greenwood JJD (1985) Frequency-dependent selection by seed predator. Oikos 44:195–210
Herrera CM, Jordano P, Lopez-Soria L, Amat JA (1994) Recruitment of a mast-fruiting, bird-dispersed tree: bridging frugivore activity and seedling establishment. Ecol Monogr 64:315–344
Hoshizaki K, Hulme PE (2002) Mast seeding and predator-mediated indirect interactions in a forest community: evidence from post-dispersal fate of rodent-generated caches. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CAB International, Wallingford, UK, pp 227–239
Hulme PE, Hunt MK (1999) Rodent post-dispersal seed predation in deciduous woodland: predator response to absolute and relative abundance. J Anim Ecol 68:417–428
Jansen PA (2003) Scatterhoarding and tree regeneration: ecology of nut dispersal in a Neotropical rainforest. Dissertation, Wageningen University, Wageningen
Jansen PA, Forget PM (2001) Scatter-hoarding rodents and tree regeneration. In: Bongers F, Charles-Dominique P, Forget PM, Théry M (eds) Nouragues: dynamics and plant–animal interactions in a neotropical rainforest. Kluwer, Dordrecht, pp 275–288
Jansen PA, Bongers F, Hemerik L (2004) Seed mass and mast seeding enhance dispersal by a noetropical scatter-hoarding rodent. Ecol Monogr 74:569–589
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Jenkins SH, Peters RA (1992) Spatial patterns of food storage by Merriam’s kangaroo rats. Behav Ecol 3:60–65
Kelly D, Sork VL (2002) Mast seeding in perennial plants: why, how, where? Annu Rev Ecol Syst 33:427–447
Kelly D, Hart DE, Allen RB (2001) Evaluating the wind pollination benefits of mast seeding. Ecology 82:117–126
Kollmann J, Coomes DA, White SM (1998) Consistencies in post-dispersal seed predation of temperate fleshy-fruited species among seasons, years and sites. Funct Ecol 12:683–690
Li HJ, Zhang ZB (2007) Effects of mast seeding and rodent abundance on seed predation and dispersal by rodents in Prunus armeniaca (Rosaceae). For Ecol Manage 242:511–517
Lin S, Li G (1989) Production situations and developmental strategies of oil tea in China. World Forest Res 4:70–75 (In Chinese)
Lu JQ, Zhang ZB (2005) Food hoarding behavior of David’s rock squirrel Sciurotamias davidianus. Acta Zool Sin 51(2):376–382
Montiel S, Montana C (2000) Vertebrate frugivory and seed dispersal of a Chihuahuan Desert cactus. Plant Ecol 146:219–227
Moore JE, Mceuen AB, Swihart RK, Contreras TA, Steele MA (2007) Determinants of seed removal distance by scatter-hoarding rodents in deciduous forests. Ecology 88:2529–2540
Ostfeld RS, Keesing F (2000) Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Tree 15:232–237
Ostfeld RS, Jones CG, Wolff JO (1996) Of mice and mast: ecological connections in eastern deciduous forests. BioScience 46:323–330
Schnurr JL, Ostfeld RS, Canham CD (2002) Direct and indirect effects of masting on rodent populations and tree seed survival. Oikos 96:402–410
Silvertown JW (1982) The evolutionary ecology of mast seeding in trees. Biol J Linn Soc 14:235–250
Smith CC, Reichman OJ (1984) The evolution of food caching by birds and mammals. Annu Rev Ecol Syst 15:329–351
Sork VL (1993) Evolutionary ecology of mast-seeding in temperate and tropical oaks (Quercus spp.). Vegetatio 107(108):133–147
Sork VL, Bramble J, Sexton O (1993) Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 74:528–541
Stapanian MA, Smith CC (1978) A model for seed scatterhoarding: coevolution of fox squirrels and black walnuts. Ecology 59:884–896
Tamura N, Hashimoto Y, Hayashi F (1999) Optimal distances for squirrels to transport and hoard walnuts. Anim Behav 58:635–642
Theimer TC (2001) Seed scatterhoarding by white-tailed rats: consequences for seedling recruitment by an Australian rain forest tree. J Trop Ecol 17:177–189
Ulft LH (2004) The effect of seed mass and gap size on seed fate of tropical rain forest tree species in Guiyana. Plant Biol 6:214–221
Vander Wall SB (1995) Dynamics of yellow pine chipmunk (Tamias amoenus) seed caches: underground traffic in bitterbrush seeds. Écoscience 2:261–266
Vander Wall SB (2002) Masting in animal-dispersed pines facilitates seed dispersal. Ecology 83:3508–3516
Wang YS, Xiao ZS, Zhang ZB (2004) Seed deposition patterns of oil tea (Camellia oleifera) influenced by seed-caching rodents. Acta Bot Sin 46:773–779
Wang W, Zhang HM, Zhang ZB (2007) Effects of predation risk on cultivated walnut (Juglans regia) seeds hoarding behavior by David’s rock squirrel (Sciurotamias davidianus) in enclosure. Acta Theriol Sin 27:358–364 (in Chinese with English abstract)
Xiao ZS (2003) Effects of small mammals on tree seed fates and forest regeneration in Dujiangyan Region, China. Ph.D. Dissertation, Institute of Zoology, Chinese Academy of Sciences, Beijing (in Chinese with English abstract)
Xiao ZS, Zhang ZB (2004) Small mammals consuming tree seeds in Dujiangyan forest. Acta Theriol Sin 24:121–124 (in Chinese with English abstract)
Xiao ZS, Wang YS, Zhang ZB, Ma Y (2002) Preliminary studies on the relationships between communities of small mammals and types of habitats in Dujiangyan Region. Chin Biodivers 10:163–169 (in Chinese with English abstract)
Xiao ZS, Zhang ZB, Wang YS (2003) Observations on tree seed selection and caching by Edward’s rat (Leopoldamys edwardsi). Acta Theriol Sin 23:208–213 (in Chinese with English abstract)
Xiao ZS, Zhang ZB, Wang YS (2004a) Impacts of scatter-hoarding rodents on restoration of oil tea Camellia oleifera in a fragmented forest. For Ecol Manage 196:405–412
Xiao ZS, Zhang ZB, Wang YS (2004b) Dispersal and germination of large and small nuts of Quercus serrata in a subtropical broad-leaved evergreen forest. For Ecol Manage 195:141–150
Xiao ZS, Zhang ZB, Wang YS (2005) The effects of seed abundance on seed predation and dispersal by rodents in Castanopsis fargesii (Fagaceae). Plant Ecol 177:249–257
Xiao ZS, Wang YS, Marvin H, Zhang ZB (2006a) Spatial and temporal variation of seed predation and removal of sympatric large-seeded species in relation to innate seed traits in a subtropical forest, Southwest China. For Ecol Manage 222:46–54
Xiao ZS, Jansen PA, Zhang ZB (2006b) Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For Ecol Manage 223:18–23
Yi XF, Xiao ZS, Zhang ZB (2008) Seed dispersal of Korean pine Pinus koraiensis labeled by two different tags in northern temperate forest, northeast China. Ecol Res 23:379–384
Zhang R (1998) Chinese oil tea. Chinese Forestry Press, Beijing (In Chinese)
Zhang ZB, Wang FS (2001) Effect of rodents on seed dispersal and survival of wild apricot (Prunus armeniaca). Acta Ecol Sin 21:839–845
Zhang HM, Zhang ZB (2006) Effects of soil depth, cache spacing and cache size of sunflower (Helianthus annuus) seeds on seed discovery by Siberian chipmunk (Tamias sibiricus senescens). Acta Theriol Sin 26:398–402 (in Chinese with English abstract)
Zhang HM, Zhang ZB (2007) Key factors affecting the capacity of David’s rock squirrels (Sciurotamias davidianus) to discover scatter-hoarded seeds in enclosures. Biodivers Sci 15:329–336 (In Chinese with English abstract)
Zhang ZB, Xiao ZX, Li HJ (2004) Impact of small rodents on tree seeds in temperate and subtropical forests, China. In: Forget PM, Lambert J, Hulme PE, Vander Wall SB (eds) Seed fates: predation, dispersal and seedling establishment. CAB International, Wallingford, UK, pp 269–282
Zhang HM, Chen Y, Zhang ZB (2008) Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For Ecol Manage 255:1243–1250
Acknowledgments
We thank XL Wang, YF Xiao, GF Chen and DG Huang for their help in animal trapping and experiment preparation, and Dr. JQ Lu for advice when preparing the manuscript. We are very grateful to the editors and to the three anonymous reviewers for their critical comments and constructive suggestions for revising and improving the manuscript. This work is partially supported by the National Natural Science Foundation of China (30430130) and the CAS Innovative Research International Partnership Project (CXTDS2005-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Janne Sundell.
Hongmao Zhang and Jinrui Cheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, H., Cheng, J., Xiao, Z. et al. Effects of seed abundance on seed scatter-hoarding of Edward’s rat (Leopoldamys edwardsi Muridae) at the individual level. Oecologia 158, 57–63 (2008). https://doi.org/10.1007/s00442-008-1114-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1114-y