Abstract
Purpose
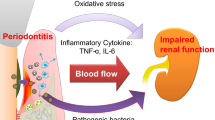
Chronic inflammation is an obvious risk factor of atherosclerotic diseases, and the presence of periodontal disease is one of the important sources of chronic inflammation in patients with chronic kidney disease (CKD) and diabetes mellitus (DM). Thus, we aimed to investigate the effects of non-surgical periodontal therapy of the patients undergoing CAPD due to diabetic nephropathy, diabetic patients without CKD, and healthy controls on inflammation exponents.
Methods
Thirty-two CAPD patients due to diabetic nephropathy (group III), 31 diabetic patients without nephropathy (group II), and 38 healthy subjects (group I) were enrolled to the study. All patients enrolled to the study (to all groups) suffered from chronic periodontitis. Plaque index, Gingival index, pocket depth (PD) measurements were recorded before and after periodontal therapy. All blood samples for biochemical parameters were measured by using standard laboratory techniques with an automatic analyser. Blood samples for TNF-α, IL-6, and PTX-3 were centrifuged, and separated serum and plasma samples were stored at − 80 °C until analysis.
Results
All inflammatory markers were significantly higher in group III than the other two at baseline. TNF-α levels were significantly decreased after periodontal treatment at 3-month visit in all groups. PTX-3, IL-6, and Hs-CRP levels were significantly reduced after periodontal treatment at 3 months in group III.
Conclusion
Periodontal disease is an important source of inflammation in diabetic CAPD patients and treatment of periodontal disease can be monitored by inflammatory markers including TNF-alpha, PTX-3, IL-6, and Hs-CRP. TNF-alpha may be useful and more sensitive monitoring inflammation in healthy patients and diabetic patients after periodontal treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD), defined as a progressive decline in renal function monitored by glomerular filtration rate (GFR), is an important public health problem all over the world and its prevalence is growing over time [1, 2]. CKD affects 10–16% of the adults around the world currently [2], and according to large population (10,872 participants)-based survey the prevalence of CKD in Turkish population was found to be 15.7% [3]. The management of CKD includes dietary changes, correction of systemic complications, and renal replacement therapy via hemodialysis, peritoneal dialysis, or renal transplantation. Several factors were defined as a risk factor for CKD including diabetes mellitus, hypertension, smoking, obesity, and genetic factors. Diabetic patients constitute majority of the newly diagnosed CKD patients [4]. One of the most important goals in patients with CKD is to improve their quality of life. Cardiovascular complications which are not explained by the traditional risk factors are still the major cause of morbidity and mortality [5]. Chronic inflammation is an obvious risk factor of atherosclerotic diseases, including CKD patients, particularly in continuous ambulatory peritoneal dialysis (CAPD) patients [6]. The presence of periodontal disease has been investigated as a potential source of chronic inflammation in patients with CKD [7, 8].
Periodontal diseases, comprising gingivitis and periodontitis, are probably the most common disease in the world. The recent Global Burden of Disease Study indicates that severe periodontitis is the sixth most prevalent disease worldwide, with an overall prevalence of 11.2% and around 743 million people affected [9].
Diabetes mellitus, heterogenous group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both, is associated with long-term damage of various systems, especially cardiovascular disease [10]. Diabetes mellitus might be associated with several diseases. Current data reveal strong evidence that a two-way relationship between diabetes and periodontitis exists [11, 12]. Various studies documented that diabetes is the risk for periodontitis, and periodontal inflammation negatively affects glycaemic control [11]. In addition, incidence of CKD is increased threefold in diabetic individuals who also have severe periodontitis and the risk of cardiorenal mortality is three times higher in diabetic people with severe periodontitis than in diabetic people without severe periodontitis [12].
In the light of these literatures, the aim of our study was to evaluate the effects of non-surgical periodontal therapy on patients undergoing CAPD due to diabetic nephropathy, to compare these possible effects among diabetic patients without CKD, healthy controls, and CAPD patients.
Materials and methods
Study population
This before/after clinical study was approved by the Erciyes University Faculty of Medicine Ethics Committee (Decision Number: 2014/606). Forty-nine CAPD patients due to diabetic nephropathy (32 of them meet the inclusion criteria) were referred from the Department of Nephrology, Erciyes University Faculty of Medicine. In addition, thirty-one patients with diabetes mellitus without nephropathy and thirty-eight healthy subjects, all patients enrolled to the study (to all group) suffered from chronic periodontitis. Informed consent was obtained from all participants. The study was conducted between December 2014 and July 2015. Twenty patients were included for each groups (Group I healthy controls, Group II Diabetic patients without nephropathy, Group III CAPD patients with diabetic nephropathy) randomly assigned by electronically generated list. Allocation concealment for each groups was done by well-sealed opaque envelopes, each number represented a patient in the study groups.
Case definitions
The clinical diagnosis of chronic periodontitis (CP) was determined based on the criteria described by Savage et al. [13] as follows: the presence of ≥ 5 teeth with ≥ 1 sites with probing depth (PD) ≥ 5 mm, clinical attachment level (CAL) ≥ 2 mm, and the presence of bleeding on probing (BOP). Type 2 Diabetes Mellitus (DM) was diagnosed when patients had fasting blood glucose (FBG) ≥ 126 mg/dL and/or ≥ 200 mg/dL glucose levels at 2 h after ingestion of 75 g glucose based on the diagnostic criteria of American Diabetes Association [9]. Diabetic nephropathy is defined by macroalbuminuria that is, a urinary albumin excretion of more than 300 mg in a 24-h collection or macroalbuminuria and abnormal renal function as represented by an abnormality in serum creatinine, or glomerular filtration rate (GFR) [14, 15].
Inclusion criteria were as follows: All patients in each groups had to have chronic periodontitis and ≥ 15 natural teeth; moreover, they needed to be > 25 years old. History of antibiotic or anti-inflammatory drugs within the previous 6 months, pregnancy or lactation, periodontal therapy within 6 months prior to the study, past or current smoking and alcohol consumption were considered to be exclusion criteria.
Clinical examinations
The following clinical parameters were evaluated at baseline and the end of the study: Plaque index [16], Gingival index [17], pocket depth (PD) measurements, bleeding on probing (BOP)—percentage of BOP (+) sites, gingival recession (GR)—from the cement–enamel junction to the gingival margin, and clinical attachment level (CAL)—the sum of PD and GR measurements. In addition, the percentage and number of deep sites were also calculated. The Decay–Missing–Filling Index (DMFT) was recorded. Clinical examinations were repeated 3 months following periodontal treatment. All clinical measurements were taken from the mid-buccal and mid-lingual sites and the buccal aspects of the interproximal contact area for the mesial and distal sites of each tooth to the nearest 0.5 mm using a 15 mm periodontal probe (CEPCN15, Nordent, IL, USA) at baseline and the 3rd and 6th months after treatment. All periodontal clinical examinations were performed by one calibrated examiner (FOT). Periapical radiographs with the parallel technique and panoramic radiographs were taken at baseline for confirmation of the diagnosis of chronic periodontitis, as well as the diagnosis of other pathological conditions within the jaws.
To estimate the reliability of the measurements during the treatment period, ten randomly selected patients were re-evaluated. The reliability of the continuous variables was expressed as the standard deviation of the differences divided by two. The range of the mean error for PD was 0.13–0.15, and this indicated stable reliability during the evaluation period. Cohen’s kappa (κ) was employed to describe the reliability of discrete GI and BOP values. Based on duplicate measurements, the κ values of GI and BOP were 0.94 ± 0.04 and 0.93 ± 0.05, respectively.
Blood sampling and laboratory analysis
At baseline and 3 after treatment, 20 mL fasting venous blood samples were collected from the antecubital fossa by an experienced nurse. All blood samples were collected in the morning at 8:00–8:30 am. Immediately after the collection, blood samples were sent to biochemical laboratory of Erciyes University Faculty of Medicine for analysis and all blood samples were measured by using laboratory techniques with an automatic analyser. Blood samples for TNF-α, IL-6, and PTX-3 were centrifuged, and separated serum and plasma samples were stored at − 80 °C until analysis.
TNF-α (Invitrogen, Cat no: KHC3011), IL-6 (Invitrogen, Cat no: KHC0061), and PTX3 (Shanghai Yehua, Cat no: YHB2259Hu) serum levels were determined using a commercial solid-phase enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. The plates were read with an Epoch microplate spectrophotometer (BioTec Winooski, VT, USA). The values were expressed as ng/mL (PTX3) and pg/mL (TNF-α and IL-6); the variation coefficients of the methods for TNF-α, IL-6, and PTX3 were 4.4, 6.2, and < 10%, respectively. TNF- α, IL-6, and PTX-3 parameters were measured in the Biochemistry Department of Erciyes University, Faculty of Medicine.
Periodontal treatment
Periodontal treatments were performed 2 h after the patients had their breakfast following the blood sampling. Standard oral hygiene instructions were given to all groups, including interdental plaque control (interdental brushes) and brushing of the dorsum of the tongue twice a day. Oral hygiene control and reinstructions were provided during all visits. After local infiltration, full-mouth scaling (scraping off the tartar from above and below the gum line) and root planning (gets rid of rough spots on the tooth roots where the bacteria gather and removes bacterial toxins that penetrate into root surfaces) (FM-SRP) was performed by the same investigator (ZT) with standard periodontal curettes and ultrasonics. Periodontal therapy was completed within 24 h in two consecutive visits. The full-mouth disinfection (FMD) protocol was performed based on Quirynen et al. [18] Periodontal therapy was scheduled in the following order: (1) brushing the dorsum of the tongue (by the patients) for 60 s with a 0.1% chlorhexidine gel; (2) rinsing twice with 0.12% chlorhexidine solution for 1 min (for the last 10 s, the patients had to gargle in an attempt for the rinsing solution to reach the tonsils); (3) subgingival irrigation of all the pockets three times within 10 min with chlorhexidine 1% gel; and (4) repeating the subgingival application on day 7. In addition, the patients were instructed to rinse twice daily for 1 min with a 0.12% solution of chlorhexidine during the 14 days after treatment. SRP was repeated if necessary at the end of the study.
During the periodontal treatment sessions, necessary tooth extractions were performed and referrals for endodontic and restorative treatments were given immediately after periodontal treatment was completed.
Statistical analysis
The sample size was calculated based on Fang et al. [19] using data relative to the mean difference and standard deviation (SD) between the IL-6 level of CKD patients during the experimental period. It was estimated that 16 patients for each group would be enough to find a decrease of 1.03 (pg/mL) at 3 months (1.00 SD, α error of 0.05, and β error of 0.2). The Kolmogorov–Smirnov test was used to test the normality of the data. The One-way ANOVA and Kruskal Wallis H tests were used to analyze the parametric and non-parametric data, respectively. The categorical variable samples’ intergroup comparisons were performed by the Chi-square analysis The repeated measures ANCOVA test was used for three groups to analyze the repeated measurements; in addition, results were adjusted for sex and the corrected p values were presented in tables. Moreover, we performed log transformation for not normally distributed variables. For the post hoc comparisons, Bonferroni correction was used. All analyses were conducted using statistical software (SPSS Version 21) with the significance level set as < 0.05.
Results
There were significant differences in terms of gender among the groups. Other demographic characteristics, oral hygiene habits, and anthropometric parameters were similar among the groups. During the study period there were no reported changes or eating habits in the lifestyle of participants; consequently, there were no significant changes in the anthropometric measurements in the groups (Table 1).
All of the periodontal parameters were similar at baseline in all groups. Periodontal treatments were without complication and no side effect was reported in the participants. There were no significant differences among groups at 3 months. However, significant improvements were observed at the 3 months compared to baseline in all groups. In addition, no significant differences were found in the DMFT total values, decay, and missing tooth number neither baseline nor 3 months of follow-up period for all groups (Table 2).
There were significant differences observed in the lipid profiles between the groups (Table 3). Triglyceride (TC) levels were significantly higher in group II than other groups both baseline and 3 months. Intragroup analysis showed no significant changes after periodontal treatment. HDL level was significantly lower in group III than other groups both baseline and 3 months. Intragroup analysis showed no significant changes after periodontal treatment for all groups in terms of HDL levels. LDL level was significantly higher in group II than other groups both baseline and after 3 months. Intragroup analysis showed significant changes after periodontal treatment for all groups.
HbA1c levels were significantly lower in group I than other groups at all baseline; however, there were no significant changes after periodontal treatment for all groups. Homeostatic model Assessment for Insulin Resistance (Homa-IR) scores were significantly higher at both baseline in group III than other groups and intragroup analysis showed significantly reduction only in group III (Table 3)
There were significant differences in nutritional parameters among groups. Also, serum albumin levels were similar for all groups at baseline and after 3 months. There were no significant differences found in the intragroup analysis (Table 3).
There were significant differences in biochemical and hematological parameters between groups. Serum Parathyroid hormone (PTH), Ca, phosphorus, uric acid, and hemoglobin levels were significantly higher in group III than other groups at both baseline, and there were no significant changes after periodontal treatment at 3 months for all groups (Table 4).
There were significant differences in terms of inflammatory markers among all groups. All inflammatory markers were significantly higher in group III than other groups at baseline. Except for PTX-3, all other parameters were significantly higher in group III than other groups after 3 months. TNF-α levels were significantly decreased after periodontal treatment at 3-month visit in all groups (graph 1). PTX-3, IL-6, and Hs-CRP levels were significantly reduced after periodontal treatment at 3 months in group III. In addition, there were no significant changes in terms of serum ferritin levels after periodontal treatment at 3 months in all groups (Table 5).
Discussion
Several findings about the relationship between periodontal disease and diabetic nephropathy were identified in this study. Firstly, a significant reduction in inflammatory markers was observed during follow-up. TNF-α decreased in all groups and PTX-3, IL-6, and Hs-CRP were reduced in group III only. Lastly, there was no significant change in HbA1c in all groups.
The present study found that most patients (Table 1) were at a dentist for over a year ago, which is a long term for patients at risk. According to literature, frequent dental visitors had more teeth than infrequent visitors. However, we investigate main periodontal parameters and Renvert et al. documented that frequency of dental visits had no impact on plaque deposits, gingival inflammation, or alveolar bone levels [20].
The recent meta-analysis results, FMD, FMS, and Q-SRP are all effective for the management of chronic periodontitis [21]. The comparisons of FMD versus Q-SRP revealed that FMD had modest supplemental clinical benefits over Q-SRP in reduction of probing depth and gain in clinical attachment level [22]. In addition, studies reported that FMD has some advantages such as greater adherence, low cost, and fewer treatment sessions, with less traveling or absence from work for the patient [23,24,25,26]. For this reasons, we preferred to apply FMD and FM-SRP as the choice of treatment to restrict the cumulative effect of bacteremia and refrain repeated acute inflammation occurring during traditional treatment modalities in this study [26, 27]. As a result, significant improvements were found for all periodontal parameters during the study period in all groups.
Monitoring of glycaemic control and changes in hemoglobin A1c level after periodontal treatment were controversial. Although some of studies reported significantly reduction of hemoglobin A1C level following periodontal treatment [28], the Cochrane review actually showed, based on meta-analysis of 14 studies (1499 participants) comparing periodontal therapy with no active intervention/usual care a mean HbA1c was 0.29% lower (95% confidence interval (CI) 0.48–0.10% lower) 3–4 months post-treatment. The Cochrane review did, however, state that there does not appear to be a notable difference between different periodontal treatments [29]. Similarly, our study showed that there were no significant changes in HgbA1c levels after periodontal treatment in all groups. Although HbA1c may be influenced by periodontal treatment, it was not the only effective factor. Diet habits and treatment compliance are the most relevant factors on glycaemic control.
We have shown that periodontal therapy can reduce HOMA-IR score in obese patients in our previous study [30]. Moreover, Sun et al. [31] reported that periodontal therapy leads to decrease of HOMA-IR levels in diabetic patients. They concluded that periodontal intervention can improve glycaemic control, lipid profile, and insülin resistance [28]. In the present study, we observed 16% reduction of HOMA-IR score levels which could not reach statistically significant in diabetic patient without CKD. Sun et al. revealed that a 22.3% reduction of HOMA-IR in their diabetic cohort. This result may be originated from the sample size differences between two studies. Sun et al. [31] included a 82 diabetic patients to their study however we enrolled 20 diabetic patients to our study. Otherwise, we found significant reduction of HOMA-IR levels in patients with CAPD with diabetic nephropathy. Although, current literature showed that periodontal therapy has reduced the HOMA-IR levels in diabetic and obese population, HOMA-IR reduction in CAPD group may not be originated from the periodontal therapy since, glucose levels were dramatically reduced in this group which is more relevant to HOMA-IR levels in our study. Otherwise, it is possible that periodontal therapy may reduce glucose levels. This result may be important because CAPD patients have CKD and treated with peritoneal dialysis and which solutions contain high glucose levels. Consequently, diagnosis and treatment of periodontal disease may play a key role in this population.
Recent studies have focused on the role of periodontal disease in patients with CKD due to diabetes mellitus to evaluate the effect of periodontal treatment on inflammatory mediators. TNF-α is one of the important cytokines that is closely associated with inflammation and frequently monitored as an inflammatory bio-marker in several studies. In this study, TNF-α serum level was highest in CAPD patients and lowest in healthy subjects at baseline, and significant reduction of TNF-α levels was observed in all groups. However, Geisinger et al. reported opposite results for diabetic patients, many studies documented that TNF-α levels were reduced after periodontal treatment in diabetic patients [32]. In addition, recent meta-analysis support the hypothesis that periodontal therapy reduces serum levels of TNF- α in diabetes mellitus [33]. It has been well established that TNF-α levels were associated with severity of inflammation. Supportingly, we found the highest level of TNF-α in patients with CAPD which are more prone to inflammation. However, there were few studies which investigated the TNF-α levels in chronic kidney disease patients with periodontitis. A study by Fang et al. [19] documented contrary results with us in terms of TNF-α levels. The opposite results may be originated from the difference of study protocols.
PTX-3 is a novel acute-phase protein that is a diagnostic and prognostic marker for inflammatory diseases additionally it is structurally linked to short pentraxins, including hs-CRP, and is highly expressed in patients with CKD [34]. While hs-CRP is derived only from hepatocytes, PTX-3 appears to be synthesized by several tissues and cells, including fat tissue, macrophages, and vascular endothelial cells [35]. To the best of our knowledge, there were only a few studies [36] investigating alterations in the PTX-3 level after 1 month of periodontal treatment. Although Mathew et al. [36] revealed a significant decrease of PTX-3 levels after 1 month of periodontal therapy, our previous study on obese patients showed no significant changes. In the present study, we did not find any significant changes in PTX-3 levels throughout the study in group with healthy controls and diabetic patients. As many clinicians would appreciate, a month may be an extremely early point to evaluate the level of alteration of PTX-3. Additionally, this reduction should be interpreted with caution since involvement of the moderate periodontitis patients, sample size, and involment of the diabetic patients with poor control may play a role on these results.
IL-6 is a polypeptide mediator produced by a variety of cell types, including T-cells, fibroblast, epithelial, and endothelial cells. Increased local level of IL-6 has been demonstrated in many inflammatory conditions [37, 38]. High-Sensitive C-reactive protein is an acute-phase protein which elevated in inflammatory conditions, was also reported [39, 40]. Although previous studies have showed that IL-6 and hs-CRP levels were reduced in diabetic patients after periodontal therapy, we observed that IL-6 and hs-CRP were not decreased after periodontal therapy in healthy controls and diabetic patients. In addition, there were no significant differences among the groups. However, Group III (diabetic nephropathy + periodontitis) had significantly higher IL-6 and Hs-CRP levels. Other non-significant differences could be explained by the inclusion of patients with moderately severe periodontal problems and obesity may play as a confounding factor.
There were some limitations of the present study. First inflammatory parameters were analyzed in serum and not in gingival crevicular fluid. Second, our study population was generally moderate periodontitis. Third, our study population consisted of some obese patients.
Within the limits of this prospective study, we concluded that clinical periodontal response to full-mouth SRP and full-mouth disinfection was successful and that TNF-α levels were significantly reduced in all groups and other inflammatory parameters (IL-6, Hs-CRP, and PTX-3) were significantly reduced in only CAPD patients.
References
Brito F, Almeida S, Figueredo CM, Bregman R, Suassuna JH, Fischer RG (2012) Extent and severity of chronic periodontitis in chronic kidney disease patients. J Periodontol Res 47(4):426–430
Coresh J, Selvin E, Stevens LA et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298(17):2038–2047
Süleymanlar G, Utaş C, Arinsoy T et al (2011) A population-based survey of chronic renal disease in Turkey–the CREDIT study. Nephrol Dial Transplant 26(6):1862–1871
Kazancioğlu R (2013) Risk factors for chronic kidney disease: an update. Kidney Int Suppl 3(4):368–371
Rodrigues VP, Libério SA, Lopes FF et al (2014) Periodontal status and serum biomarkers levels in haemodialysis patients. J Clin Periodontol 41(9):862–868
Wang AYM, Lai KN (2008) Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol 19:1643–1652
Cengiz MI, Bal S, Gökçay S, Cengiz K (2007) Does periodontal disease reflect atherosclerosis in continuous ambulatory peritoneal dialysis patients? J Periodontol 78(10):1926–1934
Kocyigit I, Yucel HE, Cakmak O et al (2014) An ignored cause of inflammation in patients undergoing continuous ambulatory peritoneal dialysis: periodontal problems. Int Urol Nephrol 46(10):2021–2028
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J (2017) Impact of the global burden of periodontal disease on health, nutrition, wellbeing of mankind: a call for global action. J Clin Perioodntol 44(5):456–462
American Diabetes Association Position Statements (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 27:5–10
Ziukaite L, Slot DE1, Van der Weijden FA (2017) Prevalence of Diabetes mellitus in people clinically diagnosed with periodontitis: a systematic review and meta-analysis of epidemiologic studies. J Clin Periodontol. https://doi.org/10.1111/jcpe.12839
Preshaw PM, Alba AL, Herrera D et al (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55(1):21–31
Savage A, Eaton KA, Moles DR, Needleman I (2009) A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 36:458–467
Tuttle KR, Bakris GL, Bilous RW et al (2014) Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis 64(4):510–533
Stanton RC (2014) Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis 63(2):3–21
Silness J, Loe H (1966) Periodontal disease in pregnancy. 3. Response to local treatment. Acta Odontol Scand 24:747–759
Loe H, Silness J (1963) Periodontal disease in pregnancy. 1. Prevalence and severity. Acta Odontol Scand 21:533–551
Quirynen M, Bollen CM, Vandekerckhove BN, Dekeyser C, Papaioannou W, Eyssen H (1995) Full- vs. partial-mouth disinfection in the treatment of periodontal infections: Short-term clinical and microbiological observations. J Dent Res 74:1459–1467
Fang F, Wu B, Qu Q et al (2015) The clinical response and systemic effects of non-surgical periodontal therapy in end-stage renal disease patients: a 6-month randomized controlled clinical trial. J Clin Periodontol 42(6):537–546
Renvert S, Persson RE, Persson GR (2011) A history of frequent dental care reduces the risk of tooth loss but not periodontal in older subjects. Sweden Dent J 35(2):69–75
Fang H, Han M, Li QL, Cao CY, Xia R, Zhang ZH (2016) Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: a systematic review and meta-analysis. J Periodontal Res 51(4):417–430
Santuchi CC, Cortelli JR, Cortelli SC, Cota LO, Fonseca DC, Alencar CO, Costa FO (2016) Scaling and root planing per quadrant versus one-stage full-mouth disinfection: assessment of the impact of chronic periodontitis treatment on quality of life—a clinical randomized, controlled trial. J Periodontol 87(2):114–123
Teughels W, Dekeyser C, Van Essche M, Quirynen M (2009) One stage, full mouth disenfection: fiction or reality? J Periodontol 50:39–51
Quirynen M, Bollen CM, Vandekerckhove BN, Dekeyser C, Papaioannou W, Eyssen H (1995) Full vs. partial mouth disinfection in the treatment of periodontal infections: short-term clinical and microbiological observations. J Dent Res 74:1459–1467
Silveira JO, Costa FO, Oliveira PAD (2016) Scaling and root planing per quadrant versus one-stage full-mouth disinfection: assessment of the impact of chronic periodontitis treatment on quality of life—a clinical randomized, controlled trial. J Periodontol 87(2):114–123
Preus HR, Dahlen G, Gjermo P, Baelum V (2015) Microbiologic observations after four treatment strategies among patients with periodontitis maintaining a high standard of oral hygiene: secondary analysis of a randomized controlled clinical trial. J Periodontol 86:8568–8575
Graziani F, Cei S, Orlandi M et al (2015) Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. J Clin Periodontol 42:843–852
Botero JE, Rodríguez C, Agudelo-Suarez AA (2016) Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Aust Dent J 61(2):134–148
Simpson TC, Weldon JC, Worthington HV et al (2015) Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev 6(11):CD004714
Taşdemir Z, Özsarı Taşdemir F, Koçyiğit İ, Yazıcı C, Gürgan CA (2016) The clinical and systemic effects of periodontal treatment in diabetic and non-diabetic obese patients. J Oral Sci 58(4):523–531
Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM (2011) Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med 50(15):1569–1574
Geisinger ML, Michalowicz BS, Hou W (2016) Systemic Inflammatory biomarkers and their association with periodontal and diabetes-related factors in the diabetes and periodontal therapy trial: a randomized controlled trial. J Periodontol 87(8):900–913
Artese HP, Foz AM, Rabelo Mde S (2015) Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PLoS ONE 26(5):e0128344
Tong M, Carrero JJ, Qureshi AR et al (2007) Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2:889–897
Suliman ME, Yilmaz MI, Carrero JJ et al (2008) Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol 3:976–985
Mathew V, Varghese S, Sankari M, Jayakumar ND (2015) Evaluation of pentraxin 3 in chronic periodontitis patients before and after the treatment. Int J Med Exercise Sci 1:9–15
Houssiau FH, Bukasa K, Sindic CJM, Van Damme J, Van Snick J (1988) Elevated levels of tge 26K human hybridoma growth factor(interleukin-6) in cerebrospinal fluid of patients with acute infection of the central nervous system. Clin Exp Immunol 71:320–323
Topley N, Jörres A, Luttmann W et al (1993) Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int 43(1):226–233
Dhingra R, Gona P, Nam BH et al (2007) C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am J Med 120(12):1054–1062
Çalapkorur MU, Alkan BA, Tasdemir Z, Akcali Y, Saatçi E (2017) Association of peripheral arterial disease with periodontal disease: analysis of inflammatory cytokines and an acute phase protein in gingival crevicular fluid and serum. J Periodontal Res 52(3):532–539
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tasdemir, Z., Özsarı Tasdemir, F., Gürgan, C. et al. The effect of periodontal disease treatment in patients with continuous ambulatory peritoneal dialysis. Int Urol Nephrol 50, 1519–1528 (2018). https://doi.org/10.1007/s11255-018-1913-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1913-y