Abstract
Diethylene glycol (DEG) is nephrotoxic, potentially resulting in high morbidity and mortality. Its main nephrotoxic by-product is diglycolic acid (DGA). This narrative overview summarizes selected literature with a focus on clinical findings, pathophysiology, diagnosis including morphological features of renal biopsies, and management. The kidney injury in DEG poisoning is secondary to proximal tubular necrosis caused by DGA. Marked vacuolization and edema of epithelial cells obstruct the lumen, reducing urine flow and, consequently, resulting in anuria and uremia. The clinical alterations due to DEG poisoning are dose-dependent. Patients may present with gastrointestinal symptoms and anion gap metabolic acidosis, followed by renal failure, and, later, encephalopathy and neuropathy. Although this three-phase pattern has been described, signs and symptoms may be overlapping. Data about DEG intoxication is scarce. Sometimes the diagnosis is challenging. The management includes supportive care, gastric decontamination, correction of acid–base disorders, and hemodialysis. The understanding of the metabolic processes related to DEG poisoning may contribute to its management, preventing death, serious sequels, or irreversible lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diethylene glycol (DEG) is an odorless and sweet substance that is toxic to humans [1]. As alcohol with peculiar physicochemical characteristics, it has several uses in the industry [2]. DEG is a solvent found in many consumer products, including antifreeze, brake fluids, lubricants, cosmetic creams, and paints [3].
This alcohol is described in the scientific literature as an agent capable of causing renal toxicity, whose intoxication produces severe metabolic acidosis with an increased anion gap and acute kidney injury [1, 2, 4,5,6,7]. These changes are associated with high morbidity and mortality. The clinical symptoms of DEG include vomiting, sensory alterations, oliguria, azotemia, and elevated levels of transaminases, which could indicate liver damage [8].
The first report of mass poisoning by DEG occurred in 1937 and, since then, some epidemics have been described [3]. Despite this, the data available in the literature are scarce and with limited analyzes regarding short- and long-term effects. [7]
In this context, we aimed to summarize the selected literature about renal toxicity associated with DEG poisoning in this narrative review.
Methodology
Data were obtained independently by four authors who carried out a non-systematic search in the PubMed database. Search strategies included Medical Subject Heading terms for “diethylene glycol” and “kidney injury’’. The selection criteria were: (i) time frame from 2015 to 2021; (ii) observational studies (case series, case–control, cohort, and cross-sectional), clinical trials, recent consensus statements, and guidelines; (iii) full text available in electronic format, written in English or Portuguese; (iv) studies with adequate methodological rigor; and (v) studies about any aspect concerning kidney injury associated with DEG intoxication. The exclusion criteria were: (i) studies that did refer to kidney injury associated with DEG; (ii) articles other than the specified inclusion criteria. Adopting the aforementioned search descriptors, 31 articles were obtained and had the title and abstract read by four authors. After this step, 14 articles were selected and completely read. In addition, the bibliographic references of the identified articles were also evaluated. To provide an overview of outcomes from selected case reports and case series, we calculated percentages of total cases reviewed that presented with certain clinical features.
The details of the selection process are displayed in Flowchart 1.

Flowchart 1. Flow diagram of studies selected for inclusion in the review
Pathophysiology
Pharmacokinetic data from DEG were not obtained directly from humans, since, in addition to the high toxicity of DEG metabolites, the diagnosis of this intoxication is usually delayed, which can make diagnosis and data collection difficult. In this sense, pre-clinical trials present an important tool for the study of the mechanisms of DEG toxicity [9].
Although DEG consists of two linked ethylene glycol molecules, its metabolism generates different products, which are not ethylene glycol (EG) and its by-products. Calcium oxalate crystals, one of the metabolites of EG responsible for renal injury, are not observed in tissues of DEG [2, 9].
After oral ingestion, DEG is rapidly absorbed by the gastrointestinal tract and is subsequently widely distributed to most tissues through blood circulation. According to an experimental study in primates, the peak of serum concentration occurs between 30 and 60 min [10]. Dermal absorption can also occur, although it is less common [11].
Experimental models with rats showed that about 50–70% of DEG is oxidized by alcohol dehydrogenase (ADH) in 2-hydroxyethoxy acetaldehyde and then, by aldehyde dehydrogenase (ALDH), in acetic 2-hydroxyethoxy acid (HEAA), whose excretion is mostly renal. HEAA is responsible for metabolic acidosis and organ dysfunction observed in DEG poisoning [12, 13]. In addition, HEAA promotes membrane destabilization and intracellular accumulation of osmotically active particles, with a consequent fluid diversion through the plasma membrane [12, 13].
Another product of DEG metabolism that has been related to renal injury is diglycolic acid (DGA), also known as oxydiacetic acid, formed from HEAA. DGA undergoes glomerular filtration and is transported to proximal tubular cells by atypical sodium dicarboxylate transporters-1 or carriers of organic anions [9, 11]. In the proximal tubule, the compound inhibits the citric acid cycle enzyme, succinate dehydrogenase, causing cell death by blocking the production of adenosine triphosphate [14]. Robinson et al. [15] demonstrated that direct administration of DGA to rats produced renal injury at a dose of 300 mg/kg. However, no toxicity was observed at a dose of 100 mg/kg. Renal histopathology of rats receiving the high dose of DGA showed marked degeneration and necrosis of the proximal tubules. These pathologic findings are also reported in cases of human toxicity [2, 9, 11, 15]. Furthermore, Landry et al. [16] showed that the DGA, at the concentration of 50 mmol/L, promoted a reduction in ATP levels of the proximal tubule cells, besides reducing oxygen consumption by these cells. Therefore, the substance acts by interfering both in energy production and in the use of mitochondrial oxygen [16, 17]. The increased urinary levels of kidney injury molecule 1 indicated proximal tubule lesions [15].
Another mechanism of injury that has been frequently identified in the cells of the proximal tubule due to the use of toxic compounds is the increased production of reactive oxygen species (ROS) in mitochondria [18, 19]. In addition to NADPH in the cytoplasm, and ketoglutarate dehydrogenase in the mitochondrial matrix, other important sources of ROS include electron carrier chain complexes I and II. In the presence of hypoxia, succinate dehydrogenase (complex II) has been shown to generate superoxide by undergoing the conversion of dehydrogenase to fumarate reductase. Thus, fumarate receives its electrons from complex I in a reverse electron transport mechanism, functioning as a final electron acceptor and generating ROS [20]. DGA, by inhibiting the cell's ability to consume oxygen, places the cell in a condition of "pseudohypoxia". This condition, then, may trigger the process described above, culminating in the generation of ROS [17].
In many cases, antioxidant compounds such as N-Acetyl-L-Cysteine and L-ascorbic acid were able to inhibit the production of ROS induced by toxic agents, in addition to stimulating the growth and regeneration of mitochondrial functions [21,22,23]. However, Landry et al. [16] showed the use of antioxidants in cells exposed to DGA was not able to reduce cell death at the highest concentration of 50 mmol/L. This result indicated that DGA-induced cytotoxicity occurred independently of ROS production, probably by an alternative mechanism. One possible alternative mechanism would be the inhibition of succinate dehydrogenase by DGA, considering that antioxidants are not able to antagonize this process. Inhibition of succinate dehydrogenase results in decreased ATP and cell death. In this case, the production of ROS would be a by-product of cell death. In the case of lower concentrations of DGA (25 mmol/L), the antioxidant can reduce DGA-induced cell death, suggesting that, at smaller concentrations, the production of ROS has a role in cell death. Inhibition of succinate dehydrogenase seems to represent the main mechanism by which DGA produces its toxic effects. This inhibition promotes ROS production, decreased oxygen consumption, and ATP levels, which result in cell death. [17]
Thus, kidney injury in DEG poisoning is secondary to proximal tubular necrosis caused by DGA. In addition, marked vacuolization and edema of epithelial cells obstruct the lumen, reducing urine flow and, consequently, resulting in anuria and uremia [17].
Figure 1 shows metabolic pathways of DEG metabolism.
Metabolic pathways of diethylene glycol. DEG is oxidized by alcohol dehydrogenase (ADH) to 2-hydroxyethoxy acetaldehyde. After that, 2-hydroxyethoxy acetaldehyde is converted by aldehyde dehydrogenase (ALDH) to 2-hydroxyethoxy acetic acid (HEAA), responsible for metabolic acidosis and organ dysfunction. As shown in Fig. 1, calcium oxalate crystals are not involved in DEG poisoning. Adapted from: Kraut JA, Kurtz I (2008) Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol 3(1): 208–225. https://doi.org/10.2215/cjn.03220807
Morphological aspects of diethylene glycol kidney toxicity
Diethylene glycol (DEG) is a similar molecule to ethylene glycol. Both molecules can be produced in analogous processes and can cause acute renal failure. Initially, DEG was thought to be metabolized by endogenous cleavage of any bond to form ethylene glycol, which would be responsible for the adverse effects. Some experimental models with rats showed oxalate crystals in the urine of animals receiving DEG suggesting that toxic effects could be caused by the formation and subsequent metabolism of the ethylene glycol [23]. However, other studies in dogs and rabbits did not show any increase in urinary oxalate concentrations after oral administration of DEG [22, 26]. Successive studies using radiolabeled DEG in rats and dogs confirmed this last observation [12]. In addition, DEG-poisoned patients did not show urinary oxalate formation. This finding supports the argument that endogenous conversion of DEG to ethylene glycol does not occur [27,28,29]. Based on these results, it appears that DEG is not metabolized to two ethylene glycol molecules, likely due to its metabolically stable structure. It was hypothesized that the experiments suggesting oxalate formation may have involved products contaminated with ethylene glycol. The overwhelming evidence is that DEG metabolism does not lead to the formation of oxalate crystals within the kidney [27].
Renal acute injuries appear to arise mainly from tubular cytoplasm degeneration, markedly presenting as diffuse/severe vacuolation. Cytoplasmic vacuolization (also called cytoplasmic vacuolation) is a well-known morphological phenomenon observed in mammalian cells after exposure to bacterial or viral pathogens as well as to various toxic natural and artificial low-molecular-weight compounds. The vacuolization can be transient, but it is more likely to cause irreversible damage. The vacuolation due to DEG poisoning is not fully understood. This process may be related to the hydropic degeneration (swelling) secondary to increased osmotic pressure, steatosis associated with basic amine-containing lipophilic compounds, complex lipids, and ion pump dysfunction as a consequence of alterations on Na, K-ATPase or Calcium-Activated Potassium Channels [30]. Vacuolization often accompanies cell death; however, its role in cell death processes remains unclear. There is an accentuated clear (negative image) dilatation of the cytoplasm like ballooned or fatty degeneration in tubular cells [27, 31]. Tubular lesions mainly occur in the proximal convoluted tubules and are restricted to the cortical regions of the kidney [24]. Toxic lesions of proximal tubules also manifest as cortical infarctions and/or necrosis, with vascular congestion, diffuse interstitial hemorrhage, and edema [25]. In addition, a profound swelling of the tubular epithelium can cause complete obliteration of the lumen [2, 32]. Depending on the amount or intensity of the exposure to the poison, vascular lesions, such as vascular necrosis and thrombotic microangiopathy, could be found (see images of renal biopsies from our personal archive in Figs. 2 and 3). Vacuolization may occur in podocytes and the parietal epithelium of Bowman's capsule cells. Swelling of the organelles, including the mitochondria, may also be seen (see images of renal biopsies from our personal archive in Figs. 2 and 3).
Renal Biopsies from patients intoxicated with Diethylene Glycol (personal archive). A Masson Trichrome (10X) shows diffuse tubular proximal tubular dilatation, with extreme cellular edema, and vacuolization, causing obliteration of the lumen. Capsular Bowman’s cells and podocytes vacuolization can also be seen. B Silver Jone´s Stain (40X) shows details of cellular vacuolization. C H&E (40X) shows diffuse interstitial edema and hemorrhage among tubular degeneration and vacuolization. D H&E (10X) shows congestion, hemorrhage, and diffuse necrosis. E H&E (10X) shows thrombotic microangiopathy of the glomerular tufts. F H&E (40X) shows arterial thrombosis and diffuse acute tubular necrosis.
Transmission Electron Microscopy of renal tissue from patients intoxicated with Diethylene Glycol (personal archive). Renal biopsy was fixed, buffered 10% formalin, and stained with osmium tetroxide, and ruthenium red. A Edema and disorganization of the podocyte cytoplasm and parietal Bowman’s capsule cells. B Diffuse tubular cells edema and vacuolization of the cytoplasm. C Proximal tubules with extensive vacuolization and eccentric nuclei seclusion. D Detail of the cytoplasmic change and multifocal vacuolization, including swollen mitochondria.
Chronic changes are nonspecific, sharing the same findings as other end-stage renal diseases, including globally sclerosed glomeruli, atrophic tubules, diffuse interstitial fibrosis and discrete infiltrate of inflammatory cells [33].
Clinical findings
Three case series and five case reports were included in this review, accounting for 69 cases [2, 7,8,9, 11, 34,35,36]. Twenty-eight patients (40.58%) were female and the unintentional ingestion of contaminated drug solutions was the major form of intoxication, corresponding to 58 (84.05%) cases. After the ingestion of DEG, the main initial symptoms were: oliguria or anuria (52–75.36%), respiratory symptoms (27–39.13%), fever (26–37.68%), diarrhea (24–34.78%), fatigue (11–15.94%), altered level of consciousness (11–15.94%) and abdominal pain (8–11.59%). Furthermore, 64 (92.75%) patients were hospitalized, of which 48 (75%) evolved with the absence of brainstem reflex. Of the analyzed group, 59 (85.5%) presented with high anion gap metabolic acidosis, 64 (92.75%) had acute kidney injury (AKI) and 63 (91.30%) required renal replacement therapy. Finally, 36 (52.17%) patients died.
High anion gap acidosis and acute kidney injury were commonly reported in the cases included in this review. The studies reported that most intoxicated individuals do not know, precisely, the amount of liquid ingested and/or the concentration of DEG in the contaminated liquid [2, 7,8,9, 34, 35]. The minimum dose reported in selected studies was 0.14 mg/kg and the reported lethal dose (based on one among eight studies) ranged from 1 to 1.63 g/ kg [34]. According to data of animal studies, O'Brien et al. suggested that the clinical repercussions of DEG metabolites are dose-dependent [37].
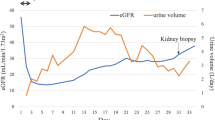
Based on our review of the selected cases series and reports, it appears there is a 3-phase pattern in presenting features of DEG poisoning (Fig. 4). Initially, the first phase consists of gastrointestinal symptoms including nausea, vomiting, abdominal pain, and diarrhea. At this stage, the patient can also have metabolic acidosis. The second phase occurs 1 to 3 days after DEG ingestion and is marked by AKI, with decreased urinary output and high anion gap metabolic acidosis. Liver injury has been described in some cases that show the elevation of serum transaminases [9]. In addition, patients may have hypertension [8, 9] and acute pancreatitis [27].
The third phase occurs 1 to 2 weeks after DEG ingestion if the patient survives the initial phases. This phase is marked by progressive and late neurological syndrome, which is characterized by encephalopathy and polyneuropathies, including bilateral facial nerve palsy, bulbar palsy, and generalized denervation of limb muscles [38].
Although this three-phase pattern has been described, signs and symptoms may be overlapping and can be modified by the ingested dose. The ingestion of DEG combined with other compounds, such as ethanol, can also modify the clinical presentation of intoxication. In this case, ethanol inhibits DEG metabolism and may delay symptoms onset for up to 48 h [5].
Table 1 summarizes the clinical findings of the studies with DEG poising.
Diagnosis
Treatment is often initiated based on history, clinical presentation, and clinical suspicion. Gas chromatographic methods can confirm exposure although these exams are not readily available [11]. During the first hours of intoxication, there is an increase in the osmolal gap, which can be greater than 20 mOsm/L, suggesting the presence of an unknown substance of low-molecular-weight in the body. After DEG is converted to 2-hydroxyethoxy acetic acid, the osmolal gap narrows, but the anion gap increases with consequent metabolic acidosis [11].
Elevations in the plasma levels of hepatic transaminases and amylases can also be detected [9, 27]. These laboratory markers, in addition to helping the diagnosis of DEG poisoning, also allow the assessment of the patient’s evolution and the poisoning severity.
Treatment
Although data are scarce, reported management of DEG poisoning includes supportive care, gastric decontamination, correction of acid–base disorders, and hemodialysis [5]. In addition, patients who survived DEG poisoning should be followed-up to assess possible medium and long-term consequences.
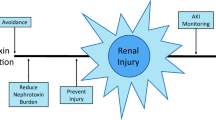
The use of fomepizole or ethanol has also been suggested [39, 40] as a means of inhibiting alcohol dehydrogenase in an attempt to block the metabolism of DEG (Fig. 5). Fomepizole has an approximately 500 times greater affinity for ADH than ethanol and can completely inhibit its activity at lower serum concentrations [39, 40]. The compound is approved for the treatment of poisoning by methanol and ethylene glycol. However, fomepizole has a high cost and low availability, while ethanol is easy to access at a low cost. In cases of DEG poisoning, fomepizole is used off-label and administered by intravenous infusion [41, 42].
Besenhofer et al. [13] demonstrated that rats receiving high doses of DEG and fomepizole had no increase in urea and creatinine, with only one of the six rats developing mild renal tubular necrosis. In contrast, rats that received only high doses of DEG had significant elevation of markers of kidney injury, and renal tubular necrosis was found in 5 of 6 rats. Thus, fomepizole may be an useful antidote for acute DEG poisoning [13, 43], especially at centers in which acute hemodialysis may not be readily available [43].
In addition, hemodialysis may also be required to correct serious metabolic abnormalities and increase the elimination of DEG and its metabolites [5, 39, 43]. Hemodialysis should be continued until metabolic acidosis [5, 43], the anion gap, and the osmolal gap improve, and the systemic signs of toxicity disappear. Thus, it seems that the early use of fomepizole together with prompt hemodialysis can minimize the effects of DEG ingestion, representing an effective management plan for these patients.
Figure 5 shows the summary of treatment of DEG poisoning.
Concluding remarks
The pathophysiology of DEG poisoning is not well understood and needs further studies. Renal biopsies of patients poisoned with DEG show diffuse interstitial edema and hemorrhage associated with tubular degeneration and vacuolization. The diagnosis of DEG poisoning is not easy, resulting in delays to start the treatment. Thus, DEG poisoning should be suspected in case of high anion gap metabolic acidosis with multisystem involvement. The treatment is mainly based on supportive care measures and the use of fomepizole seems to be beneficial, but further studies are necessary to establish its indication.
Our review has limitations. First, we have searched a single database, pubmed, which may have resulted in missed literature. Moreover, the designs of the studies, up to now, are not so robust, thus precluding definitive conclusions. Nevertheless, our study summarizes important and available data on kidney injury secondary to DEG poisoning.
In conclusion, the understanding of the metabolic processes related to DEG poisoning may contribute to its management, preventing death, serious sequels, or irreversible lesions.
Data availability
Not applicable.
References
Rentz ED, Lewis L, Mujica OJ, Barr DB, Schier JG, Weerasekera G et al (2008) Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ 86(10):749–756
Alfred S, Coleman P, Harris D, Wigmore T, Stachowski E, Graudins A (2005) Delayed neurologic sequelae resulting from epidemic diethylene glycol poisoning. Clin Toxicol (Phila) 43(3):155–159
Alkahtani S, Sammons H, Choonara I (2010) Epidemics of acute renal failure in children (diethylene glycol toxicity). Arch Dis Child 95(12):1062–1064
Asmar A, Mohandas R, Wingo CS (2012) A physiologic-based approach to the treatment of a patient with hypokalemia. Am J Kidney Dis 60(3):492–497
Minas Gerais. Secretaria de Estado de Saúde de Minas Gerais. Subsecretaria de Vigilância em Saúde (2020) Nota técnica n°02/COES-SES/MG. Protocolo de intoxicação exógena por dietilenoglicol (DEG). Belo Horizonte: Brasília (DF): Secretaria de Estado de Saúde de Minas Gerais
Song CH, Bae HJ, Ham YR, Na KR, Lee KW, Choi DE (2017) A case of ethylene glycol intoxication with acute renal injury: successful recovery by fomepizole and renal replacement therapy. Electrolyte Blood Press 15(2):47–51
Wittschieber D, Heuberger K, Schulz R, Köhler H, Varchmin-Schultheiß K (2019) Fatal poisoning with diethylene glycol in an unusual setting. Forensic Sci Med Pathol 15(4):649–652
Hari P, Jain Y, Kabra SK (2006) Fatal encephalopathy and renal failure caused by diethylene glycol poisoning. J Trop Pediatr 52(6):442–444
Gopalakrishnan N, Kamarajan M, Balasubramaniyan T, Sakthirajan R, Dhanapriya J, Dineshkumar T (2016) Diethylene glycol poisoning-induced acute kidney injury. Saudi J Kidney Dis Transpl 27:1276–1279
Clay KL, Murphy RC, Watkins WD (1975) Experimental methanol toxicity in the primate: analysis of metabolic acidosis. Toxicol Appl Pharmacol 34(1):49–61
Devoti E, Marta E, Belotti E, Bregoli L, Liut F, Maiorca P et al (2015) Diethylene glycol poisoning from transcutaneous absorption. Am J Kidney Dis 65(4):603–606
Heilmair R, Lenk W, Lohr D (1993) Toxicokinetics of diethylene glycol (DEG) in the rat. Arch Toxicol 67:655–666
Besenhofer LM, Adegboyega PA, Bartels M, Filary MJ, Perala AW, McLaren MC et al (2010) Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicol Sci 117(1):25–35
Obatomi DK, Bach PH (1996) Inhibition of mitochondrial respiration and oxygen uptake in isolated rat renal tubular fragments by atractyloside. Toxicol Lett 89(2):155–161
Robinson CN, Latimer B, Abreo F, Broussard K, McMartin KE (2017) In-vivo evidence of nephrotoxicity and altered hepatic function in rats following administration of diglycolic acid, a metabolite of diethylene glycol. Clin Toxicol (Phila) 55(3):196–205
Landry GM, Martin S, McMartin KE (2011) Diglycolic acid is the nephrotoxic metabolite in diethylene glycol poisoning inducing necrosis in human proximal tubule cells in vitro. Toxicol Sci 124(1):35–44
Landry GM, Dunning CL, Conrad T, Hitt MJ, McMartin KE (2013) Diglycolic acid inhibits succinate dehydrogenase activity in human proximal tubule cells leading to mitochondrial dysfunction and cell death. Toxicol Lett 221(3):176–184
Forkink M, Smeitink JA, Brock R, Willems PH, Koopman WJ (2010) Detection and manipulation of mitochondrial reactive oxygen species in mammalian cells. Biochim Biophys Acta 1797(6–7):1034–1044
Jezek P, Hlavatá L (2005) Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 37(12):2478–2503
Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N et al (2003) Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 284(5):L710–L719
Nowak G, Schnellmann RG (1997) Renal cell regeneration following oxidant exposure: inhibition by TGF-beta1 and stimulation by ascorbic acid. Toxicol Appl Pharmacol 145(1):175–183
Nowak G, Carter CA, Schnellmann RG (2000) Ascorbic acid promotes recovery of cellular functions following toxicant-induced injury. Toxicol Appl Pharmacol 167(1):37–45
Conrad T, Landry GM, Aw TY, Nichols R, McMartin KE (2016) Diglycolic acid, the toxic metabolite of diethylene glycol, chelates calcium and produces renal mitochondrial dysfunction in vitro. Clin Toxicol (Phila) 54(6):501–511
Schep LJ, Slaughter RJ, Temple WA, Beasley DM (2009) Diethylene glycol poisoning. Clin Toxicol (Phila) 47(6):525–35. Erratum in: Clin Toxicol (Phila) 47(8):840
Wiley FH (1938) The formation of oxalic acid from ethylene glycol and related solvents. J Ind Hyg Toxicol 20:269–277
Haag HB, Ambrose AM (1937) Studies of the physiological effect of diethylene glycol: II toxicity and fate. J Pharmacol Exp Ther 59:93–100
Morelle J, Kanaan N, Hantson P (2010) The Case: Cranial nerve palsy and acute renal failure after a “special drink.” Kidney Int 77(6):559–560
Scalzo AJ (1996) Diethylene glycol toxicity revisited: the 1996 Haitian epidemic. J Clin Toxicol 34(5):513–516
Drut R, Quijano G, Jones MC, Scanferla P (1994) Hallazgos patológicos en la intoxicación por dietilenglicol [Pathologic findings in diethylene glycol poisoning]. Medicina (B Aires) 54(1):1–5
Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, Kostrov SV (2016) Cytoplasmic vacuolization in cell death and survival. Oncotarget 7(34):55863–55869
Wordley E (1947) Diethylene Glycol Poisoning: Report on Two Cases. J Clin Pathol 1:44–46
Singh J, Dutta AK, Khare S, Dubey NK, Harit AK, Jain NK et al (2001) Diethylene glycol poisoning in Gurgaon, India, 1998. Bull World Health Organ 79(2):88–95
Heptinstall RH (1968) Pathology of end-stage kidney disease. Am J Med 44(5):656–663
Jain R, Randev S, Kumar P, Guglani V (2021) Acute Kidney Injury and Encephalopathy in a Child: Diethylene Glycol Poisoning. Indian J Pediatr 88(2):194–195
Sosa NR, Rodriguez GM, Schier JG, Sejvar JJ (2014) Clinical, laboratory, diagnostic, and histopathologic features of diethylene glycol poisoning–Panama, 2006. Ann Emerg Med 64(1):38–47
Marraffa JM, Holland MG, Stork CM, Hoy CD, Hodgman MJ (2008) Diethylene glycol: widely used solvent presents serious poisoning potential. J Emerg Med 35(4):401–406
O’Brien KL, Selanikio JD, Hecdivert C, Placide MF, Louis M, Barr DB et al (1998) Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. Acute Renal Failure Investigation Team JAMA 279(15):1175–1180
Imam YZB, Kamran S, Karim H, Elalamy O, Sokrab T, Osman Y et al (2014) Neurological manifestation of recreational fatal and near-fatal diethylene glycol poisonings: case series and review of literature. Medicine (Baltimore) 93(10):e62
Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA (2002) American academy of clinical toxicology Ad Hoc committee on the treatment guidelines for methanol poisoning. American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J Clin Toxicol. 40(4):415–46
Brent J, McMartin K, Phillips S, Aaron C, Methylpyrazole for Toxic Alcohols Study Group (2001) Fomepizole for the treatment of methanol poisoning. N Engl J Med. 344(6):424–429
Brooks DE, Wallace KL (2002) Acute propylene glycol ingestion. J Clin Toxicol 40(4):513–516
Rietjens SJ, de Lange DW, Meulenbelt J (2014) Ethylene glycol or methanol intoxication: which antidote should be used, fomepizole or ethanol? N Engl J Med. 72(2):73–9.z
Brophy PD, Tenenbein M, Gardner J, Bunchman TE (2000) Smoyer WE (2000) Childhood diethylene glycol poisoning treated with alcohol dehydrogenase inhibitor fomepizole and hemodialysis. Am J Kidney Dis. 35(5):958–62
Acknowledgements
This work was partially supported by Brazilian National Council of Research Development (CNPq—Grant # 302153/2019-5), Coordination of High Education Level Personnel (CAPES), and Foundation of Research of Minas Gerais (FAPEMIG—CDS—APQ-02541-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida Araújo, S., Faria, B.C.D., Vasconcelos, J.C. et al. Renal toxicity caused by diethylene glycol: an overview. Int Urol Nephrol 55, 2867–2875 (2023). https://doi.org/10.1007/s11255-023-03604-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03604-2