Abstract
Background
The optimal choice of dialysis modality for diabetic patients remains controversial. This study aimed to compare mortality between peritoneal dialysis (PD) and hemodialysis (HD) in end-stage renal disease (ESRD) patients with type 2 diabetes (T2D).
Methods
Our observational, longitudinal cohort consisted of all incident ESRD patients with T2D who received either PD or HD in our center from January 2012 to December 2017 and were followed until December 2019. Propensity scores were used to select a 1:1 matched cohort. Mortality was compared between dialysis modalities using Kaplan–Meier survival analysis, and risk factors for mortality were estimated using multivariate Cox regression analyses.
Results
The median follow-up times were 35.5 months in the PD group (n = 134) and 41.6 months in the HD group (n = 134, p = 0.0381). The 1-, 2-, 3-, 5-, and 7-year patient survival rates were 98%, 91%, 77%, 61%, and 35% for diabetic PD patients and 96%, 88%, 81%, 60%, and 57% for diabetic HD patients. Kaplan–Meier survival analysis showed that overall mortality did not significantly differ between modalities (log-rank = 0.9473, p = 0.6575). Using a multivariate Cox regression model, advanced age and increased cholesterol at the initiation of PD treatment were independent risk factors associated with mortality, whereas under HD therapy, the risk factors associated with mortality were lower BMI and higher HbA1c.
Conclusions
These results suggest that in patients with T2D, mortality is comparable between PD and HD irrespective of whether there are the first 2 years or over the 2-year period, and that different mortality predictor patterns exist between patients treated with PD versus HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence and prevalence of diabetes have grown rapidly worldwide, primarily driven by increases in type 2 diabetes (T2D). Diabetes is the leading cause of end-stage renal disease (ESRD), accounting for approximately 50% of cases in most developed countries [1]. In China, the overall prevalence of diabetes was recently reported to be 10.9% [2]; that is, 113.9 million Chinese adults are estimated to have diabetes, making it the world’s largest diabetes epidemic [3]. In addition, diabetes has become the second leading cause of ESRD in China. Overall, diabetes is a major public health problem in China [4].
Most diabetic ESRD patients are maintained on peritoneal dialysis (PD) or haemodialysis (HD) [5]. In general, PD has some advantages over HD, such as greater lifestyle flexibility, lower cost, improved hemodynamic stability, and better preservation of residual renal function [6]. However, compared to HD, the technique failure rate is considerably higher in PD [7]. The first-choice option of dialysis modality for ESRD patients remains controversial, especially in those with diabetes [8, 9]; indeed, modality choice has additional implications in ESRD patients who have diabetes. For example, when on PD, the constant exposure to the glucose-based peritoneal dialysate may further worsen glycemic control. Nonetheless, PD may be better tolerated than HD, mainly because the blood pressure is more stable, and vascular access is not required [8]. Although many studies have compared the impact of PD versus HD on mortality in diabetic patients, the difference in their respective mortalities remains controversial [5, 10, 11].
In China, retrospective studies of survival rates among the dialysis modalities have been reported [12,13,14,15]. However, comparisons of mortality between PD and HD in diabetic patients from mainland China have received little attention. Thus, we used a matched-pair longitudinal cohort design with matching based on the propensity of initial PD modality use. Our primary objective was to compare survival rates of ESRD patients with T2D who were initially treated with PD or HD in the matched-pair cohort. Our secondary objective was to evaluate the risk factors associated with mortality between the two modalities.
Methods
Study population and design
Patient data in this study were derived from the CO_PHD cohort between 2012 and 2019, which was registered with the Chinese Clinical Trial Registry (ChiCTR1900024059). The study protocol was complied with the principles laid down in the Declaration of Helsinki and approved by the Institutional Ethics Review Boards of Guangdong Provincial Hospital of Chinese Medicine, The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China (ZE2019-155–01).
The participants were ESRD patients with T2D who received either PD or HD as their first renal replacement therapy modality at our center; they were followed from January 1, 2012 to December 31, 2017. To be included, the patients had to have T2D, be between 18 and 80 years, and initiated dialysis treatment for at least 3 months in our center. They were excluded if data was lost at baseline, if they switched dialysis modalities, or if they did not want follow-ups after dialysis was initialized. A total of 301 patients met the inclusion criteria.
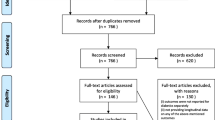
Propensity scores comparing age, sex, body mass index (BMI), albumin, hemoglobin, and estimated glomerular filtration rate (eGFR) at baseline dialysis were calculated to select a 1:1 matched cohort. 33 patients failed screening, leaving 268 patients in the study: 134 patients in the PD group and 134 patients in the HD group. The patients were followed up until either dialysis treatment stopped, death, transfer to other centers, lost to follow-up, or December 31, 2019, whichever came first. The study design is depicted in Fig. 1.
Data collection
We collected clinical information from participants by manually reviewing the hospital’s electronic medical databases and the daily new case registration report. The baseline demographic and disease characteristics collected included age, sex, mean artery pressure (MAP), BMI, primary cause of ESRD, comorbidities, and presence of cardiovascular disease (CVD). Data collected at the start of dialysis therapy included serum albumin, hemoglobin, serum glucose, hemoglobin A1c (HA1c), corrected serum calcium, phosphate, intact parathyroid hormone (iPTH), total cholesterol, triglycerides, serum creatinine, urea, urea acid, and eGFR. All data were obtained during the first 90 days of dialysis treatment.
Clinical outcomes
The primary outcome for the analysis of patient survival rates was death. To calculate patient survival rates, end events were defined as an all-cause mortality in any situation. Censored events for patient survival included recovery from renal failure, kidney transplantation, follow-up loss, transfer to another center, transition from one dialysis modality to another, and still on dialysis therapy as of December 31, 2019. Exact causes of death in PD and HD patients were collected.
Statistical analyses
Data are presented as mean ± standard deviation (SD) for normally distributed continuous variables and as the median (interquartile range) for skewed continuous variables. Results are expressed as frequencies (percentages) for categorical variables.
The Student’s t test was used for parametric data, and the Mann–Whitney U test was used for nonparametric continuous data. Either the Chi-square test or Fisher’s exact test was used for categorical data, where appropriate. Kaplan–Meier survival analysis was used to compare cumulative patient survival between the two dialysis modalities. We used the Cox proportional hazards model for univariate and multivariate regression analysis of survival rates. The results are expressed as the hazard ratio (HR) and 95% confidence intervals (CI).
Risk factors for mortality in the diabetic PD and HD cohort were first examined using univariate analysis. The variables included for the univariate Cox model were sex, age, BMI, MAP, CVD, 24-h urine output, albumin, hemoglobin, calcium, phosphorus, total cholesterol, triglyceride, HbA1c, uric acid, eGFR, ambulatory peritoneal dialysis (CAPD) in PD cohort, and autogenous arteriovenous fistula (AVF) in HD cohort. Covariates with p values < 0.25 in the univariate analysis, and demographic variables (for example age, gender, and BMI), as well as important laboratory indicators including serum albumin, hemoglobin, and HbA1c, which affects clinical outcome of diabetic dialysis patients, were used for this study.
Multivariate Cox proportional hazards regression was used to analyze risk factors for mortality in the combined and separate diabetic cohorts. The results were expressed as the hazard ratio (HR) and 95% confidence intervals (CI). Subgroup analyses for dialysis modality and mortality based on a Cox regression model were repeated in strata, according to dialysis duration time (≤ 24 months or > 24 months).
Prism5 (GraphPad Software Inc., La Jolla, CA) and SPSS version 16 (SPSS Inc., Chicago, IL, USA) software packages were used for statistical analyses. A value of p < 0.05 was considered statistically significant, and all tests were performed two-tailed.
Results
Baseline patient characteristics
The demographic and clinical characteristics of the patients at the start of dialysis therapy are shown in Table 1. To adjust for baseline variables, propensity scores were calculated, resulting in 268 patients (134 in each group). As a result, no other variables differed at baseline dialysis between the treatment groups.
The change of laboratory indexes at the 1-year and 2-year follow-up
We further analyzed the change of important laboratory indexes at the 1 year and 2 years during routine follow-up visits. As shown in Table 2, serum albumin, hemoglobin, potassium, phosphate, and eGFR were significantly lower, and serum creatinine and urea were significantly higher in the PD group compared to the HD group, whether after the first 1-year or the 2-year follow-up period. Besides, patients in the PD group tended to have a higher total cholesterol and a lower HbA1c by the completion of the 1-year period, and a lower glucose after the 2 years of treatment compared to those in the HD group. In contrast, corrected calcium, iPTH, triglyceride, and uric acid were similar between the two groups.
Clinical outcomes
The total follow-up period was 10,334 patient-months, with a mean follow-up duration of 35.5 ± 18.3 months (range 3.1–85.9 months) for diabetic PD patients and 41.6 ± 21.8 months (range 3.0–96.2 months) for diabetic HD patients. The mean duration of follow-up in the PD group was significantly shorter than the HD group (p = 0.0381).
Table 3 shows the clinical outcomes between the two groups. By the end of the study, two patients (1.5%) from the PD group recovered spontaneously and ceased dialysis. 11 patients (4.1%) received kidney transplants, of which five (3.7%) were from the PD group, and six (4.5%) were from the HD group. One patient in the PD group (0.7%) and 12 patients in the HD group (8.9%) were transferred to other dialysis centers. One patient (0.7%) in the PD group was lost to follow-up. During the follow-ups, 19 patients (14.2%) from the PD group were switched to HD. 76 patients (28.4%) died, with 35 (26.1%) in the PD group and 41 (30.6%) in the HD group.
Patient survival
Kaplan–Meier survival curves for the PD and HD groups are shown in Fig. 2. The survival rates of diabetic patients were similar between the two groups (log-rank = 0.9473, p = 0.6575). The 1-, 2-, 3-, 5-, and 7-year patient survival rates were 98%, 91%, 77%, 61%, and 35% in the PD group; and 96%, 88%, 81%, 60%, and 57% in the HD group. The most common cause of death was from cardiac events (31 patients, 40.8% of all deaths, 15 PD/16 HD), followed by cerebrovascular events (11 patients, 14.5%, 7 PD/4 HD), infection (9 patients, 11.8%, 1 PD/8 HD), and other or unknown reasons (25 patients, 32.9%, 12 PD/13 HD). The detailed causes of death for the two groups are listed in Table 4.
Risk factors for predicting mortality across dialysis modalities
Baseline variables including age, gender, BMI, MAP, presence of CVD, serum albumin, hemoglobin, calcium, phosphate, total cholesterol, triglycerides, and HA1c as well as dialysis modality (PD versus HD) were included in the multivariate Cox regression analysis. By adjusting these covariates, we found that advanced age, total cholesterol, and HbA1c at the start of dialysis were independent risk factors for increased mortality in the entire matched cohort (Table 5). In subgroup analyses, adjusted hazard ratio (CI) for mortality with interaction terms between dialysis modalities (PD versus HD) and therapy duration (≤ 2 years or > 2 years) was 0.341 (0.108–1.074); P value for interaction terms was 0.066. These results demonstrated that no statistically significant interaction was found between dialysis modalities and the subgroups of the therapy duration. Correspondingly, the subgroup analysis showed comparable survival rates for patients with T2D stratified by therapy duration irrespective of whether there are the first 2 years or over the 2-year period, as shown in Table 6. With respect to dialysis modality (Table 7), advanced age and increased cholesterol were significantly associated with mortality in the PD group, and in the HD group, mortality was significantly associated with lower BMI and higher HbA1c.
Discussion
This longitudinal cohort study is the first to compare survival rates based on initial treatment modality in adult ESRD patients with T2D receiving PD versus HD in China. To balance prognostic factors, we matched PD and HD diabetic patients based on the propensity of initial PD use. Our results suggest that PD and HD confer equivalent survival benefits in patients with T2D, but PD has a marginally worse mortality than that of HD beyond 2-year dialysis treatment.
Patients undergoing PD and HD differ in many patient characteristics when initiating treatment, and these initial characteristics are associated with mortality risk [16]. The traditional technique for controlling such differences in the risk-factor distributions between the dialysis modalities is regression adjustment. However, it only works well when all interactions between the factors are correctly specified, which in practice is unlikely due to their complex interrelationships as well as unmeasured predictors. A substitute to regression adjustment is propensity score analysis, which can be used for stratification, adjustment, or matching. Matching by propensity scores is defined here as the possibility of initial PD use given several key covariates including age, sex, BMI, serum albumin, hemoglobin, HA1c, and eGFR; it alone is sufficient in controlling for measured confounding factors and in reducing the chance of introducing a bias favoring one of the dialysis modalities. In our cohort, nine diabetic PD patients (6.3%) were unable to be matched with a diabetic HD patient, and a considerable number of diabetic HD patients (n = 24, 15.2%) were unable to be balanced with a diabetic PD patient. These unmatched patients may hold unmeasured variables (or contraindications) for one treatment modality. Although it exists different primary causes among dialysis patients with T2D, DKD accounts for most of the primary renal diseases. In our study cohort, patients with diabetes as primary renal disease were DKD (~ 84%), and patients with diabetes as a comorbid condition was 26% of the overall diabetic dialysis patients. This percentage was similar to the results from other reports [17, 18]. It was speculated that patients with diabetes as a comorbidity may have a relatively lower risk of microvascular and macrovascular complications, and thus a better clinical outcome [19, 20].
Due to conflicting results in past observational studies, it remains equivocal as to whether the dialysis modality selected as the first treatment affects survival rates in diabetic ESRD patients [5, 21, 22]. Some studies suggest better outcomes under PD treatment [16, 23, 24], whereas others indicate HD programs are more beneficial [25,26,27]. However, among these studies, patient survival rates were inconsistent and varied across study designs, follow-up periods, geographical regions, and subgroups [11]. Even though the PD group showed a higher mortality rate than the HD group beyond the 5-year follow-up in our study, this increase was not statistically significant. In subgroup analyses of therapy duration, the results showed similar mortality for patients with T2D between the two groups among stratifications of the first 2 years and above 2-year period. Overall, PD and HD show similar survival rates for patients with T2D. It was reported that 5-year survival rates of diabetics on both PD and HD are approximately 45% [1, 28]. However, the 5-year patient survival rates were 61% in the PD group; and 60% in the HD group. The patient survival rates from this study seem to be better than most other reports, suggesting that long-term survival had improved for diabetic dialysis patients in our center.
14.2% (19 patients) switched from PD to HD during the follow-up period, In contrast, patient conversion from HD to PD was zero. A previous study reported that a sign of the diminishing benefit of PD over time was the relatively high chance (57%) of modality transfer (versus 6% transfer under HD) [23]. We found that inadequate dialysis and PD-related peritonitis were two of the most common causes of modality transfer. If inadequate dialysis is noticed in a PD program, the dialysis dose and serum albumin should be increased and decreased, respectively. When dialysis adequacy and protein energy wasting could not be improved with active interventions, additional attention should be given to the timely transfer of diabetic PD patients to the HD program.
After adjusting for baseline characteristics, a multivariate Cox regression model found that advanced age, higher cholesterol, and increased HbA1c were independent risk factors for increased mortality in the combined diabetic dialysis cohort. Advanced age is well established as a risk predictor for mortality in dialysis patients. A study in Poland reported that hypercholesterolemia in diabetic PD patients is linked to atherosclerotic dyslipidemia, whereas in HD patients, it is likely more related to good nutrition [29]. In this context, the differential significance of hypercholesterolemia between diabetic PD and HD patients should be highlighted. Glycemic control, as monitored by HbA1c, is critical in reducing the risk of cardiovascular complications in diabetic patients [30, 31]. A report from Korea found that in patients with T2D who were on PD or HD, pre-dialysis glycemic control reduced the mortality risk [32], suggesting that proficient glycemic control might improve survival in PD diabetics. When we compared risk factors between the dialysis modalities in the multivariate Cox regression analysis, the significant mortality predictors in the PD group were advanced age and increased cholesterol, whereas in the HD group, they were lower BMI and higher HbA1c. In short, our results indicate that different mortality predictor patterns exist in patients with T2D treated with PD versus those treated with HD.
In the future, dialysis modality choice may become a more pressing issue, particularly in diabetic patients. The U.S. dialysis population is projected to exceed 534,000 in 2020 [33]. According to the Chinese Renal Data Registration System, the overall number of ESRD patients in mainland China receiving dialysis at the end of 2019 was 736,001 (632,653 HD and 103,348 PD). Globally, the dialysis population will continue to grow as the world’s population ages and as the prevalence of diabetes increases. This growth has a major impact on the burden of disease, making it necessary to consider the cost-effectiveness among different dialysis modalities. In general, in China, PD costs less than HD, and unique benefits are offered to patients on PD in China’s vast rural areas [28]. Because of these advantages, PD is highly valued by the Chinese government.
This study has several limitations. First, dialysis modalities were not randomly assigned; instead, the choice of modality depended not only on the patient’s clinical status but also on the physician’s practice and on government policy. Thus, because of selection biases, causality could not be evaluated, as propensity score matching is not an alternative for randomization. Second, the study was observational, longitudinal, and based on registry data. Biomarkers and predictors covariates were measured only when dialysis was initiated, so they can’t be the only parameters for the outcome. Yet it is worth noting that repetitive infectious episodes and hospitalizations are determinant of patient survival and clinical outcomes. Even though peritonitis was independently associated with higher risk of all-cause mortality in PD patients [34], the infection-related hospitalization [35] and overall hospitalization rates [36] of PD patients are similar to that of HD patients. Third, transfer to other centers and modality switches were censored in the survival analysis. The non-equal distribution of such changes between the PD and HD groups may have influenced the results. Finally, whether our results are generalizable to other populations is uncertain, because our patients were from a single-center in Southern China, and the number of samples was relatively limited. Therefore, center-specific effects are inevitable. Despite these limitations, this study is the first that compared long-term survival rates between diabetic PD and HD patients in mainland China. The patients have been carefully followed up for more than 7 years and the relatively long-term survival of patients with T2D on PD versus HD are reported. It may have clinically relevant features and help advise dialytic modality choices for diabetic patients in China.
Conclusions
In summary, this study demonstrated that the modality of dialysis itself has no effect on the survival rates of patients with T2D treated with PD or HD, whether there are the first 2 years or over the 2 years of treatment. It's worth noting that it exists a certain trend towards a higher risk of mortality in PD versus HD after a period of 2-year dialysis treatment. Based on our study and on previously published reports, we believe that dialysis modality selection should be guided by the patient’s preference following comprehensive and unbiased information.
References
Saran R, Robinson B, Abbott KC et al (2019) US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 75:A6
Wang L, Gao P, Zhang M et al (2017) Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 317(24):2515–2523
Xu Y, Wang L, He J et al (2013) Prevalence and control of diabetes in Chinese adults. JAMA 310(9):948–959
Zhang L, Long J, Jiang W et al (2016) Trends in chronic kidney disease in China. N Engl J Med 375(9):905–906
Couchoud C, Bolignano D, Nistor I et al (2015) Dialysis modality choice in diabetic patients with end-stage kidney disease: a systematic review of the available evidence. Nephrol Dial Transplant 30(2):310–320
Kim YL (2017) What is the best dialysis therapy in developed and developing countries? Peritoneal dialysis and/or hemodialysis: the trend in Korea. Contrib Nephrol 189:65–70
Lee JH, Park SH, Lim JH et al (2016) Impact of dialysis modality on technique survival in end-stage renal disease patients. Korean J Intern Med 31(1):106–115
Biesenbach G, Pohanka E (2012) Dialysis in diabetic patients: hemodialysis and peritoneal dialysis. Pros and cons. Minerva Urol Nefrol 64(3):173–182
Locatelli F, Pozzoni P, Del Vecchio L (2004) Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol 15(Suppl 1):S25–S29
Xue J, Li H, Zhou Q, Wen S, Zhou Q, Chen W (2019) Comparison of peritoneal dialysis with hemodialysis on survival of diabetic patients with end-stage kidney disease: a meta-analysis of cohort studies. Ren Fail 41(1):521–531
Elsayed ME, Morris AD, Li X, Browne LD, Stack AG (2020) Propensity score matched mortality comparisons of peritoneal and in-centre haemodialysis: systematic review and meta-analysis. Nephrol Dial Transplant 35:2172
He Z, Hou H, Zhang D et al (2020) Effects of dialysis modality choice on the survival of end-stage renal disease patients in southern China: a retrospective cohort study. BMC Nephrol 21(1):412
Wu B, Wang M, Gan L, Zhao H (2014) Comparison of patient survival between hemodialysis and peritoneal dialysis in a single Chinese center. Int Urol Nephrol 46(12):2403–2407
Chang YK, Hsu CC, Hwang SJ et al (2012) A comparative assessment of survival between propensity score-matched patients with peritoneal dialysis and hemodialysis in Taiwan. Medicine (Baltimore) 91(3):144–151
Lee CC, Sun CY, Wu MS (2009) Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int 29(2):182–190
Xue JL, Everson SE, Constantini EG et al (2002) Peritoneal and hemodialysis: II. Mortality risk associated with initial patient characteristics. Kidney Int 61(2):741–746
Schwenger V, Müssig C, Hergesell O, Zeier M, Ritz E (2001) Incidence and clinical characteristics of renal insufficiency in diabetic patients. Dtsch Med Wochenschr 126(47):1322–1326
Catalano C, Postorino M, Marino C (1996) The impact of diabetes on patients’ survival in dialysis patients with non-diabetic renal disease and in patients who develop diabetes during chronic dialysis. Nephrol Dial Transplant 11(6):1124–1128
Schroijen MA, van de Luijtgaarden MW, Noordzij M et al (2013) Survival in dialysis patients is different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. Diabetologia 56(9):1949–1957
Schroijen MA, Dekkers OM, Grootendorst DC et al (2011) Survival in dialysis patients is not different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. BMC Nephrol 12:69
Noordzij M, Jager KJ (2012) Survival comparisons between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 27(9):3385–3387
Vonesh EF, Snyder JJ, Foley RN, Collins AJ (2006) Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us. Kidney Int Suppl 103:S3-11
Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K (2013) Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol 8(4):619–628
Heaf JG, Løkkegaard H, Madsen M (2002) Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17(1):112–117
Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG (2003) Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14(2):415–424
Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S (2012) Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 27(9):3568–3575
Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ (2010) Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21(3):499–506
Yu X, Yang X (2015) Peritoneal dialysis in China: meeting the challenge of chronic kidney failure. Am J Kidney Dis 65(1):147–151
Madziarska K, Weyde W, Penar J et al (2013) Different mortality predictor pattern in hemodialysis and peritoneal dialysis diabetic patients in 4-year prospective observation. Postepy Hig Med Dosw (Online) 67:1076–1082
Duong U, Mehrotra R, Molnar MZ et al (2011) Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 6(5):1041–1048
Dronovalli S, Burney BO, Bakris GL (2009) Glycemic control and cardiovascular disease in chronic kidney disease. Curr Diab Rep 9(3):243–248
Lee MJ, Kwon YE, Park KS et al (2016) Glycemic control modifies difference in mortality risk between hemodialysis and peritoneal dialysis in incident dialysis patients with diabetes: results from a nationwide prospective cohort in Korea. Medicine (Baltimore) 95(11):e3118
Mehrotra R, Blake P, Berman N, Nolph KD (2002) An analysis of dialysis training in the United States and Canada. Am J Kidney Dis 40(1):152–160
Ye H, Zhou Q, Fan L et al (2017) The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol 18(1):186
Williams VR, Quinn R, Callery S, Kiss A, Oliver MJ (2011) The impact of treatment modality on infection-related hospitalization rates in peritoneal dialysis and hemodialysis patients. Perit Dial Int 31(4):440–449
Quinn RR, Ravani P, Zhang X et al (2014) Impact of modality choice on rates of hospitalization in patients eligible for both peritoneal dialysis and hemodialysis. Perit Dial Int 34(1):41–48
Funding
This work was financially supported by grants from the Inheritance and Innovation of Traditional Chinese Medicine platform construction for Nephropathy (No. 01030315) and the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (No. 20192028).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
This manuscript has been reviewed and approved by all authors, and no results reported in this manuscript have been published elsewhere. All the authors have declared no competing interest.
Informed consent
Written informed consent was not required because of the non-intervention and retrospective design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., He, Z., Hu, X. et al. Propensity-matched comparison of mortality between peritoneal dialysis and hemodialysis in patients with type 2 diabetes. Int Urol Nephrol 54, 1373–1381 (2022). https://doi.org/10.1007/s11255-021-03026-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03026-y