Abstract
Purpose
To perform a systematic review and meta-analysis to evaluate the cardiovascular prevention effect of aspirin among patients with chronic kidney disease (CKD).
Methods
A comprehensive literature search was conducted in Embase, PubMed, and Cochrane library (up to March 2019) without language limitations. Randomized control trials (RCT) and observational studies that met the inclusion and exclusion criteria were included. Two reviewers independently extracted data, and evaluated study quality using modified Jadad score for RCTs and Newcastle–Ottawa Scale for observational study. A meta-analysis was conducted in the Stata 15.0 software using the DerSimonian and Laird random-effects model.
Results
1768 references were identified from literature searching. Four RCTs and four cohort studies that reported the cardiovascular prevention outcome of aspirin in CKD patients (38,341 participants) were included in this review. The pooled data revealed that aspirin had no significant prevention effect on cardiovascular events among CKD patients (RR = 0.96, 95% CI, 0.59–1.13). There was also no significant reduction in cardiovascular mortality and all-cause mortality. Although we found no significant increased risk in major bleeding events, there was a statistically significant increased risk of minor bleeding events (RR = 2.57, 95% CI, 1.60–4.13) and renal events (RR = 1.30, 95% CI, 1.02–1.65) for aspirin use.
Conclusion
Our review indicated that aspirin use in CKD patients had no prevention effect on cardiovascular events and no statistically significant reduction in risk of cardiovascular or all-cause mortality, with a significant increased risk of minor bleeding and renal events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD), as an independent risk factor for coronary heart disease (CHD) [1], stroke [2], and heart failure (HF) [3], have impacted more and more patients due to aging populations. According to the Global Burden of Disease Study 2015 [4], the total estimated prevalence of CKD was 323 million people, which had a 27% increase compared with 2005. A cross-sectional study [5] in 12 countries showed that the overall prevalence of CKD was 14.3%, while in the United States, it was reported to be 14.8% [6]. The previous study had reported consistently increased cardiovascular risk of CKD patients in age, race, and sex subgroups [7]. Worst of all, cardiovascular disease (CVD) has become the leading cause of death among CKD patients, which is more common than progression to end-stage renal disease (ESRD) [6].
Aspirin, which prevents the clustering of platelet by prohibiting the generation of thromboxane, is a well-known prophylactic agent for CVD. Furthermore, low-dose aspirin use is also encouraged in the primary prevention of CVD among diabetic patients aged ≥ 50 years who have at least one additional CVD risk factor and no increased bleeding risk [8]. Currently, aspirin use is recommended in the primary prevention of CVD among high CVD risk population, but not CKD patients [9]. Due to the increased risk of CVD among CKD patients and the growing prevalence, it is urgent to evaluate the potential prevention effect of aspirin. However, few randomized control trials (RCTs) have been conducted in CKD populations. Recently, the first relevant RCT included CKD stages 3–4 patients to evaluate the primary prevention effect of aspirin, and the results indicated low-dose aspirin did not prevent CVD events except for myocardial infarction (MI) and may slow the decline of renal function without higher bleeding risk [10].
A previous meta-analysis excluded ESRD patients and included only three RCTs [11]. Since lower estimated glomerular filtration rate (eGFR) was associated with an increased ischemic stroke [12], we expanded the population to severe CKD patients including hemodialysis and peritoneal dialysis to perform a systematic review and meta-analysis on the potential prevention effect of aspirin.
Materials and methods
Study identification
In this meta-analysis, the literature retrieval was conducted in PubMed, Embase, and Cochrane library (up to March 2019) with no language limitations. We used the Boolean operator “and” to combine the subject headings. The first subject word was aspirin, and the Boolean operator “or” was used to combine the exploded versions of medical subject headings (MeSH) term acetylsalicylic acid. The second subject word was chronic kidney disease, as well as the exploded versions of MeSH terms chronic renal insufficiency or chronic kidney insufficiency or chronic kidney disease. The previous related reviews were also screened for additional related studies.
Inclusion and exclusion criteria
Two reviewers screened the titles and abstracts of retrieved studies according to the inclusion criteria: (1) randomized control trials or comparative studies evaluating the cardiovascular preventive effect of aspirin in CKD patients aged 18 years or older; (2) the cardiovascular preventive effect was defined as preventing CVD events (e.g., MI, stroke, heart failure). In addition, the exclusion criteria were: (1) head-to-head studies of aspirin versus other anticoagulant drugs; (2) studies including primary nephritic patients (e.g., membranous nephropathy, IgA nephropathy); (3) abstracts, reviews, comments, case reports, and other irrelevant studies. When the included studies enrolled duplicated patients, we only kept the most recent study with the largest sample size. All disagreements were resolved by discussion.
Data extraction and outcome
The main characteristics of included studies, including author, publication year, study design, the number of participants, distribution of gender, age, definition of CKD, eGFR, comorbidities, history of CVD, treatment regimen and follow-up time, were extracted. The incidence of major CVD events was considered as the primary outcome. Furthermore, all-cause mortality, cardiovascular mortality, MI, stroke, heart failure, renal events and minor or major bleeding events were collected. The renal events were defined as double of serum creatinine, ≥ 50% decrease in eGFR, renal failure or progress to renal replacement therapy. The major bleeding events, such as intracranial bleeding and gastrointestinal bleeding, were defined as bleeding events related to hospitalization or death. Accordingly, other bleeding events were considered as the minor bleeding events. Finally, we collected the reported hazard ratios (HRs) or relative risks (RRs) of primary and secondary outcome for aspirin users.
Evaluation of study quality
Two reviewers independently evaluated the methodological quality of all included studies, and any discrepancy was resolved through discussion between them. The quality evaluation of RCTs and comparative studies was performed using the modified Jadad score [13] and the Newcastle–Ottawa Scale (NOS) [14], respectively.
Statistical analysis
We used the Stata 15.0 software (Stata Corp, College Station, Texas) to run this meta-analysis. The RR and 95% confidence interval (CI) were used to pool the dichotomous variables using the DerSimonian and Laird random-effects model [15]. We also pooled the RRs among studies, and the HRs were directly regarded as RRs. We used the Cochrane Q statistic and the I2 test to analyze the heterogeneity among studies. We planned to conduct a subgroup analysis (age, gender, eGFR level, stage of CKD), if the heterogeneity was significant (p ≤ 0.10 or I2 > 50%). In addition, the sensitivity analysis was conducted to test the stability of the pooled data.
Results
Study identification and characteristics
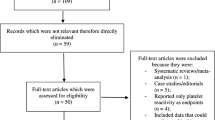
1768 references were identified from searching PubMed, Embase and Cochrane Library. After excluding 608 duplicate studies, 1134 records were removed in the title and abstract screening process. The full text of 26 studies was reviewed, and 8 studies [10, 16,17,18,19,20,21,22] were left in the qualitative and quantitative assessment (Fig. 1). There were four RCTs [10, 16,17,18] and four observational studies [19,20,21,22] that evaluated the cardiovascular prevention effect of aspirin in 38,341 CKD patients, including 8345 stages 3–4 CKD patients, 1310 stage 5 CKD patients, and 28,686 hemodialysis patients. All studies except one included patient with a mean age above 60 years old. Other characteristics of the included studies are described in Table 1.
Study quality assessment
According to the modified Jadad score, the methodological assessment of the RCTs showed relatively low level quality due to inadequate concealment of allocation and blinding method. The allocation was clearly concealed in one RCT [17], open in two [10, 16], and unclear in one RCT [18]. In addition, two RCTs [16, 17] were post hoc subgroup analyses of trials of broader populations. Due to the observational nature of cohort studies, the overall study quality was rated as intermediate. Specifically, all included cohort studies enrolled patients with a history of CVD. In addition, there was no description of outcome assessment. Only one study [20] indicated that all of the patients were followed up for 5 years.
Meta-analyses
Table 2 shows the overall results of all meta-analyses using random-effects model, including CVD, heart failure, myocardial infraction, stroke, cardiovascular mortality, all-cause mortality, major bleeding, minor bleeding, and adjusted RR of renal events. All included RCTs and two cohort studies examined CVD events, but one RCT reported that no CVD event occurred in the aspirin group and control group. Aspirin use had no statistically prevention effect on CVD events (RR = 0.96, 95% CI, 0.59–1.13), and the heterogeneity of this outcome was statistically significant (I2 = 93.9%, p < 0.001). In addition, the meta-analysis results of each single cardiovascular event indicated that no statistically significant reduction risk in aspirin group compared with control patients. In the two studies [10, 20] (517 participants) reporting heart failure event, the RR was 0.91 (95% CI, 0.43–1.90). Three trials and one observational study provided data on myocardial infraction, with the pooled RR of 0.72 (95% CI, 0.41–1.29). Furthermore, the pooled data of four trials and two cohort studies found that no statistically significant reduction in the risk of stroke with aspirin in CKD patients (6293 participants, RR = 1.11; 95% CI, 0.73–1.68).
Only three studies reported the data on cardiovascular mortality. We found no significant reduction in cardiovascular mortality with aspirin compared to control (5326 participants, RR = 0.80; 95% CI, 0.60–1.07), and without significant heterogeneity in this analysis (I2 = 2.7%, p = 0.358). Besides, there was also little or no prevention effect of aspirin use on all-cause mortality among CKD patients (6182 participants, RR = 0.88; 95% CI, 0.66–1.17), and the heterogeneity was in a relative high level (I2 = 59.4%, p = 0.043).
Six studies provided information on major and minor bleeding events. In major bleeding events, we found no significant increased risk for aspirin compared to control (6293 participants, RR = 1.15; 95% CI, 0.78–1.69), and we included one trial [10] that reported no major bleeding occurred in the aspirin group or control group. The result of the heterogeneity test (I2 = 43.6%, p = 0.131) could exclude significant heterogeneity. By contrast, there was a positive and statistically significant increased risk of minor bleeding for aspirin use (four studies, 4586 participants, RR = 2.57; 95% CI, 1.60–4.13), with a low heterogeneity (I2 = 0.0%, p = 0.567).
Finally, only one RCT and two cohort studies reported adjusted HRs as outcome measures of renal events, while only one trial [10] provided the number of occurred renal events (3 in aspirin arm and 17 in control arm, p = 0.016). The pooled RR showed that the use of aspirin was correlated with increased risk of renal events (RR = 1.30, 95% CI, 1.02–1.65), and the result of heterogeneity test (52.7%) could not deny significant heterogeneity. A subgroup analysis was not conducted due to insufficient data.
Sensitivity analyses
The sensitivity analyses were performed to calculate the pooled RR of the remaining studies by omitting each study. The result (Fig. 2) revealed a similar converged value of RR and 95% CI for the outcomes of CVD, MI, stroke, cardiovascular mortality, major bleeding, and minor bleeding. However, after omitting one cohort study [19], the pooled RR of the remaining studies for all-cause mortality was statistically significant (RR = 0.75, 95% CI, 0.60–0.94). Similarly, the sensitivity analysis of the risk of renal events was also unstable (Fig. 2).
Discussion
Our meta-analysis revealed that there was no evidence to indicate the benefit of aspirin in cardiovascular prevention among CKD patients, as the risk of CVD events, heart failure event, myocardial infraction, stroke, cardiovascular mortality and all-cause mortality is not reduced by aspirin use in patients with different stages of CKD. In contrast, aspirin use was associated with increased risk of minor bleeding (RR = 2.57, 95% CI, 1.60–4.13) and renal events (RR = 1.30, 95% CI, 1.02–1.65). There were no sufficient data to conduct a subgroup analysis.
According to the recently published US Renal Data System 2018 Annual Data Report [23], the prevalence of CVD in patients who had CKD was almost twice as those without CKD. There is no doubt that CKD is an independent risk factor for CVD. One potential explanation for the complex relationship between CKD and CVD is that they have same traditional risk factors, such as diabetes mellitus, hypertension, physical inactivity, left ventricular hypertrophy, smoking, family history and dyslipidemia [23]. Furthermore, patients with advanced renal dysfunction are often excluded from clinical trials of cardiovascular drugs, due to the fact that many drugs are cleared by the kidneys. Although our meta-analysis revealed that no preventive effect of aspirin in CVD was found in patients with CKD, the results of current studies were controversial.
Aspirin is recommended to prevent CVD among those who are at high cardiovascular risk. Apart from the diabetic patients [8], the United States Preventive Services Task Force advocates that general population aged 50–69 years old with more than 10% risk of developing CVD, and who are willing to take low-dose aspirin constantly in their residual lifetime (at least 10 years) are more likely to avail the benefit of prevention of CVD and/or colorectal cancer [24]. As mentioned above, CKD is an independent risk factor for CVD. Therefore, the use of aspirin should provide great cardiovascular prevention effects among CKD patients. The first relevant trial, British Doctors Trial, reported that aspirin had no significant prevention effect on nonfatal MI among healthy male doctors [25]. Since this study, quite a lot of researches have been concentrated on this topic, and the previous systematic review reported aspirin reduced RR (RR = 0.78, 95% CI, 0.71–0.87) of nonfatal MI among diabetes patients [26]. However, when the study subjects were limited to CKD patients, the results changed dramatically. As the first published RCT assessing primary cardiovascular prevention effect of aspirin among CKD patients, Goicoechea et al. reported no statistical significant difference between aspirin therapy group and standard treatment group in fatal or nonfatal CVD (aspirin: 5/50, control: 17/61, HR = 0.396, 95% CI, 0.146–1.076) [10]. In the post hoc subgroup analysis of hypertension optimal treatment (HOT) study, low-dose aspirin reduced the HR of primary endpoints, including major CVD, MI, stroke, cardiovascular mortality and total mortality, among patients with an eGFR less than 45 ml/min/1.73 m2 [17]. In addition, Jardine et al. reported that the cardiovascular protection effect of aspirin increased as GFR declined. Conversely, a retrospective propensity score-matched study reported that the use of aspirin among CKD patients was associated with higher incidence of any atherosclerotic CVD (p < 0.001) [21]. A previous meta-analysis, conducted by Palmer et al. [27], concluded that the use of antiplatelet agents (aspirin and clopidogrel) in CKD patients had little or no benefit on MI, or on cardiovascular or all-cause mortality. Several potential mechanisms that may explain the poor effect of antiplatelet agents in CKD population include increased platelet activation, high residual platelet reactivity, altered pharmacokinetic effects of uremia on drug transport and non-renal metabolism, and elevated Von Willebrand antigen levels in these patients [21].
Another controversial question is whether the use of aspirin increases bleeding risk among CKD patients. No differences in bleeding episodes were found between aspirin group and standard arm in the work of Goicoechea et al. [10]. And no major bleeding event occurred. The first United Kingdom Heart and Renal Protection Study (UK-HARP-1) [18], a 2 × 2 design with simvastatin as the second intervention, reported no significant differences in major bleeding events, but aspirin users developed more minor bleeds (p = 0.001). In the post hoc subgroup analysis of the HOT study, Jardine et al. [17] found that aspirin increased the risk of any bleeding episodes among CKD patients (HR = 1.61, 95% CI, 1.32–1.97), and there were no significant differences in major and minor bleeding events in CKD patients with an eGFR less than 45 ml/min/1.73 m2. Interestingly, all fatal bleeds occurred in patients with an GFR ≥ 60 ml/min/1.73 m2 in their study. However, Palmer et al. [27] concluded that the use of antiplatelet agents would increase major and minor bleeding events in patients with CKD and acute coronary syndrome who required percutaneous coronary intervention. CKD patients have complex hemostatic disorders, which caused both thrombotic predisposition [28] and bleeding diathesis [29] to occur paradoxically. In addition, prolongation of bleeding time as well as abnormal platelet aggregation and adhesion would occur when CKD patients developed uremia [30]. However, further well-designed and controlled trials are required to define the bleeding risk of aspirin use in CKD patients.
The safety of aspirin is also a fatal issue in CKD population. The pooled result of adjusted RR of renal events indicated that aspirin treatment increased renal events (doubling of serum creatinine, ≥ 50% decrease in eGFR, renal failure or progress to renal replacement therapy). The products of the enzyme cyclooxygenase, particularly thromboxane and prostacyclin, are crucial for kidney homeostasis [31]. Experimental studies concluded that aspirin or thromboxane receptor inhibitors improved renal plasma flow and GFR values, suggesting that thromboxane had a pathogenic role in the progression of renal damage [32]. However, current results of human study on the long-term effects of aspirin on renal function and progression of CKD are scarce and even contradictory. Aspirin therapy was associated with a lower risk of renal events (HR = 0.272, 95% CI, 0.075–0.955) in the study of Goicoechea et al. [10]. However, when they introduced the basal proteinuria into their regression model due to the fact that proteinuria was an important risk factor of cardiovascular and renal events in CKD patients, the aspirin treatment had no significant impact on the renal events. The UK-HARP-1 trial [18] indicated that 1 year use of low-dose aspirin was not associated with accelerating the progression of CKD. Similarly, Jardine et al. reported that aspirin treatment did not affect renal function in the overall study population nor within any eGFR category. Conversely, some observational studies supported a harmful effect of aspirin on CKD progression. Kim et al. [21] reported low-dose aspirin was significantly associated with increase of serum creatinine and ESRD progression requiring renal replacement treatment in CKD patients. In addition, another observational study, enrolled 1301 advanced CKD patients, reported that aspirin use was associated with renal failure in the patients with no stroke history [19]. However, these two studies were observational studies rather than controlled interventional trials. Thus, some important baseline characteristics were significantly different. In addition, the sensitivity analysis of the risk of renal events was unstable. Therefore, we suggest that the pooled adjusted RR of renal events should be interpreted with caution. However, the current KDIGO guideline [9] does not recommend using aspirin for primary CVD prevention in CKD patients due to the uncertain benefit and the potential harm.
As compared to the previous meta-analysis which only concentrated on non-end-stage CKD patients, our systematic review included the first RCT [10] of evaluating the primary cardiovascular prevention effect of aspirin among CKD patients, and expanded the study objects to collect stage 5 CKD patients [19] and CKD patients in dialysis [20, 22]. Limitations in our reviews must be considered. First, only one small sample size RCT [10] related to this topic was included, and the remaining studies were subgroup analysis of RCTs or observational study with relative lower quality. Second, the multi-factorial designs of both HARP and HOT study, inconsistent definition, and different characteristics of patients may be a potential source of bias and heterogeneity. Finally, the data extracted from included studies were not sufficient to conduct a further subgroup analysis.
Conclusions
In conclusion, our review indicated that aspirin use in CKD patients had no preventive effect on CVD events and no statistically significant reduction in risk of cardiovascular or all-cause mortality. Aspirin use in CKD patients was related to a significantly increased risk of minor bleeding and renal events. However, we did not find enough evidence to not use aspirin in CKD or ESRD patients. So, further large-scale controlled interventional trials are required to generate validated evidence.
References
Baber U, Gutierrez OM, Levitan EB, Warnock DG, Farkouh ME, Tonelli M, Safford MM, Muntner P (2013) Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am Heart J 166(2):373–380. https://doi.org/10.1016/j.ahj.2013.05.008
Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC (2015) Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transpl 30(7):1162–1169. https://doi.org/10.1093/ndt/gfv009
Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3(7):514–525. https://doi.org/10.1016/s2213-8587(15)00040-6
Global Burden of Disease Study 2015 (GBD 2015) results. Seattle, WA: Institute for Health Metrics and Evaluation (IHME), University of Washington
Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo RF, Aleckovic-Halilovic M, Zou H, Zhang L, Gouda Z, Tchokhonelidze I, Abraham G, Mahdavi-Mazdeh M, Gallieni M, Codreanu I, Togtokh A, Sharma SK, Koirala P, Uprety S, Ulasi I, Remuzzi G (2016) Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 4(5):e307–e319. https://doi.org/10.1016/s2214-109x(16)00071-1
Unite States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States (2018). National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J (2013) CKD and cardiovascular disease in the atherosclerosis risk in communities (ARIC) study: interactions with age, sex, and race. Am J Kidney Dis 62(4):691–702. https://doi.org/10.1053/j.ajkd.2013.04.010
American Diabetes Association (2018) 9. Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes care 41(Suppl 1):S86–S104. https://doi.org/10.2337/dc18-s009
Levin A, Stevens PE, Bilous RW, Coresh J, Francisco A, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb E, Levey AS, Riella M, Shlipak MG, Wang H, White CT, Winearls CG, (2013) Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150. https://doi.org/10.1038/kisup.2012.73
Goicoechea M, de Vinuesa SG, Quiroga B, Verde E, Bernis C, Morales E, Fernandez-Juarez G, de Sequera P, Verdalles U, Delgado R, Torres A, Arroyo D, Abad S, Ortiz A, Luno J (2018) Aspirin for primary prevention of cardiovascular disease and renal disease progression in chronic kidney disease patients: a multicenter randomized clinical trial (AASER Study). J Thromb Haemost JTH 32(3):255–263. https://doi.org/10.1007/s10557-018-6802-1
Major RW, Oozeerally I, Dawson S, Riddleston H, Gray LJ, Brunskill NJ (2016) Aspirin and cardiovascular primary prevention in non-endstage chronic kidney disease: a meta-analysis. Atherosclerosis 251:177–182. https://doi.org/10.1016/j.atherosclerosis.2016.06.013
Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, Woodward M, Longstreth WT Jr, Psaty BM, Shlipak MG, Folsom AR, Gansevoort RT, Coresh J (2014) Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke 45(7):1925–1931. https://doi.org/10.1161/strokeaha.114.004900
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst. https://doi.org/10.2307/632432
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Saito Y, Morimoto T, Ogawa H, Nakayama M, Uemura S, Doi N, Jinnouchi H, Waki M, Soejima H, Sugiyama S, Okada S, Akai Y (2011) Low-dose aspirin therapy in patients with type 2 diabetes and reduced glomerular filtration rate: subanalysis from the JPAD trial. Diabetes care 34(2):280–285. https://doi.org/10.2337/dc10-1615
Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, Zoungas S, Lambers Heerspink HJ, Chalmers J, Zanchetti A (2010) Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 56(12):956–965. https://doi.org/10.1016/j.jacc.2010.02.068
Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, Cairns HS, Collins R, Foley RN, Frighi V, Kourellias K, Ratcliffe PJ, Rogerson M, Scoble JE, Tomson CR, Warwick G, Wheeler DC (2005) First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis 45(3):473–484. https://doi.org/10.1053/j.ajkd.2004.11.015
Hsiao KC, Huang JY, Lee CT, Hung TW, Liaw YP, Chang HR (2017) Different impact of aspirin on renal progression in patients with predialysis advanced chronic kidney disease with or without previous stroke. Eur J Intern Med 39:63–68. https://doi.org/10.1016/j.ejim.2016.11.009
Liu J, Pan Y, Chen L, Qiao QY, Wang J, Pan LH, Gu YH, Gu HF, Fu SK, Jin HM (2016) Low-dose aspirin for prevention of cardiovascular disease in patients on hemodialysis: a 5-year prospective cohort study. Hemodial Int 20(4):548–557. https://doi.org/10.1111/hdi.12409
Kim AJ, Lim HJ, Ro H, Ko KP, Han SY, Chang JH, Lee HH, Chung W, Jung JY (2014) Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PLoS One 9(8):e104179. https://doi.org/10.1371/journal.pone.0104179
Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, Gillespie BW, Young EW (2007) Aspirin prescription and outcomes in hemodialysis patients: the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 50(4):602–611. https://doi.org/10.1053/j.ajkd.2007.07.007
Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V (2019) US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 73(3s1):A7–A8. https://doi.org/10.1053/j.ajkd.2019.01.001
Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. (2017) https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed 4 Jan 2019
Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, Warlow C, Hafner B, Thompson E, Norton S, Gilliland J, Doll R (1988) Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 296(6618):313–316. https://doi.org/10.1136/bmj.296.6618.313
Desai D, Ahmed HM, Michos ED (2015) Preventing cardiovascular disease in patients with diabetes: use of aspirin for primary prevention. Curr Cardiol Rep 17(3):13. https://doi.org/10.1007/s11886-015-0566-z
Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G, Jardine M, Webster A, Nicolucci A, Zoungas S, Strippoli GFM (2012) Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 156(6):445–459. https://doi.org/10.7326/0003-4819-156-6-201203200-00007
Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR (2008) Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol JASN 19(1):135–140. https://doi.org/10.1681/asn.2007030308
Mezzano D, Tagle R, Panes O, Pérez M, Downey P, Muñoz B, Aranda E, Barja P, Thambo S, González F, Mezzano S, Pereira J (1996) Hemostatic disorder of uremia: the platelet defect, main determinant of the prolonged bleeding time, is correlated with indices of activation of coagulation and fibrinolysis. Thromb Haemost 76(3):312–321
Washam JB, Adams GL (2008) Risks and benefits of antiplatelet therapy in uremic patients. Adv Chronic Kidney Dis 15(4):370–377. https://doi.org/10.1053/j.ackd.2008.07.006
Harris RC, Breyer MD (2001) Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 281(1):F1–F11
Boffa JJ, Just A, Coffman TM, Arendshorst WJ (2004) Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol 15(9):2358–2365
Acknowledgements
We thank Dr. Qing He for providing very useful comments while performing this review.
Author information
Authors and Affiliations
Contributions
BQ and YH contributed equally to this review. They had full access to all the study data and take responsibility for the integrity of the data and the accuracy of the results. They contributed to the study development, quality assessment, data analysis and manuscript writing. LW and HL were responsible for literature search, identifying relevant studies and data collection. Hu was responsible for chart production and creating the figures. ML, the corresponding author, managed the study development and guided the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Ethical approval
This review was based on published studies. Therefore, ethical approval and informed consent are not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, B., He, Y., Wu, L. et al. Is there a cardiovascular protective effect of aspirin in chronic kidney disease patients? A systematic review and meta-analysis. Int Urol Nephrol 52, 315–324 (2020). https://doi.org/10.1007/s11255-019-02350-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02350-8