Abstract
Purpose
We investigated the clinical efficacy of the Triple D score (TrD-S) on stone-free rate (SFR) prediction following shockwave lithotripsy (SWL) for renal stones 10–20 mm in diameter and modified the scoring system to improve outcome prediction.
Methods
We retrospectively examined clinical data from the medical records of 226 consecutive patients who underwent SWL for 10–20 mm kidney stones. The TrD-S was calculated according to the cutoffs of < 150 mm3 for stone volume, < 600 Hounsfield unit for stone density, and < 12 cm for skin-to-stone distance on computed tomography. The Quadruple D score was defined as the sum of the TrD-S and stone location (0/1 point for intrarenal stone distribution at lower/non-lower poles, respectively). Complete clearance 3 months after the final SWL was considered the stone-free status.
Results
The residual group (n = 102) had significantly older age, larger stones, higher stone density, higher lower-pole stone incidence, and lower TrD-S than the stone-free group (n = 124). In the multivariate analysis, age, TrD-S, and non-lower-pole stones independently predicted the SFR. The TrD-Ss of 0, 1, 2, and 3 points showed SFRs of 40.0%, 51.9%, 73.0%, and 100.0%, respectively. The Quadruple D scores of 0, 1, 2, 3, and 4 points showed SFRs of 0.0%, 37.9%, 54.5%, 84.4%, and 100.0%, respectively, with better prediction accuracy than the TrD-S (p = 0.01).
Conclusions
The TrD-S is successfully validated for use in Japanese patients with 10–20-mm renal stones. Simple addition of the stone location to the TrD-S could reinforce SFR prediction after SWL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first-line surgical treatment for renal stones has shifted to endourological procedures, such as ureteroscopy (URS) and percutaneous nephrolithotomy (PNL); consequently, extracorporeal shockwave lithotripsy (SWL) has lost its place as a paramount therapeutic modality despite its proven efficacy [1, 2]. However, SWL remains a primary therapy for solitary renal stones sized < 20 mm according to recent guidelines [1, 2].
Several parameters affecting the stone-free rates (SFRs) after SWL have been determined; these include stone size and location [1, 3, 4], SWL-resistant stone composition (calcium oxalate monohydrate, brushite, or cystine) [5, 6], stone attenuation values on computed tomography (CT) [7], skin-to-stone distance (SSD) [8, 9], spatial pelvicalyceal and lower pole anatomy of the kidney [1, 4], patients’ body mass index (BMI) and obesity [10], and shockwave delivery frequency [11]. Although some combined parameters are useful for clinically predicting SWL outcomes [3, 12,13,14,15], no consensus exists as to the best prediction model probably because of the complexity in modelling for clinical practice and/or heterogeneous recommendations in practice guidelines [16, 17]. Inconsistency among guidelines would lead to clinical confusion among urologists in determining treatment modalities for renal stones, especially those 10–20 mm in diameter [16, 17].
Recently, Tran et al. [14] reported a novel and simple nomogram (Triple D scoring system), which constitutes three CT-based parameters [SSD, stone density, and stone volume (SV)] to screen for the most appropriate patients for SWL. Its clinical usefulness has been externally validated in different retrospective studies [18, 19]. These reports described a high area under the curve (AUC) of 0.751–0.845 [14, 18, 19] for the Triple D score (TrD-S) in predicting successful outcomes of SWL therapy for renal stones. However, they included 4–10-mm kidney stones [18, 19]. For ≤ 10-mm kidney stones, SWL generally achieves SFRs of ~ 50–90% [4]. Therefore, the European Association of Urology guideline on urolithiasis recommends SWL as the preferred first-line therapy for all kidney stones smaller than 10 mm, with URS as an alternative for selected cases and PNL reserved for when SWL and URS have failed [1, 4]. In evaluating the clinical relevance of the TrD-S in routine practice to date, no attention has been paid to 10–20-mm renal stones, the sizes of which relate to overlapping indications for SWL and endourological surgery [14, 18, 19].
Herein, we investigated the clinical efficacy of the TrD-S on SFR prediction following SWL for 10–20-mm renal stones and presented a prediction model modified from this score.
Patients and methods
Patient data collection
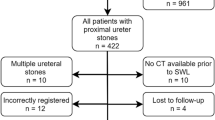
We retrospectively reviewed the medical archives of 2063 consecutive patients who underwent the first SWL session for upper urinary stones at South Miyagi Medical Center (n = 797, from August 1, 2002 to May 31, 2015), Yamagata City Hospital Saiseikan (n = 951, from August 1, 2008 to March 31, 2016), and Nihonkai General Hospital (n = 315, from January 1, 2009 to January 31, 2016). The inclusion criterion was 10–20-mm renal stones (n = 375). The exclusion criteria were as follows: (1) partial staghorn calculi (n = 9), (2) calyceal diverticular stone (n = 1), (3) horse-shoe kidney (n = 3), (4) ureteral stricture (n = 3), (5) bed-ridden status (n = 2), (6) unavailable CT images before SWL (n = 85), (7) endourology prior to SWL (n = 9), (8) incomplete treatment owing to mechanical disorders of shockwave lithotripter during sessions (n = 4), (9) follow-up loss 3 months after the final SWL (n = 27), and (10) no stone status evaluation 3 months after the final SWL owing to inadequate medical follow-up timing (n = 6). Finally, 226 patients were eligible for the present study.
The study protocol was approved by the Ethical Committees of Yamagata University School of Medicine (No. 535; March 2, 2018), South Miyagi Medical Center (No. 30-6; October 3, 2018), Yamagata City Hospital Saiseikan (No. 430-014, September 14, 2018), and Nihonkai General Hospital (No. 5; September 25, 2018).
Preoperative evaluation of renal stones targeted with SWL
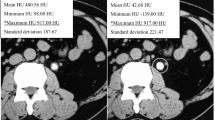
Radiopaque kidney stones were evaluated before SWL using plain abdominal X-ray imaging of the kidney, ureter, and bladder (KUB) and CT. Stone diameters were measured as the maximum longitudinal diameters. SV was calculated using the following formula: SV = π/6 × (anteroposterior × transverse × cranio-caudal diameters) [14, 18, 19]. Stone density was presented in Hounsfield unit (HU), and SSD was calculated as the average distance from the body surface to a targeted stone at 0°, 45°, and 90° on CT [8].
The TrD-S was calculated as the sum of the numbers of components matching the cutoffs of < 150 mm3 for SV, < 600 HU for stone density, and < 12 cm for SSD as described by Tran et al. [14]. We defined the Quadruple D score as the TrD-S combined with the stone location (i.e., distribution). The location was allocated 0 and 1 point if a certain stone was placed at the lower calyces and other sites, respectively. Thus, the Triple and Quadruple D scores could range from 0 (worst) to 3 (best) points [14] and 0 (worst) to 4 (best) points, respectively.
SWL and postoperative stone status evaluation
The lithotripters used were electromagnetic shockwave ones, the Storz Modulith SLX-MX (South Miyagi Medical Center) and Siemens Lithoskop (Yamagata City Hospital Saiseikan and Nihonkai General Hospital). SWL was performed with a gradual ramping up of shockwave energy at a rate of 60, 90, or 120 shocks per minute, according to the therapist’s preference and manufacturers’ instructions. Treatment efficacy was evaluated on KUB X-ray after each SWL session. Postoperative CT and/or intravenous urography were performed when small calcification shadows on KUB X-ray were not definitely determined as stone residuals. Repeated sessions were conducted when a single session was unsuccessful. Stone-free status was defined as complete absence of stone remnants 3 months after the final SWL session.
Statistics
Continuous variables were compared using Student’s t test or Mann–Whitney U-test; their correlations were assessed using Pearson’s correlation analysis. Cross charts between two categories were analyzed using Fisher’s exact test, Chi-square test, or Cochran–Armitage trend test. Variables that may be differential and predictive in the univariate analyses were further investigated in multivariate logistic regression analyses. P-values of < 0.2 in the univariate analysis were set as the threshold for variable entering, and a stepwise regression method was used with the significance level set at 0.05 for exclusion of variables in the multivariate analysis. All p-values were based on two-sided statistical analyses. P-values of < 0.05 were considered statistically significant. All analyses were performed using the R statistical software version 3.4.1 (http://cran.rproject.org/, accessed on July 28, 2017). Two receiver-operating characteristic (ROC) curves were compared using the pROC package version 1.12.1 (https://cran.rproject.org/web/packages/pROC/index.html, accessed on July 10, 2018).
Results
The patient demographics are presented in Table 1. The patients were classified into two groups according to stone status 3 months after the final SWL sessions: stone-free (n = 124) and residual (n = 102) groups. The residual group had significantly older age, larger stones, higher stone density on CT attenuation than the stone-free group. Stone location was significantly different between the groups, with a higher lower-pole stone incidence in the residual group (27.5% vs. 10.5%, p = 0.002, Fisher’s exact test). No differences in the BMI, the number of SWL sessions, total shockwave energy delivered per patient, shockwave frequency, stone composition, and SSD were observed between the groups. There was a moderately positive correlation between the BMI and SSD in the entire cohort (Pearson’s correlation coefficient; r = 0.534, p < 0.001). In the residual group, 19 (18.6%) patients were completely cleared of stone fragments and thus attained the stone-free status. The TrD-S was significantly lower in the residual group than in the stone-free group.
Table 2 shows the results of the multivariate logistic regression model for predicting the stone-free status; age, stone location (non-lower vs. lower-pole stones), TrD-S (0, 1, 2, or 3 points), drainage with ureteral stents (yes vs. no), and number of SWL sessions were initially incorporated to the model because they had p-values of < 0.2 (Table 1). Age, TrD-S, and non-lower-pole stones were independent predictors of the stone-free status in the multivariate analysis, yielding a sufficient AUC of 0.736 [95% confidence interval (CI), 0.670–0.803] in the multivariate logistic regression model.
The TrD-Ss of 0, 1, 2, and 3 points showed SFRs of 40.0%, 51.9%, 73.0%, and 100.0%, respectively (Cochran–Armitage test, p = 0.001; Fig. 1a, left). Conversely, the Quadruple D scores of 0, 1, 2, 3, and 4 points showed SFRs of 0.0%, 37.9%, 54.5%, 84.4%, and 100.0%, respectively (Cochran–Armitage test, p < 0.001; Fig. 1a, right). The AUC for the Quadruple D score was significantly higher than that for the TrD-S (AUC, 0.596 vs. 0.651; 95% CI 0.539–0.654 vs. 0.590–0.712; p = 0.01; Fig. 1b).
a SFRs based upon the TrD-S (left) and Quadruple D score (right). The Quadruple D score was defined as the sum of the TrD-S and intrarenal location of a targeted stone (0 points: lower-pole stone or 1 point: non-lower-pole stone). b ROC curves for the TrD-S and Quadruple D score. AUC area under the curve
Discussion
In this study, we demonstrated that the TrD-S, lower pole location, and age were independent predictors of the SFR after SWL for 10–20-mm renal stones. The SFRs significantly improved as the number of positive components consisting of the Triple and Quadruple D scores increased. These findings support the successful validation of the TrD-S for use in Japanese patients with 10–20-mm renal stones treated with SWL; these also indicate that the Quadruple D score may be more relevant than the TrD-S in clinical decision-making of SWL for medium-sized renal stones.
The ROC curve analysis revealed a low AUC (0.596) of the TrD-S for SFR prediction. This was because the SSD, a component of the TrD-S, was not a significant factor for discriminating stone-free or residual outcomes after SWL. Moderately correlated with the SSD (r = 0.534), the BMI was not different between the groups in the present study. The SSD and BMI, which are clinical indicators of obesity, have been reported as significant predictors of SWL outcomes in univariate analyses [13, 20]. In multivariate analyses, either the SSD or BMI is often excluded from the final models for outcome prediction [8, 13, 20, 21] probably owing to the correlation between them [22]. However, neither the SSD nor BMI was related to the SWL outcomes in the present study. It may be partially because most patients were not obese (BMI, 24.6 ± 3.8 kg/m2), reflecting racial backgrounds discrete from those in previous studies [8, 13, 20, 21]. Generally, the BMI varies among races, and the prevalence of obesity, defined by the World Health Organization as a BMI of ≥ 30 kg/m2, is no more than 2–3% in the Japanese population, in contrast to the 10–20% in Europe and the USA [23]. Based upon the increased incidence of obesity-related morbidities, obesity is specified as a BMI of ≥ 25 kg/m2 in Japan, where the prevalence and degree of obesity remain mild [23]. Moreover, it may be because of the sampling bias resulting from the study design in which the patients with renal stones had similar anthropometric characteristics. In the present study, we investigated patients with 10–20-mm renal stones.
A lower pole location was a significant factor relating to poor SFRs after SWL, consistent with previous reports [1, 2, 4]. A steep infundibular-pelvic angle, long lower-pole calyx (> 10 mm), and narrow infundibulum (< 5 mm) are depicted as unfavorable factors for SWL [1]; however, we did not incorporate these specific conditions in the Quadruple D score, which is the sum of the lower pole location (distribution) and TrD-S, in pursuit of sufficient ease of use in clinical practice. Despite such a simplification, the Quadruple D score significantly improved SFR prediction after SWL for renal stones compared with the TrD-S. Ozgor et al. [19] revealed that the stone location and TrD-S were independent factors affecting SWL success in their multivariate analysis. Larger stone burdens located in lower pole calyces, increasing SSD, and unfavorable lower pole anatomy all decrease the success rates of SWL and URS but have limited influence on PNL outcomes [4]. Thus, for 10–20-mm renal calculi, stone and anatomical factors must be carefully considered when weighing the relative outcomes and invasiveness of each procedure [4].
For renal stones, age is reported as an independent predictor of SWL outcomes in multivariate analyses [13, 24], which is consistent with the present result. In a prospective study [20], the effects of age on SWL outcomes for kidney stones reached a significant marginal level (p = 0.06) in the univariate analysis; it was not confirmed as a significant predictor in the multivariate analysis. Thus, considering that the relationship between age and SWL outcomes remains controversial, age was not considered as an additional component to the TrD-S in the present study. We previously reported that age had no significant effects on the SFR after SWL for ureteral stones [25], which is consistent with other reports [13, 26, 27]. Renal stones planned for surgical treatment are usually larger than ureteral stones [1, 2, 4]. As a general principle, the efficacy of SWL decreases, while the need for ancillary procedures and re-treatment increases as the stone burden enlarges [4]. Interestingly, Ikegaya et al. [28] demonstrated that renal stones were more difficult to be disintegrated with SWL in older patients than in younger patients. The probability of renal hematoma after SWL for kidney stones increased significantly with age, indicating the dose limitation of shockwaves in older patients [29]. Taken together, age might have negative impacts on the SFR (renal stones) owing to resistance to fragmentation rather than stone clearance, unless the kidneys have unfavorable anatomical factors for SWL, such as lower pole configuration.
Many researchers have reported nomograms predicting successful outcomes after SWL for upper urinary stones [3, 12,13,14,15, 24]. Although some nomograms present with excellent outcome prediction accuracy, they are often too complex to calculate in the clinical setting, e.g., because of exponential functions [12, 13, 24]. Based on stone length, location, and number, Kanao et al. [3] reported a simple prediction nomogram of the SFR after a single SWL session; the SFRs were ~ 56.8% (11–15 mm) and 35.1% (16–20 mm) for calyceal stones and 64.4% (11–15 mm) and 42.7% (16–20 mm) for renal pelvic stones. However, this nomogram [3] does not include CT attenuation and the SSD already proven to affect SWL outcomes [1, 2]. Recently, Kim et al. [15] constructed nomograms to predict the SFR after SWL, which are characterized by manual scoring of four or six clinical variables on graphical charts in a CT-independent or -dependent manner. Besides the four variables, sex; stone location, number, and maximal diameter; hydronephrosis grade; and stone CT attenuation are included in the CT-dependent nomogram. Their nomograms and the TrD-S seem to be very practical and easy to use in clinical practice and remain to be externally validated.
There are limitations in the present study. The lower-pole stone morphology and hydronephrosis grade were not assessed [15]. Other limitations include the retrospective design of the study, relatively small number of patients, and lack of a validation dataset. It is unclear whether the Quadruple D score could be extrapolated to ureteral stones. Further studies are needed to confirm the validity of the present findings.
In conclusion, the TrD-S was successfully validated for use in Japanese patients treated with SWL for 10–20-mm renal stones, showing a parallel increase in the SFR with the number of positive components consisting of the TrD-S. Simple addition of the stone location (lower-pole or non-lower-pole stones) to the TrD-S could reinforce SFR prediction after SWL, without losing its simplicity and ease of use for urologists.
References
Turk C, Petrik A, Sarica K et al (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69(3):475–482
Assimos D, Krambeck A, Miller NL et al (2016) Surgical management of stones: American Urological Association/Endourological Society guideline, PART II. J Urol 196(4):1161–1169
Kanao K, Nakashima J, Nakagawa K et al (2006) Preoperative nomograms for predicting stone-free rate after extracorporeal shock wave lithotripsy. J Urol 176(4 Pt 1):1453–1456
Leavitt D, de la Rosette J, Hoenig D (2016) Strategies for nonmedical management of upper urinary tract calculi. In: Wein A, Kavoussi L, Partin A, Peters C (eds) Campbell-Walsh’s urology. Elsevier, Philadelphia, pp 1235–1259
Dretler SP (1988) Stone fragility—a new therapeutic distinction. J Urol 139(5):1124–1127
Ringdén I, Tiselius HG (2007) Composition and clinically determined hardness of urinary tract stones. Scand J Urol Nephrol 41(4):316–323
Pareek G, Armenakas NA, Fracchia JA (2003) Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J Urol 169(5):1679–1681
Pareek G, Hedican SP, Lee FT Jr, Nakada SY (2005) Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 66(5):941–944
Ng CF, Siu DY, Wong A, Goggins W, Chan ES, Wong KT (2009) Development of a scoring system from noncontrast computerized tomography measurements to improve the selection of upper ureteral stone for extracorporeal shock wave lithotripsy. J Urol 181(3):1151–1157
Pareek G, Armenakas NA, Panagopoulos G, Bruno JJ, Fracchia JA (2005) Extracorporeal shock wave lithotripsy success based on body mass index and Hounsfield units. Urology 65(1):33–36
Madbouly K, El-Tiraifi AM, Seida M, El-Faqih SR, Atassi R, Talic RF (2005) Slow versus fast shock wave lithotripsy rate for urolithiasis: a prospective randomized study. J Urol 173(1):127–130
Vakalopoulos I (2009) Development of a mathematical model to predict extracorporeal shockwave lithotripsy outcome. J Endourol 23(6):891–897
Wiesenthal JD, Ghiculete D, Ray AA, Honey RJ, Pace KT (2011) A clinical nomogram to predict the successful shock wave lithotripsy of renal and ureteral calculi. J Urol 186(2):556–562
Tran TY, McGillen K, Cone EB, Pareek G (2015) Triple D Score is a reportable predictor of shockwave lithotripsy stone-free rates. J Endourol 29(2):226–230
Kim JK, Ha SB, Jeon CH et al (2016) Clinical nomograms to predict stone-free rates after shock-wave lithotripsy: development and internal-validation. PLoS ONE 11(2):e0149333
Pradere B, Doizi S, Proietti S, Brachlow J, Traxer O (2018) Evaluation of guidelines for surgical management of urolithiasis. J Urol 199(5):1267–1271
Zumstein V, Betschart P, Abt D, Schmid HP, Panje CM, Putora PM (2018) Surgical management of urolithiasis—a systematic analysis of available guidelines. BMC Urol 18(1):25
Gokce MI, Esen B, Gulpinar B, Suer E, Gulpinar O (2016) External validation of Triple D Score in an elderly (>/=65 years) population for prediction of success following shockwave lithotripsy. J Endourol 30(9):1009–1016
Ozgor F, Tosun M, Kayali Y, Savun M, Binbay M, Tepeler A (2017) External validation and evaluation of reliability and validity of the Triple D Score to predict stone-free status after extracorporeal shockwave lithotripsy. J Endourol 31(2):169–173
El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ (2007) A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. Eur Urol 51(6):1688–1693
Perks AE, Schuler TD, Lee J et al (2008) Stone attenuation and skin-to-stone distance on computed tomography predicts for stone fragmentation by shock wave lithotripsy. Urology 72(4):765–769
Allard CB, Shuster A, Pinthus JH et al (2012) Obesometric factors associated with increased skin-to-stone distances in renal stone patients. Can J Urol 19(6):6554–6559
Examination Committee of Criteria for ’Obesity Disease’ in Japan, Japan Society for the Study of Obesity (2002) New criteria for ‘obesity disease’ in Japan. Circ J 66(11):987–992
Abdel-Khalek M, Sheir KZ, Mokhtar AA, Eraky I, Kenawy M, Bazeed M (2004) Prediction of success rate after extracorporeal shock-wave lithotripsy of renal stones—a multivariate analysis model. Scand J Urol Nephrol 38(2):161–167
Ichiyanagi O, Nagaoka A, Izumi T, Kawamura Y, Kato T (2015) Age-related delay in urinary stone clearance in elderly patients with solitary proximal ureteral calculi treated by extracorporeal shock wave lithotripsy. Urolithiasis 43(5):419–426
Halachmi S, Meretyk S (2006) Shock wave lithotripsy for ureteral stones in elderly male patients. Aging Male 9(3):171–174
Abdel-Khalek M, Sheir K, Elsobky E, Showkey S, Kenawy M (2003) Prognostic factors for extracorporeal shock-wave lithotripsy of ureteric stones—a multivariate analysis study. Scand J Urol Nephrol 37(5):413–418
Ikegaya H, Kato A, Kumano S, Tominaga T (2005) Correlation between age and the efficacy of ESWL. BJU Int 96(7):1145
Dhar NB, Thornton J, Karafa MT, Streem SB (2004) A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol 172(6 Pt 1):2271–2274
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for the English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required, because the present study was retrospective and the anonymity of the participants was ensured.
Informed consent
For this type of study formal consent is not required, because the present study was retrospective and the anonymity of the participants was ensured.
Rights and permissions
About this article
Cite this article
Ichiyanagi, O., Fukuhara, H., Kurokawa, M. et al. Reinforcement of the Triple D score with simple addition of the intrarenal location for the prediction of the stone-free rate after shockwave lithotripsy for renal stones 10–20 mm in diameter. Int Urol Nephrol 51, 239–245 (2019). https://doi.org/10.1007/s11255-018-02066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-02066-1