Abstract
Purpose
Protein-energy wasting, characterized by decreased muscle mass, is one of the strongest predictors of mortality in patients on maintenance hemodialysis (MHD). As people get older, their muscle strength usually declines faster than muscle mass. However, the association between lower-limb muscle strength and all-cause mortality remains unclear. We aimed to evaluate risk factors for decreased upper-limb muscle strength in MHD patients and its impact on patient survival.
Methods
The cross-sectional part of the study included 174 MHD patients. Subsequently, they were followed up for 52 weeks. Biceps muscle strength, anthropometry, body composition, dietary intake, daily steps, and biochemical indicators of malnutrition and inflammation were evaluated. Risk factors for muscle weakness were screened by multiple linear regression analysis, and patient survival was analyzed by Kaplan–Merier and Cox multivariate analysis.
Results
The 174 MHD patients (93 men; 63.05 ± 12.29 years) were classified as a young (< 65 years, n = 97) group and an elderly group (≥ 65 years, n = 77). Gender, daily steps, muscle mass, 25(OH)D level and IL-6 in young group, and muscle mass, 25(OH)D, daily steps, and NT-proBNP in elderly group were associated with the decreased biceps muscle strength. The survival rate in high muscle strength group was significantly higher than that in low muscle strength group (P = 0.002). The association between low muscle strength and high mortality risk remained strong in the fully adjusted model.
Conclusion
Risk factors of muscle weakness were different between young and elderly MHD patients. There was a strong correlation between strong biceps muscle strength and high patient survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein-energy wasting (PEW), a pathological condition characterized by a progressive reduction of protein and energy stores, has been increasingly reported in patients with chronic kidney disease (CKD) and is associated with adverse clinical outcomes [1, 2]. It was reported that PEW occurred in approximately 18–75% CKD patients [3,4,5]. Henceforth, early recognition and early intervention of PEW may improve the clinical outcome.
Decreased muscle mass is one of the most important criteria for the diagnosis of PEW [1]. Actually, both muscle mass and muscle strength are vital prognostic indicators in CKD patients [6, 7]. When people get older, the loss of muscle mass is associated with the decline in muscle strength. This strength decline is more prominent than the concomitant loss of muscle mass [8]. In some cases, muscle strength reduction occurs when muscle mass remains constant or is even increased [9]. It has been recently documented that low muscle strength is more unequivocally associated with PEW and mortality than low muscle mass [10]. Muscle strength assessment may give extra diagnostic and prognostic data in CKD patients from different age groups.
Serum 25(OH)D is a reliable indicator of the vitamin D status [11]. Vitamin D deficiency is defined as a 25(OH)D level of less than 50 nmol/L (20 ng/mL) and is a highly prevalent condition in the older population [12]. Vitamin D deficiency is also common among CKD patients, and as CKD progresses, vitamin D deficiency becomes more pronounced [13, 14]. It has been documented that the vitamin D status is positively correlated with physical performance and muscle size in CKD patients [15]. Our previous work also showed that vitamin D receptor activators (VDRAs) increased serum creatinine (SCr) levels in non-dialysis patients, but had no effect on renal function [16]. We speculated that VDRAs may be directly involved in Cr metabolism in the body muscles by increasing its release. A considerable number of dialysis patients need to use VDRAs for the treatment of CKD–mineral and bone disorder (CKD-MBD). However, whether 25(OH)D level or application of VDRAs is related to muscle strength in dialysis patients remains unclear.
The prevalence of chronic disease increases with age. The burden of chronic disease increases the risk of disability in the elderly who often need special support and care [17, 18]. In China, some elderly MHD patients are usually less active and have been confined to bed for many years or have to rely on wheelchairs. So we hypothesized that upper-limb muscle strength might be a more convincing indicator to assess the patient’s overall muscle strength than lower-limb muscle strength. The aim of the present study was to determine risk factors for decreased biceps muscle strength in MHD patients of different age groups and evaluate the impact of muscle strength change on the mortality rate of MHD patients.
Methods
Participants and study design
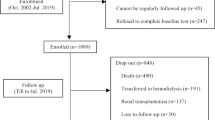
The single-centre study has a cross-sectional and a longitudinal part. The cross-sectional study was carried out in January 2016 in the dialysis centre of Fudan University Huashan Hospital (Shanghai, China), then the patients were followed up for 52 weeks for subsequent survival analysis. The selection criteria were patients (1) ≥ 18 years; (2) who underwent thrice-weekly hemodialysis for more than 3 months; (3) without residual renal function (urine volume less than 200 mL/day). Patients with evidence of serious infectious diseases and severe anemia disease were excluded from the study. The included patients were first categorized into two groups (low muscle strength group, n = 87; high muscle strength group, n = 87) according to biceps muscle strength, and then divided into a young MHD group (age < 65 years, n = 97) and an elderly MHD group (age ≥ 65 years, n = 77). Biocompatible membranes (polysulfone), ultrapure water and bicarbonate buffered dialysis fluid were used. The study was approved by the ethics committee of Huashan Hospital. Written informed consent was obtained from each patient.
Study outcome
Patients were followed for 52 weeks up to death, kidney transplant, or the end of follow-up period. The endpoint of the study was all-cause mortality. Data for endpoints were obtained from hospital charts and through telephone interview with patients.
Data collection
Bicep muscle strength was measured with a digital hand-held dynamometer (Hoggan) in the nonfistula hand if implanted or in the dominant hand. Handgrip strength was assessed with a handgrip dynamometer. Each measurement was repeated three times, and the mean value of these three measurements was recorded for the analysis. Body composition was tested by estimating muscle mass and fat mass using bioelectrical impedance analysis (TANITA) 30 min after a midweek dialysis session. Patients were asked to remove all the accessories and stand on the body composition analyzer with bare feet. Body composition was measured by the prediction equations of manufacturer within the analyzer as previously reported [19,20,21]. Body mass index (BMI), waist-to-hip ratio (WHR), triceps skinfold thickness (TSF), midarm circumference (MAC), midarm muscle circumference (MAMC), and calf circumference were measured and calculated according to the standard techniques. Digital Pocket Pedometer (Omron) was used to record daily steps. Each patient was asked to keep a food diary for 3 consecutive days. Three-day-dietary diaries were collected and analyzed by dieticians using Keto nutritional assessment software (vision 2.0, Fresenius Kabi Pharmaceutical Co., Ltd, Beijing, China). The nutritional status was assessed by subjective global assessment (SGA).
Biochemical methods
Pre-dialysis blood specimens were taken during a midweek session for laboratory assessment by standard techniques. 25(OH)D levels were detected by enzyme immunoassay. Interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured by Elisa (ANOGEN). The levels of total superoxide dismutase (T-SOD) and malondialdehyde (MDA) were measured by Oxidative Stress Kit (Nanjing Jiancheng). The normalized protein catabolic rate (nPCR) was calculated using the formula described previously [22].
Statistical analyses
Continuous variables were summarized as the mean ± standard deviation (SD) or median. The differences between groups were analyzed using two independent-sample t tests or Wilcoxon–Mann–Whitney test, as appropriate. Categorical variables were described using proportions and analyzed with the Chi square test. The correlation between muscle strength and other nutritional markers was assessed through Pearson’s correlation coefficient. Univariable linear regression was used to analyze the predictors of decreased upper-limb muscle strength. Values of P < 0.20 were included in multivariate regression analysis. Survival curves were estimated using the Kaplan–Meier method, and differences in survival distribution were evaluated by the log-rank test. The Cox proportional-hazards regression model was used to estimate hazard ratios. We included covariates in multivariable-adjusted models if they were biological relevance or clinical interest. Cox regression analyses are presented as hazard ratios with 95% confidence intervals. Statistical analysis was carried out using SPSS17.0. The level of significance was set at < 0.05.
Results
Of the 174 MHD patients included, 93 were male and 81 were female with a mean age of 63.05 ± 12.29 years and a dialysis vintage of 9.19 ± 6.06 years. The leading cause of end-stage renal disease was glomerulonephritis (44.83%), followed by hypertension (17.82%), diabetic nephropathy (17.24%), and other causes (20.11%). Paticipants were first categorized into two groups (low muscle strength group, n = 87; high muscle strength group, n = 87) according to biceps muscle strength. Those with low muscle strength were older, more of women, had higher SGA scores and the smaller number of daily steps (P < 0.001) (Table 1).
Baseline characteristics of young and elderly MHD patients by biceps muscle strength
To examine biceps muscle strength of the MHD patients of different age groups, they were categorized into a young MHD group (< 65 years, n = 97) and an elderly MHD group (≥ 65 years, n = 77). Then, the patients were further divided according to the age-specific median biceps muscle strength values, and the baseline characteristics are depicted in Table 1. It was found that patients with low muscle strength had higher SGA scores and the smaller number of daily steps in both groups. In young MHD group, compared with patients with high biceps muscle strength, those with low muscle strength were more of women (P < 0.001) and had longer dialysis vintage (P = 0.003). As expected, patients with low muscle strength were older in elderly MHD group (P = 0.004), but not in young MHD group (P = 0.54). There was no significant difference in Kt/V, diabetes (%), calcitriol dosage, daily energy intake (DEI) and daily protein intake (DPI) between the high and low muscle strength patients in both groups.
Muscle-related parameters in young and elderly MHD patients by biceps muscle strength
As shown in Table 2, patients with low muscle strength had lower handgrip strength, less midarm muscle circumference (MAMC), calf circumference, and muscle mass in both groups. The levels of muscle metabolism-related parameters SCr were also lower in patients with low muscle strength. Pearson’s correlation analysis showed that biceps muscle strength had a significant positive correlation with muscle mass (r = 0.403, P < 0.001), MAMC (r = 0.394, P < 0.001), calf circumference (r = 0.314, P < 0.001) and SCr (r = 0.417, P < 0.001) (Fig. 1).There was no significant difference in BMI, WHR and fat mass between the patients with high and low muscle strength.
Laboratory characteristics of young and elderly MHD patients by biceps muscle strength
As summarized in Table 3, there were no significant differences in nutritional parameters, serum calcium, phosphorus, iPTH, C-reactive protein (CRP), oxidative stress parameters, and bicarbonate between the patients with high and low muscle strength. In young MHD group, compared with patients with high biceps muscle strength, those with low muscle strength had high levels of IL-6 (P = 0.04) and TNF-α (P = 0.04). In elderly MHD group, those with low muscle strength had higher concentrations of N-terminal-proBNP (NT-pro BNP) (P = 0.04).
25(OH)D levels of young and elderly MHD patients by biceps muscle strength
In both young and elderly MHD groups, compared with patients with high biceps muscle strength, those with low muscle strength had lower concentrations of 25(OH)D (Table 3). In addition, 25(OH)D levels were significantly positively correlated with biceps muscle strength (r = 0.362, P < 0.001) (Fig. 2a). Patients were further stratified into four groups according to 25(OH)D levels (< 25 nmol/L, 25–50 nmol/L, 50–75 nmol/L and ≥ 75 nmol/L). Compared with 25(OH)D < 25 nmol/L group, muscle strength of the biceps gradually improved with the increase of 25(OH)D level in 25–50 nmol/L group (P < 0.05) and 50–75 nmol/L group (P < 0.05), but not in ≥ 75 nmol/L group (P > 0.05) (Fig. 2b).
Major determinants of biceps muscle strength in young and elderly MHD patients
In young MHD patients, univariate variable screening indicated that gender, dialysis vintage, SGA score, daily steps, muscle mass, 25(OH)D, IL-6, and TNF-α were significant at P < 0.20 and considered as potential predictors of low biceps muscle strength. Multivariable analysis showed that gender (β = − 1.98, P = 0.004), daily steps (β = 4.03, P = 0.001), muscle mass (β = 0.07, P = 0.03), 25(OH)D (β = 0.04, P = 0.04), and IL-6 (β = − 0.08, P = 0.004) were significantly correlated with biceps muscle strength (Table 4). In elderly MHD patients, univariate analysis showed that gender, age, SGA score, muscle mass, DPI, serum albumin, 25(OH)D, CRP, NT-proBNP, daily steps, and bicarbonate were significant at P < 0.20 and considered as potential predictors. Multivariate analysis showed that muscle mass (β = 0.09, P = 0.01), 25(OH)D (β = 0.06, P = 0.004), daily steps (β = 3.91, P = 0.001), and NT-proBNP (β = − 0.97, P = 0.04) were independent factors association with biceps muscle strength (Table 5).
Effect of biceps muscle strength on survival
During the 52-week follow-up period, 16 patients (9.20%, 16/174) died of cardiovascular disease (14) and tumors (2). 27(15.52%, 27/174) patients developed non-mortal cardiovascular events. Figure 3a indicates that MHD patients with high biceps muscle strength (97.70%, thick dark line) had significantly better survival than those with low biceps muscle strength (83.90%, dotted line) (P = 0.002 by log-rank test). Figure 3b further shows the survival rate stratified according to the presence of high or low biceps muscle strength in different age groups. Elderly patients with low muscle strength had the worst prognosis (P = 0.001). We also indicates that non-mortal cardiovascular events were more common in patients with low biceps muscle strength (Supplementary Fig. 1).
As continuous variables, after adjustment for Model 1 (age and sex), Model 2 (age, sex and muscle mass), and Model 3 [age, sex, muscle mass, diabetes, hemoglobin, cholesterol, CRP and 25(OH)D], the HR became 0.76 (95% CI 0.63–0.91, P = 0.003), indicating that low biceps muscle strength still had a significant negative effect on survival after adjustment (Table 6). With categorical variables against low biceps muscle strength group taken into consideration, high biceps muscle strength group was still associated with a lower mortality risk (HR 0.14, 95% CI 0.02–0.78, P = 0.03). Low biceps muscle strength was also associated with an increased risk of non-mortal cardiovascular events after adjustment (Supplementary Table 1).
Discussion
Compared with younger patients, a higher proportion of hemodialysis patients older than 65 years were malnourished [23]. With age progressing, the reduction in lean body mass (LBM) and muscle area further aggravates in MHD patients [24, 25]. In the current study, we examined biceps muscle strength in MHD patients older and younger than 65 years and evaluated the risk factors of muscle weakness, finding that gender, daily steps, muscle mass, 25(OH)D, and IL-6 were associated with the decreased biceps muscle strength in young MHD group, while in elderly MHD group, muscle mass, 25(OH)D, daily steps, and NT-proBNP were associated with the decreased biceps muscle strength. This study also confirmed that biceps muscle strength was an independent risk factor for survival in MHD patients.
In this study, we found a significant difference in muscle mass between high and low muscle strength at both age groups. However, although muscle mass and muscle strength are highly correlated, loss of muscle mass can only partly explain the decrease of muscle strength [26]. Our study also demonstrated a significant difference in SGA score, inflammatory factors, 25(OH)D, and NT-proBNP between the high and low strength groups. A cohort study showed that muscle strength, as a marker of muscle quality, was more important than muscle mass in estimating mortality risk [27]. Another recent study [10] including 330 incident dialysis patients who were followed up for 5 years reported that patients with low muscle strength (by handgrip) were more likely to die, irrespective of their muscle mass. We further noticed that after adjusting for muscle mass and other factors, biceps strength was still significantly associated with all-cause mortality in the survival analysis. Hence, muscle mass quantification and muscle strength assessment are of importance for MHD patients.
According to a recent meta-analysis, exercise training was shown to significantly increase 6-min walk distance, lower extremity muscle strength, and quality of life in hemodialysis patients [28]. Intra-dialytic, low-intensity progressive strength training was safe and effective to improve physical performance in hemodialysis patients [29]. In this study, we also found that daily steps were significantly correlated with biceps muscle strength in both young group and elderly group. Further studies are needed to establish the generalizability of exercise interventions in dialysis patients.
Vitamin D deficiency or insufficiency is common among CKD patients or dialysis patients. Studies [30,31,32] have demonstrated that vitamin D deficiency is associated with atherosclerosis, vascular calcification, stroke, cardiovascular death and all-cause mortality. The 2009 KDIGO CKD-MBD guidelines suggest that vitamin D deficiency and insufficiency be corrected using treatment strategies recommended for the general population in patients with CKD stages 3-5D [33]. A cross-sectional study [34] indicated that suboptimal levels of 25(OH)D were associated with reduced quadriceps muscle strength and increased fall risk in dialysis patients. A "dose–effect" relationship was also identified between 25(OH)D levels and handgrip strength under 75 nmol/L (30 ng/mL) in hemodialysis patients, which was no more present above 75 nmol/L [35]. Hemodialysis patients supplemented with cholecalciferol for 6 months had higher 25(OH)D [36]. However, no effect on muscle strength was detected, suggesting that the process of muscle adaptation to improved 25(OH)D levels may require more than 6 months. Active vitamin D administration is also associated with increased muscle mass and health-related quality of life in men [37]. Our study revealed that 25(OH)D level was independently associated with biceps muscle strength. Interestingly, weekly dosage of calcitriol was similar between high and low strength groups, suggesting that calcitriol supplementation for the treatment of CKD-MBD is not sufficient to improve vitamin D deficiency in MHD patients and other sources of vitamin D may be needed. It was found in our study that biceps muscle strength improved with the gradual increase in circulating 25(OH)D level, while 25(OH)D level ≥ 75 nmol/L failed to confer additional benefits for muscle strength. But as the sample size of the present study is relatively small and most of the enrolled patients had 25(OH)D insufficiency or deficiency, more larger sample randomized studies are needed to further evaluate the effect of natural or active vitamin D administration on muscle strength.
Proinflammatory cytokines play a role in the development of systemic inflammation in patients with end-stage renal disease [38]. The presence of an inflammatory state may be closely related to mortality in dialysis patients [39, 40]. Previous data suggest that IL-6 was positively correlated with age, and IL-6 elevation was associated with decreased muscle power but not with decreased muscle fibre size [41]. Similar to previous studies, we found that IL-6 level in elderly MHD patients was higher than that in young MHD group. IL-6 is known as a determinant of biceps muscle strength in young MHD patients, but we failed to observe significant differences in IL-6 level between high and low muscle strength groups in elderly patients in the present study.
Natriuretic peptide is recommended as an aid to the diagnosis of heart failure [42]. Observational studies [43] have shown that serum level of B-type natriuretic peptide (BNP) has a strong relationship to both the volume status and survival in HD patients. The relationship between natriuretic peptide and muscle strength is rarely reported. A population-based cohort study [44] demonstrated that greater lean mass rather fat mass was associated with low BNP and N-terminal-proBNP levels. It was recently reported that plasma BNP was negatively correlated with mid-arm circumference and grip strength in male patients [45]. It was found in the present study that NT-proBNP was an independent risk factor for biceps strength in elderly MHD group, probably because the normal metabolic balance between catabolism and anabolism is altered in patients with cardiac volume overload [46]. Achieving and maintaining dry weight might be a strategy in improving muscle strength among elderly patients on hemodialysis.
This study has some limitations. First, it is a single-centre study with a relatively small sample size. The study followed an observational design and cannot provide direct cause-and-effect associations. Second, the study only included prevalent patients. It lacked age-matched healthy control group for comparative analysis. Third, the follow-up period is only 1 year in the survival analysis and, therefore, the results should be interpreted with caution. Fourth, polyneuropathy in hemodialysis patients is a very disabling condition. There also may be an interaction between the polyneuropathy and muscle strength. However, we did not do the neurophysiological examinations. Finally, we only tested the strength of biceps brachii muscle; although it showed a good correlation with grip strength, whether it can represent muscle strength of the upper limbs needs to be verified.
In conclusion, risk factors of muscle weakness were different in young and elderly MHD patients. Biceps muscle strength was an independent risk factor for survival of MHD patients. Appropriate amount of exercise, dry weight control, and increasing 25(OH)D levels might be beneficial to improve muscle strength in hemodialysis patients.
Availability of data and materials
Data that support the findings of this study are available upon request from the corresponding author.
References
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C (2008) A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73(4):391–398
Kalantar-Zadeh K, Kopple JD (2001) Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 38(6):1343–1350
Mehrotra R, Kopple JD (2001) Nutritional management of maintenance dialysis patients: why aren't we doing better? Annu Rev Nutr 21:343–379
Kopple JD (1997) McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr 65(5):1544–1557
Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD (2003) Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42(5):864–881
Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimburger O, Barany P, Suliman ME, Lindholm B, Stenvinkel P, Qureshi AR (2008) Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 27(4):557–564
Heimburger O, Qureshi AR, Blaner WS, Berglund L, Stenvinkel P (2000) Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis 36(6):1213–1225
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA (2001) Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56(5):B209–217
Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, Cederholm T, Stenvinkel P, Carrero JJ (2014) Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 9(10):1720–1728
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19(2):73–78
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C (2009) Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75(1):88–95
Krassilnikova M, Ostrow K, Bader A, Heeger P, Mehrotra A (2014) Low dietary intake of vitamin D and vitamin D deficiency in hemodialysis patients. J Nephrol Ther 4(3):1
Gordon PL, Doyle JW, Johansen KL (2012) Association of 1,25-dihydroxyvitamin D levels with physical performance and thigh muscle cross-sectional area in chronic kidney disease stage 3 and 4. J Ren Nutr 22(4):423–433
Zhang Q, Li M, Zhang T, Chen J (2016) Effect of vitamin D receptor activators on glomerular filtration rate: a meta-analysis and systematic review. PLoS ONE 11(1):e0147347
Xie H, Cheng C, Tao Y, Zhang J, Robert D, Jia J, Su Y (2020) Quality of life in Chinese family caregivers for elderly people with chronic diseases. Health Qual Life Outcomes 14(1):99
Liao CC, Li CR, Lee SH, Liao WC, Liao MY, Lin J, Yeh CJ, Lee MC (2020) Social support and mortality among the aged people with major diseases or ADL disabilities in Taiwan: a national study. Arch Gerontol Geriatr 60(2):317–321
Al-Qaoud TM, Nitsch D, Wells J, Witte DR, Brunner EJ (2011) Socioeconomic status and reduced kidney function in the Whitehall II Study: role of obesity and metabolic syndrome. Am J Kidney Dis 58(3):389–397
Bansal N, Zelnick LR, Himmelfarb J, Chertow GM (2018) Bioelectrical impedance analysis measures and clinical outcomes in CKD. Am J Kidney Dis 72(5):662–672
Wilson FP, Xie D, Anderson AH, Leonard MB, Reese PP, Delafontaine P, Horwitz E, Kallem R, Navaneethan S, Ojo A, Porter AC, Sondheimer JH, Sweeney HL, Townsend RR, Feldman HI, Investigators CS (2014) Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol 9(12):2095–2103
Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, Group NS (2004) Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 15(4):1061–1070
Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, Bergstrom J (1998) Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int 53(3):773–782
Biasioli S, Foroni R, Petrosino L, Cavallini L, Zambello A, Cavalcanti G, Talluri T (1993) Effect of aging on the body composition of dialyzed subjects: comparison with normal subjects. ASAIO J 39(3):M596–601
Kaizu Y, Ohkawa S, Odamaki M, Ikegaya N, Hibi I, Miyaji K, Kumagai H (2003) Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis 42(2):295–302
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH, Health A (2009) Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90(6):1579–1585
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Matsuzawa R, Hoshi K, Yoneki K, Harada M, Watanabe T, Shimoda T, Yamamoto S, Matsunaga A (2017) Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep 2(6):1096–1110
Chen JL, Godfrey S, Ng TT, Moorthi R, Liangos O, Ruthazer R, Jaber BL, Levey AS, Castaneda-Sceppa C (2010) Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant 25(6):1936–1943
London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F (2007) Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18(2):613–620
Matias PJ, Ferreira C, Jorge C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A (2009) 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant 24(2):611–618
Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, Marz W, Ritz E, Wanner C (2010) Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 31(18):2253–2261
Kidney Disease: Improving Global Outcomes CKDMBDWG (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (113):S1–S130
Boudville N, Inderjeeth C, Elder GJ, Glendenning P (2010) Association between 25-hydroxyvitamin D, somatic muscle weakness and falls risk in end-stage renal failure. Clin Endocrinol (Oxf) 73(3):299–304
Bataille S, Landrier JF, Astier J, Giaime P, Sampol J, Sichez H, Ollier J, Gugliotta J, Serveaux M, Cohen J, Darmon P (2016) The "dose-effect" relationship between 25-hydroxyvitamin D and muscle strength in hemodialysis patients favors a normal threshold of 30 ng/mL for plasma 25-hydroxyvitamin D. J Ren Nutr 26(1):45–52
Hewitt NA, O'Connor AA, O'Shaughnessy DV, Elder GJ (2013) Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol 8(7):1143–1149
Mori A, Nishino T, Obata Y, Nakazawa M, Hirose M, Yamashita H, Uramatsu T, Shinzato K, Kohno S (2013) The effect of active vitamin D administration on muscle mass in hemodialysis patients. Clin Drug Investig 33(11):837–846
Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, Kudla M, Teplan V, Haluzik M (2009) Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition 25(7–8):762–768
Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, Zhang W, Zhao Y (2013) Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol 26(2):243–253
Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL (1998) Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 32(1):107–114
Molsted S, Eiken P, Andersen JL, Eidemak I, Harrison AP (2014) Interleukin-6 and vitamin D status during high-intensity resistance training in patients with chronic kidney disease. Biomed Res Int 2014:176190
Booth RA, Hill SA, Don-Wauchope A, Santaguida PL, Oremus M, McKelvie R, Balion C, Brown JA, Ali U, Bustamam A, Sohel N, Raina P (2014) Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev 19(4):439–451
Sivalingam M, Vilar E, Mathavakkannan S, Farrington K (2015) The role of natriuretic peptides in volume assessment and mortality prediction in Haemodialysis patients. BMC Nephrol 16:218
Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH Jr, de Lemos JA (2005) Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 112(14):2163–2168
Chen SF, Li YJ, Song HM, Wu P, Zhang XS, Cui CL (2016) Impact of protein nutritional status on plasma BNP in elderly patients. J Nutr Health Aging 20(9):937–943
Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ (1997) Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 96(2):526–534
Acknowledgements
This work was partly presented as a poster at the annual meeting of the American Society of Nephrology, Oct 31–Nov 5, 2017, New Orleans, LA, and has been published in an abstract form (J Am Soc Nephrol 28, 2017:723). Part of this work was also accepted as an oral communication during the XIX International Congress on Nutrition and Metabolism in Renal Disease held in Genoa (Italy) on June 29, 2018.
Funding
This work was supported by the China Natural Science Foundation (81570665, 81400745), State Key Program of National Natural Science Foundation of China (81730017), Program for Outstanding Medical Academic Leader (2019LJ03) and Shanghai Science and Technology Committee (17411950700). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
QZ, JZ and WZ collected and interpreted the data; QZ and MW analyzed data and prepared figures; BH and MZ helped with data interpretation; QZ wrote and edited the manuscript. JC designed research and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (the ethics committee of Huashan Hospital 2016–193) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11255_2020_2468_MOESM1_ESM.doc
Supplementary Figure 1: Kaplan–Meier curves for non-mortal cardiovascular events according to the presence of high or low biceps muscle strength (A) and in different age groups (B) (DOC 147 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, J., Zhang, W. et al. Risk factors for decreased upper-limb muscle strength and its impact on survival in maintenance hemodialysis patients. Int Urol Nephrol 52, 1143–1153 (2020). https://doi.org/10.1007/s11255-020-02468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02468-0