Abstract
Background
This study investigated the association between insulin-like growth factor-1 and nutritional status indicators in patients undergoing maintenance hemodialysis (MHD).
Methods
Patients undergoing MHD for > 3 months were included in this single-center cross-sectional study in March 2021. Clinical, demographic, and body mass data and blood samples were collected before the hemodialysis sessions. Serum insulin-like growth factor-1 (IGF-1) levels were measured using a radioimmunoassay, and serum IGF-1 standard deviation score (SDS) was calculated for MHD patients according to age and sex. The nutritional status of patients was assessed using serum albumin, serum prealbumin, handgrip strength, pinching strength, upper arm muscle circumference, lean body mass, phase angle, seven-point subjective global assessment (SGA) score, and geriatric nutritional risk index (GNRI). The patients were divided into groups according to tertiles of serum IGF-1 SDS levels. Spearman correlation analyses and univariate and multivariate binary logistic regression analyses were used to determine the association between serum IGF-1 SDS and nutritional status parameters.
Results
A total of 155 MHD patients (male: female = 90:65) were enrolled in the study, with a median dialysis vintage of 28.0 (11.0, 55.0) months, and an average age of 66 (65.5 ± 13.0) years. The median of IGF-1 SDS was − 0.1 (− 0.6 to 0.6). Compared to patients with higher IGF-1 SDSs, patients with lower IGF-1 SDSs had lower levels of serum ceruloplasmin (341.0 [287.5, 416.0] vs 395.0 [327.0, 451.0] vs 409.0 [349.5, 507.5], p = 0.002), serum albumin (34.7 ± 3.0 vs 37.0 ± 3.1 vs 37.8 ± 2.6, p < 0.001), serum prealbumin (270.3 [233.7, 327.8] vs 326.0 [279.3, 355.6] vs 363.0 [324.2, 398.2], p < 0.001), handgrip strength (13.8 [10.0, 20.7] vs 17.7 [10.7, 22.5] vs 23.3 [16.6, 27.8], p < 0.001), pinch strength (4.6 [3.9, 6.0] vs 4.9 (3.9, 6.9) vs 6.5 [4.7, 8.7], p = 0.002), phase angle (3.3 [3.0, 3.8] vs 3.9 [3.4, 4.7] vs 4.3 [3.6, 5.2, p < 0.001), modified Creatinine Index (83.1 ± 19.7 vs 93.1 ± 23.4 vs 113.9 ± 24.3, p < 0.001), intracellular water (14.5 ± 4.4 vs 16.1 ± 4.9 vs 16.9 ± 4.4, p = 0.031), higher extracellular water (26.9 ± 5.8 vs 25.7 ± 5.5 vs 25.1 ± 3.1, p = 0.042), and higher malnutrition risk as defined by GNRI (49.0% vs 15.7% vs 11.5%, p < 0.001) and SGA (53.9% vs 23.5% vs 7.7%, p < 0.001).
Conclusions
Lower IGF-1 SDSs are independently associated with higher malnutrition risk in patients with MHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic kidney disease (CKD) are prone to malnutrition or protein-energy wasting (PEW), which is a strong risk factor for poor prognoses in patients undergoing maintenance hemodialysis (MHD) [1]. The worldwide prevalence of PEW ranges from 11 to 54% in patients with CKD stages 3–5 [2], and between 28 and 54% in patients undergoing dialysis [3]. The prevalence of PEW varies in different studies, depending on the assessment method. The Seven-Point Subjective Global Assessment (SGA) [4] and Geriatric Nutritional Risk Index (GNRI) [5] are widely used tools for PEW assessment. Abnormalities in growth hormone (GH)/insulin-like growth factor-1 (IGF-1) are considered significant factors in the development of skeletal muscle wasting in patients with CKD via inflammation [6]. Studies have shown that lower serum IGF-1 levels are significantly associated with biochemical and anthropometric markers of malnutrition [7, 8]. Researchers have also observed an inverse association between serum IGF-1 levels and nutritional status assessed by traditional SGA in patients undergoing MHD [9]. Since serum IGF-1 levels are strongly related to age or sex, serum IGF-1 SDSs were calculated using the method described by Isojima et al. to minimize the bias of these confounding factors [10].
To the best of our knowledge, no study has analyzed the relationship between standardized IGF-1 levels and indicators of comprehensive nutritional assessment such as the 7-point SGA and GNRI, in patients with MHD. Hence, we designed the present cross-sectional study to explore the relationship between IGF-1 SDS levels and the overall nutritional status indicators in these patients.
Methods and materials
Population and study design
This was a single-center, observational, cross-sectional study of patients undergoing MHD at Guangzhou Red Cross Hospital in March 2021. Patients included (1) had end-stage renal disease (ESRD); (2) performed maintenance hemodialysis regularly for more than 3 months, 3 times a week, and for four hours each time; (3) aged 18–80 years; and (4) provided informed consent. The exclusion criteria were: (1) neurological or mental illness or inability to complete the questionnaire; (2) history of acute heart failure, severe infection, or malignant tumor within 3 months; (3) history of surgery within 3 months; (4) pacemaker use; and (5) refusal to participate in the study.
Research methodology
Demographic, clinical, and laboratory parameters
Data on the following demographic and clinical parameters were collected: history of diabetes, sex, age, and dialysis vintage. Venous blood samples were collected by nurses shortly before the hemodialysis sessions. They were sent to the clinical laboratory of Guangzhou Red Cross Hospital within 2 h. Routine blood tests, serum biochemical tests, and enzyme immunity measurements were performed. Further, the following parameters were measured: Serum creatinine, Kt/V, serum albumin, serum prealbumin, serum IGF-1, serum interleukin-6 (IL-6), serum ceruloplasmin, and serum hypersensitive C-reactive protein (hs-CRP).
Measurement and calculation of IGF-1
Serum IGF-1 was measured by high-performance liquid chromatography-mass spectrometry using a Thermo Q Exactive Focus instrument (Thermo Fisher Scientific, Waltham, MA, USA). The assay was calibrated using standards prior to testing. All reagents used in this study (including inoculated strains, reagent wedges, calibrators, and dilutions) were obtained from the same batch. The intra-group coefficients of variation ranged from 2.4% to 6.3%, and the inter-group coefficients of variation ranged from 3.0% to 7.6%. The sensitivity was 20 μg/L, and the upper limit of detection was 1600 μg/L. The SDS of serum IGF-1 was calculated using the following formula [10]:
Nutritional indicators
Body mass
A body composition analyzer (Bodystat5000, UK) was used to measure the body composition of patients. According to the manufacturer's guidelines, measurements were performed in the supine position, with electrodes attached to the hands and feet on the side without a fistula for hemodialysis. Resistance (R in ohms) and reactance (Xc in ohms) values were recorded at 50 kHz. Patients with implantable electronic devices (e.g., electronic heart pacemakers) were excluded. Lean body mass, phase angle, extracellular water (ECW), ECW%, intracellular water (ICW), ICW%, and the ECW/ICW ratio were measured during the process.
Calculation of nutritional indices
According to the 7-point SGA, a total score of 6 to 7 was classified as normal nutritional status, 3 to 5 as mild to moderate malnutrition, and 1 to 2 as severe malnutrition. A 7-point SGA score ≤ 5 points was used as the diagnostic criterion for malnutrition or PEW [11].
The GNRI was calculated according to the following formula:
where the ideal body weight was calculated as \(22\;({\text{kg/m}}^{2} ) \times {\text{height}}\) [12]. If the actual body weight was above the ideal body weight, the value of “(actual body weight/ideal body weight)" was set to 1. At present, some studies suggest that a GNRI in MHD patients of < 91.2 can be defined as a risk of malnutrition [13].
The mCI was calculated using parameters including sex, age, spKt/V (for urea clearance), and pre-hemodialysis creatinine level, using the following formula [14]:
Grip strength and pinch strength
Grip strength and pinch strength were measured using the BASELINE digital Grip Force Tester (12-0091, Fabrication Enterprises Inc., USA) and the BASELINE digital Pinch Force tester (12-0081, Fabrication Enterprises Inc., USA). The participants were instructed to apply as much grip or pinch force as possible on the instruments with the dominant hand or the hand without fistulae. The measurement was repeated thrice, and the maximum value was recorded.
Anthropometry
Upper arm circumference was measured according to the criteria developed by Frisancho [15]. The upper arm muscle circumference was calculated according to the following formula:
Parameters of hemodialysis
Patients in this study were treated on a Braun Dialog + (B. Braun Co., Ltd., Melsungen, Germany) dialysis machine using a REXEED-15L high-throughput polysulfone membrane dialyzer (Asahi Kasei Corp., Tokyo, Japan) with a membrane area of 1.5 m2, dialysis blood flow rate of 200–300 ml/min, dialysis fluid flow rate of 500 ml/min, and dialysis duration of 4 h.
Statistical analysis
Continuous variables with a normal distribution were described as \(\overline{x }\) ± s, and variables with a non-normal distribution were described as the median (25–75% interquartile range). Categorical variables were described as percentages. Patients were divided into three groups based on their serum IGF-1 SDS levels. Differences in clinical and demographic data among the three groups were assessed by one-way analysis of variance, Kruskal–Wallis test, or χ2 test, as appropriate. Spearman analyses were used to evaluate the correlation between the IGF-1 SDS and nutritional indicators. Univariate analyses were conducted to select potential explanatory variables with dependent variables of malnutrition defined as SGA ≤ 5 and GNRI ≤ 91.2. In multivariate binary logistic regression analyses, potentially relevant variables or known to be important in the physiology of malnutrition: sex, age, dialysis vintage, Kt/V (≥ 1.2), DM, IL-6, phosphorus, and 25-OH-D values, were included to determine whether IGF-1 SDS levels was independently associated with malnutrition. SPSS (version 22.0; IBM Corp, Armonk, NY, USA) was used for statistical analyses. Significance was set at p < 0.05.
Results
Characteristics of the study population
A total of 155 patients with MHD (Fig. 1) were included (male/female = 90:65), with a median dialysis vintage of 28.0 (11.0, 55.0) months and an average age of 66 (65.5 ± 13.0) years. The sample selection flowchart is shown in Fig. 1. The baseline characteristics of the total study population and those after stratification by serum IGF-1 tertile are presented in Table 1. The median serum IGF-1 level was 186.0 (140.0, 248.7) ng/mL. The median of IGF-1 SDS levels were levels − 0.1 (− 0.6 to 0.6). Malnourished patients, as defined by the 7-point SGA and the GNRI, accounted for 28.4% (n = 44) and 25.3% (n = 39) of the study population, respectively. Patients with lower serum IGF-1 levels were older and had lower levels of serum creatine, serum ceruloplasmin, serum albumin, serum prealbumin, handgrip strength, pinch strength, upper arm circumference, upper arm muscle circumference, phase angle, modified Creatinine Index (mCI), ICW, higher levels of ECW%, serum IL-6, and higher malnutrition risk, as defined by the SGA and GNRI values (all p < 0.05).
Correlations between IGF-1 SDS and nutritional indicators
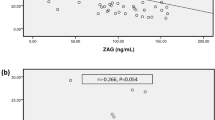
In Spearman’s analyses, we observed that the levels of serum IGF-1 SDS were positively correlated with the values of grip strength (r = 0.32, p < 0.001), pinch strength (r = 0.28, p < 0.001), phase angle (r = 0.33, p < 0.001), lean body mass (r = 0.30, p < 0.001), serum albumin (r = 0.42, p < 0.001), serum prealbumin (r = 0.52, p < 0.001), mCI (r = 0.52, p < 0.001), ICW (r = 0.21, p = 0.012), and were negatively correlated with the levels of ECW% (r = − 0.22, p = 0.010) (Fig. 2). There were no associations between ECW (r = 0.13, p = 0.141), ICW% (r = 0.02, p = 0.800), and ECW/ICW (r = − 0.11, p = 0.180).
Associations between the serum IGF-1 and nutrition risk defined by different nutritional indicators
The results of univariate binary logistic regression analysis are shown in Table 2. We found that the lowest tertile of IGF-1 SDS levels (SGA ≦ 5: OR = 13.71, 95% CI = 4.31–43.61, p < 0.001; GNRI ≦ 91.2: OR = 7.21, 95% CI = 2.62–19.87, p < 0.001), mCI, and ICW values were related to malnutrition defined by the 7-point SGA and GNRI. Higher levels of serum hs-CRP and IL-6 were related to the malnutrition as defined by the GNRI.
The multivariate binary logistic regression results are presented in Table 3. After partial or full adjustment for sex, age, dialysis vintage, Kt/V (≥ 1.2), diabetes mellitus(DM), serum IL-6, serum 25 hydroxyvitamin D3, and serum phosphorus levels, we found that the lowest tertile of IGF-1 SDS levels ( SGA ≦ 5: OR = 9.69, 95% CI = 2.49–37.62, p = 0.001; GNRI ≦ 91.2: OR = 5.71, 95% CI = 1.64–19.89, p = 0.006) were independently positively associated with malnutrition defined as SGA ≤ 5 and GNRI ≤ 91.2.
Discussion
Our study showed that the patients with lower serum IGF-1 SDS levels had lower serum levels of creatine, ceruloplasmin, albumin, and prealbumin; higher serum levels of IL-6; lower values of grip strength, pinch strength, upper arm circumference, upper arm muscle circumference, phase angle, and modified Creatinine Index (mCI); and higher malnutrition defined by the two nutrition indicators, SGA and GNRI. After adjusting for confounding factors in multivariate binary logistic regression models, we found that the lowest quartile of IGF-1 SDS levels were independently associated with malnutrition defined by SGA and GNRI.
Disorder of the GH/IGF-1 axis may occur at any stage of CKD. Notably, IGF-1 is mainly produced in the liver. Circulating insulin-like growth factor-binding proteins (IGFBPs), which are transport proteins, modulate IGF-1 bioavailability, prolong its half-life, and regulate its activity in target tissues [16]. Normal total IGF levels in uremia are thought to result from decreased IGF degradation caused by enhanced IGFBP binding. Binding to IGFBP protects IGF1 from metabolic degradation, but could also inhibit IGF1 interaction with its receptor, resulting in impaired IGF1 bioactivity [17]. It has been shown that serum IGF-I levels vary with age, gender, puberty, physiological status, and ethnicity [18]. In one study, serum IGF-1 was evaluated as the SDS of IGF-1 levels based on a Japanese population reference range established according to age and sex [10].
GH and IGF-1 resistance may adversely affect metabolism and nutritional status in adults with ESRD [19]. In another study, researchers observed that protein catabolism was also associated with impaired IGF-I signaling pathways in ESRD-related sarcopenia [20]. Serum IGF-1 is a positive marker of skeletal muscle strength and mass in patients undergoing hemodialysis [21].
In addition, phase angle (PhA) is a parameter obtained from direct measurements of bioelectrical impedance analysis (BIA). It is widely used as a marker of cellular health and has been recognized as a valuable measure for nutritional assessment, reflecting both muscle mass and muscle function [22, 23]. Extracellular body water (ECW) and intracellular body water (ICW) measured using BIA have also been introduced as markers, and studies have shown that the ECW/ICW ratio is associated with malnutrition [24]. The IGF-deficient group has been found to have a lower PhA, ICW%, and higher ECW values [25]. Other researchers have also found a correlation between serum IGF-1 and biochemical and anthropological parameters of malnutrition in patients with MHD [26, 27]. Our results showed that serum albumin, serum prealbumin, grip strength, pinch strength, body phase angle, lean body mass, mCI, and ICW were all significantly and positively correlated with serum IGF-1 SDS, and ECW% was negatively correlated with serum IGF-1 SDS, which is consistent with the results of a previous study [24,25,26,27]. However, our study did not find a correlation between serum IGF-1 SDS and the ECW/ICW ratio.
However, the underlying factors associated with these findings are complex. First, IGF-1 induces protein synthesis and myogenesis by activating the Akt/mTOR pathway, resulting in the growth and repair of skeletal muscles [20]. Together with our results, these findings indicate that serum IGF-1 levels may reflect protein anabolism and skeletal muscle function in MHD patients. Second, lower serum IGF-1 levels may reflect inflammatory status. Chronic inflammation has been reported to disrupt the GH/IGF-1 axis through relative GH and/or IGF-1 insufficiency, peripheral resistance to GH/IGF-1 receptors, inhibition of GH/IGF-1 signaling, dysregulation of IGF-binding proteins, reduced IGF-1 bioavailability, and altered gene regulation through the microRNA system [28,29,30,31,32].
Clinical data showed significantly higher levels of circulating IL-6 and IL-6 receptors in older individuals [33], with IL-6 levels being a significant predictor of sarcopenia [34]. Lastly, there is a negative correlation between plasma levels of CRP and IL-6 and rates of mixed muscle and myosin heavy chain protein synthesis in a population-based study of the general community, further illustrating the likelihood of inflammation contributing to reduced protein synthesis in sarcopenia [35]. Researchers have observed that inflammation and malnutrition in patients with ESRD often coexist and are mutually causal, a condition termed malnutrition–inflammation complex syndrome (MICS) [36]. Possible causes of MICS include comorbidities, oxidative and carbonyl stress, nutrient loss through dialysate, anorexia, loss of appetite, uremic toxins, decreased clearance of inflammatory cytokines, and volume overload [37]. In conclusion, a possible mechanism for attenuated skeletal muscle protein synthesis in CKD may be suppression of insulin/IGF-1 signaling due to metabolic acidosis, upregulated pro-inflammatory cytokine expression, and malnutrition due to anorexia [38].
Finally, in persistent systemic and tissue inflammation, the impairment of IGF-I-mediated signaling pathways preferentially increases muscle protein catabolism and inhibits muscle protein anabolism, resulting in excessive muscle protein loss, high hospitalization rates, and high rates of cardiovascular events and mortality among patients with CKD and ESRD [37, 39]. In our study, we also found that the group with a lower IGF-1 SDS had higher IL-6 levels; therefore, the results support the above notion.
This study has some limitations. First, this was a cross-sectional observational study, and causal conclusions could not be drawn. Second, given that our study had a small sample size, bias cannot be ruled out. Third, our results cannot exclude the possibility of residual confounding. Lastly, we did not measure serum IGFBPs and growth hormone levels; therefore, the relationship between the GH/IGF-1 axis and nutritional status in patients with MHD remains unclear.
Although a crossover clinical trial and randomized controlled trial have shown that the application of recombinant human growth hormone and IGF-1 could improve protein synthesis and metabolism in patients with ESRD [40, 41], these studies included only a small number of patients with short follow-up periods. However, there are currently no prospective studies with large enough sample sizes or sufficient evidence to determine the potential long-term side effects of recombinant IGF-1 or GH supplementation in patients with MHD.
Our study confirmed that higher serum IGF-1 SDS levels are significantly associated with better nutritional status as assessed by biochemical markers, anthropometric measures, body composition parameters, and comprehensive nutrition assessment. Future observational and interventional studies with larger sample sizes and multicenter are still needed to provide a theoretical basis for routine clinical application of IGF-1 preparations to improve nutritional status in patients with ESRD and dialysis.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- MHD:
-
Maintenance hemodialysis
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- SGA:
-
Seven-point subjective global assessment
- GNRI:
-
Geriatric Nutritional Risk index
- GH:
-
Growth hormone
- IGFBPs:
-
Insulin-like growth factor bind proteins
- IGF-1:
-
Insulin-like growth factor-1
- IGF-1 SDS:
-
Insulin-like growth factor-1 standard deviation score
- PEW:
-
Protein-energy wasting
- DM:
-
Diabetes mellitus
- IL-6:
-
Interleukin-6
- hs-CRP:
-
Hypersensitive C-reactive protein
- mCI:
-
Modified Creatinine Index
- ECW:
-
Extracellular body water
- ICW:
-
Intracellular body water
References
Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K (2015) Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 18:254–262. https://doi.org/10.1097/MCO.0000000000000171
Koppe L, Fouque D, Kalantar-Zadeh K (2019) Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle 10:479–484. https://doi.org/10.1002/jcsm.12421
Carrero JJ, Thomas F, Nagy K et al (2018) Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of renal nutrition and metabolism. J Ren Nutr 28:380–392. https://doi.org/10.1053/j.jrn.2018.08.006
Fouque D, Kalantar-Zadeh K, Kopple J et al (2008) A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73:391–398. https://doi.org/10.1038/sj.ki.5002585
Takahashi H, Inoue K, Shimizu K et al (2017) Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Ren Nutr 27:201–206. https://doi.org/10.1053/j.jrn.2016.12.005
Gungor O, Ulu S, Hasbal NB, Anker SD, Kalantar-Zadeh K (2021) Effects of hormonal changes on sarcopenia in chronic kidney disease: where are we now and what can we do? J Cachexia Sarcopenia Muscle 12:1380–1392. https://doi.org/10.1002/jcsm.12839
Besbas N, Ozaltin F, Coşkun T et al (2003) Relationship of leptin and insulin-like growth factor I to nutritional status in hemodialyzed children. Pediatr Nephrol 18:1255–1259. https://doi.org/10.1007/s00467-003-1264-4
Wang X, Tian F, Sun H et al (2019) Insulin-like growth factor-1 as a nutritional monitoring factor in patients with chronic intestinal failure. Clin Nutr 38:1737–1744. https://doi.org/10.1016/j.clnu.2018.07.031
Jia T, Gama Axelsson T, Heimbürger O et al (2014) IGF-1 and survival in ESRD. Clin J Am Soc Nephrol 9:120–127. https://doi.org/10.2215/CJN.02470213
Isojima T, Shimatsu A, Yokoya S et al (2012) Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J 59:771–780. https://doi.org/10.1507/endocrj.EJ12-0110
Cuppari L, Meireles MS, Ramos CI, Kamimura MA (2014) Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J Ren Nutr 24:385–389. https://doi.org/10.1053/j.jrn.2014.05.004
Shah B, Sucher K, Hollenbeck CB (2006) Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract 21:312–319. https://doi.org/10.1177/0115426506021003312
Yamada K, Furuya R, Takita T et al (2008) Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr 87:106–113. https://doi.org/10.1093/ajcn/87.1.106
Canaud B, Granger Vallée A, Molinari N et al (2014) Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS ONE 9:e93286. https://doi.org/10.1371/journal.pone.0093286
Frisancho AR (1974) Triceps skin fold and upper arm muscle size norms for assessment of nutrition status. Am J Clin Nutr 27:1052–1058. https://doi.org/10.1093/ajcn/27.10.1052
Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB (2019) The insulin like growth factor and binding protein family: novel therapeutic targets in obesity and diabetes. Mol Metab 19:86–96. https://doi.org/10.1016/j.molmet.2018.10.008
Tönshoff B, Kiepe D, Ciarmatori S (2005) Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr Nephrol 20:279–289. https://doi.org/10.1007/s00467-005-1821-0
Löfqvist C, Andersson E, Gelander L et al (2001) Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab 86:5870–5876. https://doi.org/10.1210/jcem.86.12.8117
Johannsson G, Ahlmén J (2003) End-stage renal disease: endocrine aspects of treatment. Growth Horm IGF Res 13(Suppl A):S94–S101. https://doi.org/10.1016/s1096-6374(03)00063-7
Mori K (2021) Maintenance of skeletal muscle to counteract sarcopenia in patients with advanced chronic kidney disease and especially those undergoing hemodialysis. Nutrients 13:1538. https://doi.org/10.3390/nu13051538
Delanaye P, Bataille S, Quinonez K et al (2019) Myostatin and insulin-like growth factor 1 are biomarkers of muscle strength, muscle mass, and mortality in patients on hemodialysis. J Ren Nutr 29:511–520. https://doi.org/10.1053/j.jrn.2018.11.010
Norman K, Stobäus N, Pirlich M, Bosy-Westphal A (2012) Bioelectrical phase angle and impedance vector analysis—clinical relevance and applicability of impedance parameters. Clin Nutr 31:854–861. https://doi.org/10.1016/j.clnu.2012.05.008
Sukackiene D, Rimsevicius L, Miglinas M (2022) Standardized phase angle for predicting nutritional status of hemodialysis patients in the early period after deceased donor kidney transplantation. Front Nutr 9:803002. https://doi.org/10.3389/fnut.2022.803002
Yajima T, Yajima K, Takahashi H, Yasuda K (2019) Combined predictive value of extracellular fluid/intracellular fluid ratio and the geriatric nutritional risk index for mortality in patients undergoing hemodialysis. Nutrients 11(11):2659. https://doi.org/10.3390/nu11112659
Muscogiuri G, Barrea L, Laudisio D et al (2019) Somatotropic axis and obesity: is there any role for the Mediterranean diet? Nutrients 11(9):2228. https://doi.org/10.3390/nu11092228
Jacob V, Le Carpentier JE, Salzano S et al (1990) IGF-I, a marker of undernutrition in hemodialysis patients. Am J Clin Nutr 52:39–44. https://doi.org/10.1093/ajcn/52.1.39
Qureshi AR, Alvestrand A, Danielsson A et al (1998) Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int 53:773–782. https://doi.org/10.1046/j.1523-1755.1998.00812.x
Witkowska-Sędek E, Pyrżak B (2020) Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Cent Eur J Immunol 45:469–475. https://doi.org/10.5114/ceji.2020.103422
Cirillo F, Lazzeroni P, Catellani C et al (2018) MicroRNAs link chronic inflammation in childhood to growth impairment and insulin-resistance. Cytokine Growth Factor Rev 39:1–18. https://doi.org/10.1016/j.cytogfr.2017.12.004
Cirillo F, Lazzeroni P, Sartori C, Street ME (2017) Inflammatory diseases and growth: effects on the GH-IGF axis and on growth plate. Int J Mol Sci 18:1878. https://doi.org/10.3390/ijms18091878
Wong SC, Dobie R, Altowati MA et al (2016) Growth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr Rev 37:62–110. https://doi.org/10.1111/j.1365-2265.2010.03799.x
Soendergaard C, Young JA, Kopchick JJ (2017) Growth hormone resistance–special focus on inflammatory bowel disease. Int J Mol Sci 18:1019. https://doi.org/10.3390/ijms18051019
Roubenoff R, Harris TB, Abad LW et al (1998) Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci 53:M20–M26. https://doi.org/10.1093/gerona/53A.1.M20
Payette H, Roubenoff R, Jacques PF et al (2003) Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham heart study. J Am Geriatr Soc 51:1237–1243. https://doi.org/10.1046/j.1532-5415.2003.51407.x
Toth MJ, Matthews DE, Tracy RP, Previs MJ (2005) Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288:E883–E891. https://doi.org/10.1152/ajpendo.00353.2004
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263. https://doi.org/10.1053/ajkd.2001.29222
Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD (2003) Malnutrition–inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42:864–881. https://doi.org/10.1016/j.ajkd.2003.07.016
Gordon BS, Kelleher AR, Kimball SR (2013) Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45:2147–2157. https://doi.org/10.1016/j.biocel.2013.05.039
Zha Y, Qian Q (2017) Protein nutrition and malnutrition in CKD and ESRD. Nutrients 9(3):208. https://doi.org/10.3390/nu9030208
Fouque D, Peng SC, Shamir E, Kopple JD (2000) Recombinant human insulin-like growth factor-1 induces an anabolic response in malnourished CAPD patients. Kidney Int 57:646–654. https://doi.org/10.1046/j.1523-1755.2000.00886.x
Feldt-Rasmussen B, Lange M, Sulowicz W et al (2007) Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 18:2161–2171. https://doi.org/10.1681/ASN.2006111207
Acknowledgements
The authors are indebted to all nephrologists and nurses in the nephrology department of Guangzhou Red Cross Hospital, Jinan University for their excellent management of hemodialysis patients. We thank the patients and staff involved in this cross-sectional study. We especially thank Shilin Xu, B.S. Nurs, because all the laboratory data in the study were derived from the electronic management system for blood purification center (Hope®, software) developed by him, which can import the laboratory test results according to the patients’ IDs included in the study.
Funding
This work was supported by the Science and Technology Program of Guangdong Province (Grant No. 2017B090904027), the Research Program of Sports Bureau of Guangdong Province (Grant No. GDSS2020M003), the Foundation for Young Talents of Chinese Nutrition Society (Chinese Nutrition Society Office (2020) No. 51), the Research-oriented Hospital Program of Guangzhou (Grant No. 2022RHPG05), And the Guangzhou Clinically Characteristic Technology Project (Grant No. 2019TS60).
Author information
Authors and Affiliations
Contributions
TX contributed to the study design, partial data collection, and drafting of the manuscript. Yao Xu was involved in the analysis and interpretation of the data. JL, LW, QX, WL, and PL collected data. YL, RT, YL, and XZ were involved in the study design and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This study was approved by the Ethics Committee of Guangzhou Red Cross Hospital [No. 2021-066-01]. This study adhered to the tenets of the Declaration of Helsinki and the Guidance on Sample Collection of Human Genetic Diseases by the Ministry of Public Health of China and does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, T., Xu, Y., Li, J. et al. Associations between insulin-like growth factor-1 standard deviation score and overall nutritional parameters in patients with maintenance hemodialysis: a cross-sectional study. Int Urol Nephrol 55, 2257–2266 (2023). https://doi.org/10.1007/s11255-023-03526-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03526-z