Abstract
Background
This cohort study investigates the association between insulin growth factor-1 (IGF-1), bone mineral density, and frailty phenotype in children with chronic kidney disease (CKD).
Methods
Forty-six patients (median age 14.5 years) were prospectively enrolled. Frailty phenotype was defined as the presence ≥ 3 of the following indicators: suboptimal growth/weight gain (body mass index height age < 5th percentile or height < 3rd percentile or loss of ≥ 10 percentiles/year in at least one parameter), low muscle mass (lean tissue mass height age < 5th percentile or loss of ≥ 10 percentiles/year), general fatigue reported by parent or child, and C-reactive protein > 3 mg/l. Lumbar bone mineral apparent density (LBMAD) was measured by dual-energy X-ray absorptiometry, body composition by bioimpedance spectroscopy, and IGF-1 by enzyme-labeled chemiluminescent immunometric assay.
Results
Frailty phenotype (seven patients) was more frequent in advanced CKD (estimated glomerular filtration rate < 30 ml/min/1.73m2) (p = 0.014). IGF-1 and LBMAD z-scores were lower in patients with suboptimal growth/weight gain (14 patients) (p = 0.013, p = 0.012), low muscle mass (nine patients) (p = 0.001, p = 0.009), and general fatigue (eight patients) (p < 0.001, p = 0.004). IFG-1 and LBMAD z-scores were associated with frailty phenotype (OR 0.109, 95% CI 0.015–0.798 and OR 0.277, 95% CI 0.085–0.903) after adjustment for CKD stage. IGF-1 z-score was associated with LBMAD < 5th percentile (six patients) (OR 0.020, 95% CI 0.001–0.450) after adjustment for CKD stage. The association between LBMAD and frailty phenotype lost significance after adjustment for IGF-1.
Conclusion
Frailty phenotype is more frequent in advanced pediatric CKD. IGF-1 is negatively associated with frailty phenotype and interferes in the association between frailty and LBMAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a condition, initially recognized in elderly populations, characterized by a decline in resistance to minor stress events due to reduced biological reserves [1]. It is the outcome of accelerated and cumulative damage across multiple homeostatic mechanisms and physiological processes, at a molecular and cellular level, resulting in increased vulnerability to adverse health outcomes, such as falls, disability, fractures, hospitalization, and mortality [1]. Genetic factors, aging, environmental factors, and chronic diseases are the major underlying pathogenetic mechanisms. Frailty phenotype, including weight loss, muscle weakness, poor endurance, and low physical activity, is frequently reported in adult patients with chronic kidney disease (CKD); it concerns more than 60% of patients with chronic kidney disease stage 5 treated by dialysis (CKD 5D) [2]. According to a recent large-scale study, this condition may also affect pediatric patients and is associated with increased infection and hospitalization rates [3]. Understanding the multifaceted etiology of frailty is of high interest in the pediatric population, because it may open windows toward early recognition, prevention, and management of this condition. In general, chronic inflammation, musculoskeletal damage, alterations in the hypothalamic-pituitary axis, nutritional changes, and physical impairment have been implicated in the pathogenesis of frailty [4,5,6]. In adult CKD, anorexia and reduced physical activity combined with the accumulation of pro-inflammatory cytokines, metabolic acidosis, and hormonal imbalance, such as vitamin D deficiency and insulin-like growth factor-1 (IGF-1) signaling derangement, may contribute to the development of frailty [2, 7]. Data regarding the risk factors of frailty in pediatric CKD are lacking.
IGF-1 is synthesized by the liver, in response to growth hormone (GH) signaling, as well as by the muscle, bone, and nerve cells. This hormone plays a significant role in the maintenance of skeletal muscle mass and strength by promoting myoblast proliferation and differentiation and inhibiting muscle protein breakdown [5, 8], exerts potential neurotrophic and neuroprotective effects [5], and regulates bone growth and remodeling, mediating a muscle-bone cross-talk [8]. In CKD, IGF-1 secretion and bio-availability are decreased through impaired GH signaling and increased accumulation of insulin growth factor binding proteins [9]. In detail, free bioactive IGF-1 reduction due to increased hepatic production of inhibitory IGF-1 binding proteins may be accompanied by IGF-1 insufficiency, secondary to decreased expression of GH-receptor in target organs and/or defective GH-induced activation of cellular JAK/STAT pathway [10]. According to clinical studies, children with early CKD present low or normal IGF-1 levels, when compared to healthy children [11], while reduced IGF-1 levels may be more prevalent in advanced CKD [12]. Frailty has been associated with low IGF-1 levels [13, 14] and is considered a predictive risk factor of osteoporosis and fracture risk in the adult population [15, 16]. Pediatric CKD is a weakening bone disease, impairing bone mineralization and turnover [17], possibly leading to low bone mineral density (BMD) and increased fracture risk [18, 19]. The purpose of this study is to investigate the association between IGF-1, BMD, and frailty phenotype in children with CKD.
Methodology
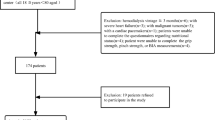
We conducted a cohort study from January 2019 to March 2020 on the prospective collection of data of children and adolescents with CKD, followed up at the 1st and 3rd Department of Pediatrics at the Hippokration General Hospital of Thessaloniki. Inclusion criteria included the following: (i) age of participants ranging from 6 to 19 years old; (ii) estimated glomerular filtration rate (eGFR) < 90 ml/min/1.73 m2, as calculated by the revised Schwartz formula; and (iii) absence of mobility impairment due to neurological disorders. Advanced CKD was defined as eGFR < 30 ml/min/1.73m2.
Frailty phenotype definition was adapted using the model proposed by the prospective multi-center cohort study of North American children with CKD, CKiD (Chronic Kidney Disease in Children),and was determined as the presence of ≥ three of the four indicators: suboptimal growth/weight gain, low muscle mass, fatigue, and inflammation [4]. Suboptimal growth/weight gain was defined as the presence of body mass index adjusted to height age (BMI HA) lower than the 5th percentile (z-score − 1.65) or height lower than the 3rd percentile (z-score − 1.88 SD), or loss of ≥ 10 percentiles/year in one of two parameters. Weight, height, and BMI z-scores were calculated according to the World Health Organization (WHO) reference values for healthy children of the same sex and age. Height age (HA) corresponded to the child’s height when plotted at the 50th percentile on the WHO height for age growth charts. Low muscle mass was defined as the presence of lean tissue mass adjusted to height age (LTM HA) lower than the 5th percentile (z-score − 1.65) or loss of ≥ 10 percentiles/year, based on the body composition data using a portable multi-frequency bioimpedance spectroscopy (BIS) device (body composition monitor, BCM, Fresenius) and application of body composition reference curves for healthy children in the UK [20]. The participants were instructed to refrain from eating and drinking 8 h before measurement. All measurements in patients on dialysis were performed at their dry weight. For patients on peritoneal dialysis, BIS measurement was effectuated 3 h post-dialysis session with an empty abdomen, and for patients on hemodialysis 1 h after the end of the session. Electrodes were placed on the left hand and foot with the patient in the supine position. Fat tissue mass (FTM) and LTM were derived from the impedance data. The annual assessment of general fatigue was estimated by using the Greek version of the PedsQL Multidimensional Fatigue Scale questionnaire for the “general fatigue” subscale, which includes six items, evaluated on a 5-point Likert scale, where 0 = never a problem, 1 = rarely a problem, 2 = sometimes a problem, 3 = often a problem, and 4 = always a problem. The items were reverse-scored and transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0) so that higher scores indicate less fatigue symptoms. Subscale score was computed as the sum of the item scores divided by the number of items. General fatigue was recognized for a score ≤ 50, corresponding to a moderate/severe response to the questionnaire reported by the child or parent [21]. A similar approach for the definition of fatigue has been used in other studies regarding children with CKD [3, 22]. Finally, inflammation was defined as the presence of C-reactive protein (CRP) > 3 mg/dl during the last year [3].
Lumbar bone mineral density was assessed by dual-energy X-ray absorptiometry (GE Health, Lunar Prodigy), which was performed within 1 month from the body composition assessment. We used lumbar (L1–L4) bone mineral apparent density (LBMAD) z-scores, based on reference levels of healthy children of the same age and sex [23], while LBMAD z-score < − 1.65 (< 5th percentile) was considered low. LBMAD was calculated by dividing the sum of bone mineral content (BMC) of the lumbar spine vertebrae by the sum of lumbar spine vertebral volume, which was estimated for each vertebra by the following equation: projected area of the vertebra ^ 1.5 [23]. Finally, insulin growth factor-1 (IFG-1) was measured in all patients in the same laboratory on the day of body composition assessment with the patients on an empty stomach, using a solid-phase enzyme-labeled chemiluminescent immunometric assay on the Siemens Immulite 2500 analyzer (Siemens Healthcare Medical Diagnostics, Bad Nauheim, Germany). For the purposes of this study, we used IGF-1 z-scores according to sex, age, and pubertal status of the participants based on the reference values of a large cohort study of Swedish healthy children and adolescents [24]. Finally, treatment with recombinant GH (rGH) therapy was recorded.
Data are expressed as median values and ranges. All statistical tests were performed using SPSS Statistics software for Windows. Spearman correlation test was used to assess the correlations between the on-study parameters. Mann Whitney and Fisher’s exact tests were used to compare the distribution of the on-study parameters between different patient groups. Univariate and multiple logistic regression tests were performed to detect risk factors of frailty. A p value of < 0.05 was considered statistically significant.
Results
Forty-six children and adolescents—30 males and 16 females—were included in this study with a median age of 14.5 years (6.2–18.9 years). The etiology of CKD was congenital abnormalities of kidney and urinary tract in 28 (60.9%), hemolytic uremic syndrome in five (10.9%), ciliopathy in four (8.7%), focal segmental glomerulosclerosis in three (6.5%), autosomal recessive polycystic kidney disease in one (2.2%), familiar hyperuricemic nephropathy in one (2.2%), chronic interstitial nephritis in one (2.2%), and unknown in three (6.5%) patients respectively. Advanced CKD was present in 13 (28.3%) patients. Among them, nine patients were on dialysis; seven on peritoneal dialysis, and two on hemodialysis. Of note, rGH therapy was administered to six patients for a median duration of 15 months (5–28 months). In total, frailty indicators, including suboptimal growth/weight gain, low muscle mass, general fatigue, and inflammation, were remarked in 14 (30.4%), nine (19.6%), eight (17.4%), and zero patients respectively, leading to frailty phenotype in seven patients (15.2%). Presence of at least one frailty indicator was present in 16 (34.8%) patients. Suboptimal growth/weight gain and low muscle mass were more frequent in patients with general fatigue (p < 0.001 and p < 0.001, respectively) (Fig. 1).
Patient’s age or sex did not influence the presence of frailty phenotype (Table 1). The distribution of anthropometric parameters and frailty indicators according to CKD stage is illustrated in Table 2. Frailty indicators, involving suboptimal growth, low muscle mass, and general fatigue, were more often observed in patients with advanced CKD (p = 0.041, p = 0.092, and p = 0.031, respectively), while frailty phenotype was significantly more frequent in patients with advanced CKD (38.5%) compared to early-stage CKD (6.1%). It is worth noting that children with ≥ 1 frailty indicators or frailty phenotype were more frequently under rGH therapy (six patients) (p = 0.015 and p = 0.037 respectively).
Distribution of IGF-1 levels did not differ between patients with or without advanced CKD (Table 2). IGF-1 z-score level was significantly lower in patients with suboptimal growth, low muscle mass, and general fatigue (p = 0.013, p = 0.001, and p < 0.001, respectively) (Fig. 2) and was associated with suboptimal growth (OR 0.482, 95% CI 0.241–0.966, p = 0.04), low muscle mass (OR 0.218, 95% CI 0.061–0.780, p = 0.019), and general fatigue (OR 0.144, 95% CI 0.028–0.747, p = 0.021) after adjustment for CKD stage. Moreover, a significant negative association was observed between IGF-1 z-score and presence of ≥ 1 frailty indicators (p = 0.012) or frailty phenotype (p < 0.001) (Table 1). Among the correlation tests between IGF-1 z-score and anthropometric parameters, we found that IGF-1 z-score was significantly correlated to height (rs = 0.378, p = 0.010) and LTM HA (rs = 0.353, p = 0.016) z-score. No correlation was observed between IGF-1 and weight (rs = 0.211, p = 0.159), FTM (rs = 0.066, p = 0.661), or BMI HA (rs = − 0.023, p = 0.881) z-scores. Of note, no statistically significant association was observed between rGH therapy and IGF-1 z-score levels (p = 0.108).
In the current study, LBMAD level was low (< 5th percentile) in six patients (13%) and did not differ between patients presenting or not advanced CKD (Table 2). LBMAD z-score level was significantly lower in patients with suboptimal growth, low muscle mass, and general fatigue (p = 0.012, p = 0.009, and p = 0.004, respectively) (Fig. 2) and was associated with suboptimal growth (OR 0.501, 95% CI 0.275–0.911, p = 0.024), low muscle mass (OR 0.394, 95% CI 0.175–0.889, p = 0.025), and general fatigue (OR 0.308, 95% CI 0.112–0.818, p = 0.023) after adjustment for CKD stage. LBMAD z-score was significantly lower in patients with ≥ 1 frailty indicators (p = 0.011) or frailty phenotype (p = 0.005) (Table 1). Among the correlation tests between LBMAD and anthropometric parameters, we found that LBMAD z-score was significantly correlated only to LTM HA (rs = 0.324, p = 0.028). No correlation was observed between LBMAD and weight (rs = 0.210, p = 0.161), height (rs = 0.103, p = 0.496), FTM (rs = 0.197, p = 0.190), or BMI HA (rs = 0.153, p = 0.309) z-scores. Of note, no statistically significant association was observed between rGH therapy and LBMAD z-score levels (p = 0.115).
In this study, LBMAD was correlated to IGF-1 z-score level (rs = 0.437, p = 0.002), and patients with low LBMAD z-score levels presented significantly lower IGF-1 z-score levels (p = 0.002) (Fig. 3). IGF-1 was significantly associated with low LBMAD z-score after adjustment for CKD stage (OR 0.020, 95% CI 0.001–0.450, p = 0.014). In univariate logistic regression analysis, IGF-1 z-score, LBMAD z-score, and CKD stage were identified as risk factors for frailty phenotype (OR 0.065, 95% CI 0.008–0.558, p = 0.013; OR 0.343, 95% CI 0.138–0.850, p = 0.021; and OR 3.076, 95% CI 1.263–7.489, p = 0.013, respectively) (Table 3). Both IGF-1 z-score and LBMAD z-score were significantly associated with frailty phenotype after adjustment for CKD stage. Nevertheless, the association between frailty phenotype and LBMAD or CKD stage lost significance after adjustment for IGF-1 z-score level.
Discussion
Chronic diseases are considered a major factor incriminated for the development of frailty in adult patients, but data in pediatric populations are scarce [25]. According to a recent multi-center pediatric study in children with liver disease, 46% of patients with end-stage liver disease were frail. Data on the prevalence of frailty in children with CKD are limited to one large-scale North American pediatric study, where frailty concerned 13% of the pediatric population with early-stage CKD. After application of the proposed Sgambat et al. frailty model in our study, we found that only 6.1% of children with early-stage CKD presented frailty, whereas the overall prevalence was 15.2%. The discrepancy between the two studies may be due to the different ethnicity, physical activity, or nutritional status between the populations. To our knowledge, this is the first European pediatric single-center study on frailty in children with CKD. Further large-scale studies are needed to clarify the origin of the differences in the frailty prevalence among the pediatric nephrology units in Europe. Moreover, the larger proportion of frail patients with advanced CKD in our study emphasizes the urgent need for frailty screening in these patients. Our results are in concordance with adult studies, where frailty is more frequent in CKD 5D patients [26]. This observation may be attributed to concomitant decreased physical activity and impaired nutritional status combined with the decline in residual kidney function [26]. In any case, taking into consideration the critical role of frailty on the overall morbidity, regular monitoring for frailty parameters is worth applying in all children with CKD.
Although frailty phenotype has been already introduced as a clinical syndrome by Fried et al. in the early 2000s, frailty indicators in CKD are not universal and are adapted to the available screening tools and the particular characteristics of the study population [1, 26]. The frailty indicators recently proposed for pediatric populations are suboptimal growth/weight gain, sarcopenia or enhanced muscle mass loss, fatigue, and inflammation. Among them, general fatigue was the predominant factor of frailty in our study; all but one patient with general fatigue presented frailty. Fatigue has been reported in children with CKD, with a higher prevalence in advanced CKD [22]. This clinical finding was observed in our study as well, emphasizing the need for regular assessment of fatigue by appropriate questionnaires in this at-risk population. Moreover, we remarked that general fatigue was significantly associated with suboptimal growth/weight gain and low muscle mass, indicating the role of growth disorders on the occurrence of low energy in pediatric populations. Interestingly, FTM distribution did not differ between frail and not frail patients, which may help differentiate frailty from protein and energy wasting (PEW) syndrome, where adipose tissue is generally reduced [27]. In detail, PEW is defined as loss of body protein and energy reserves, partly induced by low protein and energy intake, leading to loss of muscle and ultimately adipose tissue [27]. Therefore, although frailty and PEW may be combined in certain cases and lead to cachexia phenotype, frailty is a distinct condition and should be separately examined.
In the current study, IGF-1 was significantly inversely associated with all frailty indicators, and with frailty phenotype, regardless of CKD stage. Although IGF-1 has been associated with frailty in elderly populations, this is the first study to demonstrate such a link in a pediatric CKD population [13, 14]. Apart from its role in growth, IGF-1 is widely considered a valuable nutritional marker, while lower IGF-1 levels have been associated with several adverse events including mortality and malnutrition in CKD 5D patients [28,29,30,31]. In fact, this hormone may reflect muscle protein reserves, since it mediates growth-stimulating effects of circulating growth hormone on muscle tissue and exerts independent autocrine and paracrine effects as well [8]. At a molecular level, IGF-1 isoforms are suggested to promote mitochondrial biogenesis, autophagy, and neuromuscular junction integrity in muscle tissue and induce local antioxidant and anti-inflammatory activity, ultimately leading to muscle growth and sarcopenia prevention [32]. Moreover, in our study, IGF-1 z-score levels were significantly lower in patients with fatigue. Association between fatigue and IGF-1 has already been observed in various populations, such as in adult patients with chronic fatigue syndrome [33] and in children with inflammatory bowel disease [34]. This finding is probably due to the exhaustion secondary to the poor muscle performance observed in these patients. Nevertheless, newly explored pathways may additionally justify the link between IGF-1 and fatigue. Depressive behavior, frequently reported in patients with fatigue, may be due to the reduced neurotrophic effects of IGF-1 [35]. In addition, sleep disorders, which are relatively common in exhausted children with CKD, may interact with GH/IGF-1 axis leading to IGF-1 reduction [22, 36]. Further longitudinal studies are needed to explore the role of IGF-1 monitoring on the improvement of prompt diagnosis of physical function impairment and fatigue in children with CKD.
Another main finding of this study is the significant negative association between LBMAD and frailty. The relationship between low BMD and frailty, already observed in elderly populations [37, 38], indicates the bidirectional association between frailty and osteoporosis [15], which is mainly attributed to muscle-bone interactions [4]. To evaluate BMD in our study, we used the ante-posterior lumbar site as recommended for children [39], and adjusted lumbar BMC to corresponding vertebral volume, to overcome possible underestimation of BMD in patients with growth retardation [19]. As expected, LBMAD was significantly lower in patients with low muscle mass, justifying the displayed link between LBMAD and frailty. Nevertheless, muscle-bone cross-talk may be modulated by several factors in CKD [40]. In this study, we highlighted that IGF-1 interferes in the correlation between LBMAD and frailty, pointing out the possible role of IGF-1 in the impairment of the muscle-bone axis in CKD. Apart from its role in muscle growth, IGF-1 contributes to bone growth and remodeling through stimulation of chondrocyte expansion, enhancement of osteoblastic bone formation, and inhibition of osteoclastic bone resorption [8]. As a result, IGF-1 was significantly correlated to LBMAD in this study. Similar correlations between BMD and IGF-1 have been found in children [41] and adults [28] with CKD. The results of our study suggest that the IGF-1 level may serve as an indicator of muscle-bone integrity in children with CKD. Additional large-scale studies are necessary to confirm our conclusions.
Targeting the GH/IGF-1 axis is probably the key to assure optimal musculoskeletal health in children with CKD. Pediatric studies have shown that rGH therapy leads to an increase in BMD in parallel with enhancement of muscle mass in patients with CKD [42, 43], primarily by increasing serum IGF-1 levels, while concomitant treatment with recombinant IGF-1 has been shown to act additively on growth in uremic rats [44]. Interestingly, in our study, rGH therapy was more frequently administered in frail children and was not significantly associated with IGF-1 z-score levels. The limited number of patients, the short duration of rGH therapy, the higher rate of rGH therapy in patients with advanced CKD, and the possible resistance to rGH therapy may explain this result. Resistance exercise has been shown to trigger skeletal muscle IGF-1 gene expression in vivo [45] and increase circulating IGF-1 levels in CKD adult patients [46]. Whether in combination with long-term physical exercise, rGH therapy, as well as recombinant IGF-1, if necessary, guarantees prevention of frailty in children with CKD, is a question that needs to be answered in the future [47].
Our study has several limitations. The single-center type of study, which includes a limited number of patients with a broad spectrum of CKD severity, precludes us from making definite conclusions. Moreover, the utility of DXA in the estimation of bone health in pediatric CKD is limited. It cannot distinguish between trabecular and cortical bone, which is of high importance in patients with secondary hyperparathyroidism; it does not provide information on bone microarchitecture, compared to bone biopsy; while its effectiveness at predicting fracture risk has not been proven [19, 48]. Besides, this technique is based on the areal rather than volumetric BMC, resulting in an artificial underestimation of BMD in children with growth retardation. According to the Revised 2013 International Society for Clinical Densitometry Pediatric Official Positions, the best methods for reporting spine BMD results in children with growth delay include BMAD or BMD adjusted to HA [49]. Currently, there is no clinical evidence for preference between the two methods regarding the estimation of BMD in children with short stature. In our study, we divided BMC by the calculated vertebrae volume to present the estimated volumetric BMD. Furthermore, although BIS is a bedside, inexpensive, and non-irradiant method to assess nutritional status, there is a large variance of agreement between BIS and reference techniques in the estimation of lean mass, especially in dialysis patients [50], limiting its accuracy for the evaluation of body composition at individual level. Considering that bioimpedance analysis measurements are affected, in part, by the hydration status of the examined patient, BIS was realized in our study at the end of a dialysis session in all CKD 5D patients, in order to minimize the risk of misestimation of body composition indices.
In conclusion, frailty phenotype occurs in children with CKD and especially in those with advanced stage. General fatigue is a key factor of this condition and should be regularly investigated with appropriate questionnaires in this vulnerable population. IGF-1 seems to negatively predict frailty phenotype and is likely to interfere in the relationship between LBMAD and frailty in children with CKD. Further multi-center studies are needed to confirm the role of maintenance of normal IGF-1 levels on the prevention of frailty phenotype in these patients.
References
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–MM56
Nixon AC, Bampouras TM, Pendleton N, Woywodt A, Mitra S, Dhaygude A (2018) Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J 11:236–245
Sgambat K, Matheson MB, Hooper SR, Warady B, Furth S, Moudgil A (2019) Prevalence and outcomes of fragility: a frailty-inflammation phenotype in children with chronic kidney disease. Pediatr Nephrol 34:2563–2569
Greco EA, Pietschmann P, Migliaccio S (2019) Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne) 10:255
Clegg A, Hassan-Smith Z (2018) Frailty and the endocrine system. Lancet Diabetes Endocrinol 6:743–752
Leng S, Chen X, Mao G (2014) Frailty syndrome: an overview. Clin Interv Aging 9:433
Kim JC, Kalantar-Zadeh K, Kopple JD (2013) Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 24:337–351
Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER (2015) Role of IGF-I signaling in muscle bone interactions. Bone 80:79–88
Oh Y (2012) The insulin-like growth factor system in chronic kidney disease: pathophysiology and therapeutic opportunities. Kidney Res Clin Pract 31:26–37
Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K (2012) The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant 27:3063–3071
Blum WF (1991) Insulin-like growth factors (IGFs) and IGF binding proteins in chronic renal failure: evidence for reduced secretion of IGFs. Acta Paediatr Scand Suppl 379:24–31
Tönshoff B, Blum WF, Mehls O (1997) Derangements of the somatotropic hormone axis in chronic renal failure. Kidney Int Suppl 58:S106–SS13
Doi T, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Makino K, Suzuki T, Shimada H (2018) Association between insulin-like growth factor-1 and frailty among older adults. J Nutr Health Aging 22:68–72
Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD (2004) Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 16:153–157
Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD (2017) An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord 18:46
Kojima G (2016) Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone 90:116–122
Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, Salusky IB (2010) Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol 5:1860–1866
Denburg MR, Tsampalieros AK, de Boer IH, Shults J, Kalkwarf HJ, Zemel BS, Foerster D, Stokes D, Leonard MB (2013) Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98:1930–1938
Lalayiannis AD, Crabtree NJ, Fewtrell M, Biassoni L, Milford DV, Ferro CJ, Shroff R (2019) Assessing bone mineralisation in children with chronic kidney disease: what clinical and research tools are available? Pediatr Nephrol 35:937–957
Wells JCK, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K, Haroun D, Wilson C, Cole TJ, Fewtrell MS (2012) Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr 96:1316–1326
Crichton A, Knight S, Oakley E, Babl FE, Anderson V (2015) Fatigue in child chronic health conditions: a systematic review of assessment instruments. Pediatrics 135:e1015–e1031
Roumelioti ME, Wentz A, Schneider MF, Gerson AC, Hooper S, Benfield M, Warady BA, Furth SL, Unruh ML (2010) Sleep and fatigue symptoms in children and adolescents with CKD: a cross-sectional analysis from the chronic kidney disease in children (CKiD) study. Am J Kidney Dis 55:269–280
Crabtree NJ, Shaw NJ, Bishop NJ, Adams JE, Mughal MZ, Arundel P, Fewtrell MS, Ahmed SF, Treadgold LA, Högler W, Bebbington NA, Ward KA (2017) Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults—the ALPHABET study. J Bone Miner Res 32:172–180
Löfqvist C, Andersson E, Gelander L, Rosberg S, Blum WF, Albertsson Wikland K (2001) Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab 86:5870–5876
Onder G, Vetrano DL, Marengoni A, Bell JS, Johnell K, Palmer K (2018) Accounting for frailty when treating chronic diseases. Eur J Intern Med 56:49–52
Worthen G, Tennankore K (2019) Frailty screening in chronic kidney disease: current perspectives. Int J Nephrol Renovasc Dis 12:229–239
Kim JC, Kalantar-Zadeh K, Kopple JD (2013) Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 24:337–351
Jia T, Axelsson TG, Heimbürger O, Bárány P, Lindholm B, Stenvinkel P, Qureshi AR (2014) IGF-1 and survival in ESRD. Clin J Am Soc Nephrol 9:120–127
Rashid Qureshi A, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B, Bergström J (2001) Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13:S28–S36
Fernández-Reyes MJ, Alvarez-Ude F, Sánchez R, Mon C, Iglesias P, Díez JJ, Vázquez A (2002) Inflammation and malnutrition as predictors of mortality in patients on hemodialysis. J Nephrol 15:136–143
Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, Bergström J (1998) Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int 53:773–782
Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musarò A (2019) Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 18:e12954
Allain TJ, Bearn JA, Coskeran P, Jones J, Checkley A, Butler J, Wessely S, Miell JP (1997) Changes in growth hormone, insulin, insulin-like growth factors (IGFs), and IGF-binding protein-1 in chronic fatigue syndrome. Biol Psychiatry 41:567–573
Lucia Casadonte CJ, Brown J, Strople J, Neighbors K, Fei L, Alonso EM (2018) Low insulin-like growth factor-1 influences fatigue and quality of life in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 67:616–621
Szczêsny E, OElusarczyk J, Glombik K, Budziszewska B, Kubera M, Lasoń W, Basta-Kaim A (2013) Possible contribution of IGF-1 to depressive disorder. Pharmacol Rep 65:1622–1631
Chennaoui M, Léger D, Gomez-Merino D (2020) Sleep and the GH/IGF-1 axis: consequences and countermeasures of sleep loss/disorders. Sleep Med Rev 49:101223
Bartosch P, McGuigan FE, Akesson KE (2018) Progression of frailty and prevalence of osteoporosis in a community cohort of older women—a 10-year longitudinal study. Osteoporos Int 29:2191–2199
Kenny AM, Waynik IY, Smith JA, Fortinsky R, Kleppinger A, McGee D (2006) Association between level of frailty and bone mineral density in community-dwelling men. J Clin Densitom 9:309–314
Wasserman H, O’Donnell JM, Gordon CM (2017) Use of dual energy X-ray absorptiometry in pediatric patients. Bone 104:84–90
Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, Shults J, Zemel BS, Foster BJ, Foerster DL, Leonard MB (2013) Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int 83:495–502
Swolin-Eide D, Magnusson P, Hansson S (2007) Bone mass, biochemical markers and growth in children with chronic kidney disease: a 1-year prospective study. Acta Paediatr 96:720–725
van der Sluis IM, Boot AM, Nauta J, Hop WC, de Jong MC, Lilien MR, Groothoff JW, van Wijk AE, Pols HA, Hokken-Koelega AC, de Muinck Keizer-Schrama SM (2000) Bone density and body composition in chronic renal failure: effects of growth hormone treatment. Pediatr Nephrol 15:221–228
Van Dyck M, Gyssels A, Proesmans W, Nijs J, Eeckels R (2001) Growth hormone treatment enhances bone mineralisation in children with chronic renal failure. Eur J Pediatr 160:359–363
Kovács GT, Oh J, Kovács J, Tönshoff B, Hunziker EB, Zapf J, Mehls O (1996) Growth promoting effects of growth hormone and IGF-I are additive in experimental uremia. Kidney Int 49:1413–1421
Sun DF, Chen Y, Rabkin R (2006) Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int 70:453–459
Moinuddin I, Leehey DJ (2008) A comparison of aerobic exercise and resistance training in patients with and without chronic kidney disease. Adv Chronic Kidney Dis 15:83–96
Bacchetta J, Salusky IB (2016) Combining exercise and growth hormone therapy: how can we translate from animal models to chronic kidney disease children? Nephrol Dial Transplant 31:1191–1194
Weber LT, Mehls O (2010) Limitations of dual x-ray absorptiometry in children with chronic kidney disease. Pediatr Nephrol 25:3–5
Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM, International Society for Clinical Densitometry (2014) Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD pediatric official positions. J Clin Densitom 17:225–242
Milani GP, Groothoff JW, Vianello FA, Fossali EF, Paglialonga F, Consolo S, Edefonti A, Consonni D, van Harskamp D, van Goudoever JB, Schierbeek H, Agostoni C, Oosterveld MJS (2018) Bioimpedance spectroscopy imprecisely assesses lean body mass in pediatric dialysis patients. J Pediatr Gastroenterol Nutr 67:533–537
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karava, V., Dotis, J., Christoforidis, A. et al. Association between insulin growth factor-1, bone mineral density, and frailty phenotype in children with chronic kidney disease. Pediatr Nephrol 36, 1861–1870 (2021). https://doi.org/10.1007/s00467-021-04918-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04918-y