Abstract
Computed tomography (CT) attenuation value of ureteral stones is one of the predictors of shockwave lithotripsy (SWL) outcome. It is common to use the mean Hounsfield units (HU) to describe the CT attenuation value. However, an observer bias can occur when measuring the mean HU in the conventional method. On the other hand, our way to obtain only the maximum HU is simpler and less biased. We retrospectively evaluated 464 patients with ureteral stones who underwent SWL and compared predictive accuracy of various factors including maximum and mean HU. Results were determined after a single SWL. Predictors of SWL success were examined by the statistical analysis of successful and failed groups. 324 of the 464 patients who underwent SWL were stone-free after a single SWL. Significant differences were found in factors related to CT attenuation value and stone size. As a result of receiver operating characteristic analysis, it was found that maximum HU and mean HU, major diameter and volume have equivalent prediction accuracy, respectively. Multivariate analysis revealed that maximum HU and major diameter were included in independent predictors. We also examined the new original indicators using maximum HU and major diameter. Stone-resistant probability obtained from the logistic model and Maximum HU and Major diameter Index obtained by multiplying maximum HU by major diameter were useful for predicting SWL success, respectively. In conclusion, maximum HU and mean HU have equivalent predictive accuracy, and maximum HU is easier to measure and less biased than mean HU.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shockwave lithotripsy (SWL) is a safe and effective treatment method for ureteral stones. Predicting the outcomes of patients after SWL treatment will reduce the physical and economic burden of patients. Various factors have been reported as predictors of SWL success [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Stone size, stone number, stone location, non-contrast computed tomography (CT) attenuation value of stones measured by Hounsfield unit (HU) density [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15], skin-to-stone distance (SSD) [1,2,3,4], and body mass index (BMI) [5, 6] have all been reported as indicators to predict successful SWL.
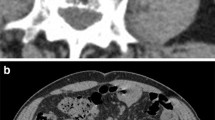
Among these predictors, the CT attenuation value has been favorably evaluated in various studies. When measuring the CT attenuation value of a stone, the region of interest (ROI) is often set inside the stone so as not to include the soft tissue surrounding the stone, as shown in Fig. 1a [1, 4, 6,7,8,9,10]. Using this method, the maximum value (maximum HU), minimum value (minimum HU), mean value (mean HU), and standard deviation (SD) of CT attenuation values within the ROI are calculated. Most studies have used the mean HU to describe the CT attenuation value of stones [1,2,3, 5,6,7,8,9,10,11,12,13,14,15,16]. However, this method of ROI determination can cause observer bias. For example, the mean HU depends on how far the observer sets the ROI close to the stone’s edge. In addition, this method has a drawback that it takes time to measure.
Setting ROI of ureteral stones on axial non-contrast CT images. a Set the maximum ROI inside the stone so as not to include the soft tissue around the stone. b Set the ROI including the soft tissue around the stone to include the whole stone. *The same maximum HU can be obtained by the method described in a, b
To improve those points, we focused on maximum HU in this study. The correlation between the maximum HU and the operative time of flexible ureteroscopy with lithotripsy for renal stones has already been reported [17], but there are no reports about comparing maximum HU and mean HU as a predictor of the SWL outcome. To obtain only maximum HU, the ROI had better be simply set to include the whole stone, as shown in Fig. 1b. The same maximum HU can be obtained by the method described in Fig. 1a, b because the maximum HU generally represents CT attenuation values at the center of the stone. Using this new measuring method, it is possible to reduce the observer bias more than using the conventional method. When prediction accuracy is similar among factors, it is practical to choose a factor that is easy to measure and reproduce.
In this study, we hypothesized that identifying maximum HU by our simple and quick method would predict the success of SWL with the same accuracy as mean HU. We reviewed the conventional factors known to predict the SWL success and compared the prediction accuracy of maximum and mean HU. Based on the results of this study, we propose new predictors of SWL outcome.
Patients and methods
Of the total number of ureteral stone cases in which SWL was performed between January 2015 and December 2016 at Yokkaichi Hazu Medical Hospital, 464 cases that did not meet exclusion criteria were evaluated. Exclusion criteria included cases in which the major diameter of the stone was less than 5 mm, cases in which non-contrast CT was not obtained before the first SWL session, cases in which SWL was performed on a residual calculus after transurethral lithotripsy (TUL), and cases in which outcome after treatment was unknown.
Non-contrast CT images were preoperatively obtained with the Toshiba Aquilion ONETM CT scanner (Toshiba Medical Systems, Otawara-shi, Japan). Mean value of total effective tube current time product was 4633.81 ± 878.70 mAs (range 1056–7126 mAs) with a fixed tube voltage of 120 kV. The window width and level were 290 and 45 HU, respectively. The CT dose output was controlled for individual patients through automatic exposure regulation and tube current modulation. CT dose index volume (CTDIvol) and dose-length product (DLP) values were obtained from 262 of the 464 patients as a measure of radiation dose output. Effective dose (ED) was calculated from the DLP by multiplying it by the conversion coefficient (k = 0.015 mSv/mGy cm).
The image reference application was EV Insite ver. 2.10.7.9 of PSP Corporation.
All patients underwent SWL using MODULITH SLX TM (Stortz Medical; Tägerwilen, Switzerland) under analgesia. Shockwave power was given up to 18 kV. Frequency of generating shockwaves was set at a rate of 60–120 shots per minute, and maximum number of shockwaves was limited to 4000 shots. Each patient’s surgical position (supine position or prone position) was determined by stone location. That is, upper ureteral stones were treated in the prone position as much as possible. This is because the treatment result of upper ureteral stones at our hospital was significantly better in the prone position. All middle ureteral stones were treated in the prone position and all lower ureteral stones were treated in the supine position.
Various factors were evaluated on non-contrast CT images prior to the first SWL session. Factors related to CT attenuation value were measured by a single radiologist using the method shown in Fig. 1a. Stone size was evaluated by measuring major diameter (i) and minor diameter (ii) on axial images and longitudinal diameter (iii) on coronal images. Stone volume was defined as (i × ii × iii)/2. SSD was defined as the distance from the center of the stone to skin at 90° measured on an axial image. SSD was measured from the dorsal or ventral side based on treatment position.
Additional SWLs were performed when residual stones remained after the first SWL. The shortest interval between treatments was 1 week (mean 2–3 weeks). SWLs were repeatedly performed until the patient became stone-free on non-contrast CT or KUB images, or until more treatments were judged ineffective.
The usefulness of each predictor was statistically verified. We defined patients who achieved stone-free status on non-contrast CT or KUB images with a single SWL as the “Successful” group and patients who required multiple SWLs as the “Failed” group. Cases that transitioned to TUL after first SWL were classified as Failed. Results were analyzed using Student’s t test, Mann Whitney’s U test, the Chi-square test, and Fisher’s exact test. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to compare the predictive accuracy of each factor. Multivariate analysis was performed using a logistic regression model and new indicators were devised based on the result of the multivariate analysis. Validities of the results were verified using ROC analysis and Spearman’s rank correlation coefficient. All tests considered P < 0.05 as significant difference. Statistical analyses were performed with R Statistical Language version 3.0.2.
Results
Patients’ characteristics are shown in Table 1. A total of 324 (69.8%) and 140 (30.2%) patients were classified as Successful and Failed, respectively. In comparing the Successful and Failed groups, significant differences were found in factors related to stone size and CT attenuation value. Among these factors demonstrating significant differences in the univariate analysis (Table 1), factors relating to CT attenuation value, such as maximum HU, mean HU, and SD were strongly correlated with one another. Similarly, factors relating to stone size, such as major diameter, minor diameter, and volume are strongly correlated with one another. ROC analyses were performed to identify which of these factors should be selected as an explanatory variable for multivariate analysis.
Figure 2a shows ROC curves of factors related to CT attenuation value (maximum HU, mean HU, and SD). The ROC curves, AUCs, and 95% confidence intervals (95% CI) of these three factors were almost identical (maximum HU: AUC 0.691, 95% CI 0.64–0.742, mean HU: AUC 0.682, 95% CI 0.632–0.733, SD: AUC 0.689, 95% CI 0.637–0.741). Similarly, three factors related to stone size (major diameter, minor diameter, and volume) also had similar prediction accuracy (Fig. 2b) (major diameter: AUC 0.671, 95% CI 0.618–0.724, minor diameter: AUC 0.642, 95% CI 0.588–0.695, volume: AUC 0.668, 95%CI 0.615–0.720). Based on this result, maximum HU was selected as the CT attenuation value-related factor and major diameter was selected as the size-related factor in the subsequent multivariate analysis.
Table 2 shows the results of the logistic regression analysis of predicting successful SWL. Model 1 selected clinically useful factors based on the results of the univariate analysis. The variables of Model 2 were obtained by applying stepwise backward selection to Model 1. In Model 2, four factors, including stone location/treatment position, major diameter, maximum HU, and SSD, were significant. However, appropriate treatment position varies depending on the SWL device. To suggest as more universal indicators that do not depend on the device, we verified a model limited to two factors, major diameter and maximum HU (Model 3).
Using the formula obtained from the logistic model in Table 2, it was possible to directly calculate the probability of failure with a single SWL. We defined this probability as the stone resistance probability (SRP). The probability obtained from four factors in Model 2 (stone location/treatment position, major diameter, maximum HU, and SSD) was defined as SRP4, and the probability obtained from two factors (major diameter and maximum HU) in Model 3 was defined as SRP 2. SRP4 and SRP2 were calculated with the following formulas:
We next calculated “Maximum CT attenuation values and Major diameter Index (MMI)” as a simpler index. MMI is a new index calculated by the following formula:
MMI cannot directly represent the probability of failure with a single SWL such as SRP, but it can bring important information for the difficulty of SWL with a simple and quantitative way. Figure 3 shows five ROC curves, including SRP 4, SRP 2, MMI, maximum HU, and major diameter. Comparing AUCs of these factors, the prediction accuracy of MMI was inferior to that of SRP4, but it was comparable to SRP2. Furthermore, MMI was slightly superior to each of maximum HU and major diameter.
In comparison of more than three groups, corrections of multiple testing were done.
We next calculated Spearman’s rank correlation coefficient and compared the correlation between each predictor and the number of SWL sessions required to become stone-free. The results were SRP4: 0.407, SRP2: 0.326, MMI: 0.327, maximum HU: 0.303 and major diameter: 0.278. These results were consistent with the ROC curves.
The new 110 cases were set to validate those results. The AUCs in the validation cases were SRP4: 0.725, SRP2: 0.656, MMI: 0.675, maximum HU: 0.621 and major diameter: 0.651. The validation cases demonstrated the same trend as the test cases, suggesting that SRP and MMI may be useful new predictors of successful SWL.
Regarding parameters according the radiation dose, the mean CTDIvol was 33.01 ± 7.17 mGy (range 6.60–72.2 mGy). On average, DLP amounted to 1669.27 ± 416.87 mGy cm (range 308.8–2809.6 mGy cm). By applying conversion coefficient of k = 0.015 mSv/mGy cm, this resulted in an overall mean ED of 25.04 ± 6.25 mSv.
Discussion
There are many studies on factors predicting the efficacy of SWL [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. In this study, we found that a simple measured value, maximum HU of the ureteral stone on non-contrast CT, can be used to accurately predict SWL success. In addition, we identified two new indicators, SRP and MMI as reliable predictors of treatment success.
Non-contrast CT is an essential diagnostic tool for urolithiasis. The relationship between CT attenuation value and successful SWL has been reported for years, but most studies have evaluated mean HU, with a cutoff value of 1000HU [5, 11,12,13].
There are various opinions on how to set ROI within a stone. Several reports have used ellipses or free-hand curves so as not to include soft tissue surrounding the stone [1, 4, 6,7,8,9,10]. Other reports have drawn three small ROI within a single stone and calculated the average [2, 14].
It is conventional to measure on a maximum axial image, and it is also possible to measure using three slices [3, 5]. However, how to determine the ROI was not unified among urologists or radiologists, errors have been caused by the difference in method to measure. Furthermore, since mean HU varies depending on how far from the stone edge to the ROI, different values will be obtained even when a single urologist measures the same stone twice. On the other hand, maximum HU can be reliably obtained the same value regardless of how it is measured. Nevertheless, few studies use maximum HU [4, 9, 15, 17] as a predictor of SWL outcome. This may be because the maximum HU may not be representative of stone heterogeneity. However, it remains unclear if there is any value in expressing stone heterogeneity when predicting SWL success.
Heterogeneous stones may have some “weakness”, which can be identified by low CT attenuation values, making easier to break. Some reports have utilized SD [7] or variation coefficient of CT attenuation values [8] in the ROI to evaluate stone heterogeneity. SD is an indicator of numerical variation. At first glance it seems that there are many weak points with low HU within the stone if the SD is large. However, we found that the larger the SD was, the more SWL sessions were required. In our study, the minimum HU was approximately 150 HU with very little variation among stones. Mean HU (r = 0.943, p < 0.01) and SD (r = 0.967, p < 0.01) were strongly correlated with maximum HU. Hence, we concluded that SD does not accurately reflect stone heterogeneity and its clinical significance is unclear. Although the coefficient of variation was also verified, our data suggests that does not have a prediction accuracy above maximum HU.
In 1988, Baron et al. reported the usefulness of CT pattern as a predictor of success in crushing gallstones with shock waves in vitro [18]. In their study, they listed CT attenuation values of the cross section of stones, graphed them, classified the waveforms into six patterns, and examined the relationship between the waveform patterns and the crushing effects. The author found a significant difference in ease of crushability depending on the waveform patterns. Prior to this study, we considered whether the waveform patterns could be used to evaluate the structure and crushability of ureteral stones and verified in our case. To evaluate the distribution of CT attenuation values in the cross section of a stone, we drew multiple profile curves in a lattice pattern on the stone. We tried to visually search for “weak points” with a low CT value by looking at the waveform of the profile curve. We found no correlations between the number of waveform differences and the number of weak points. Gallstones, especially those that contain a cholesterol component, have dynamic numerical changes in HU. In contrast, urinary stones have a relatively uniform internal structure. Hence the distribution of CT attenuation values does not always correlate with easy crushability and does not appear to be clinically important. Based on the above results, we ignored stone heterogeneity and focused on maximum HU to devise a more versatile and efficient index.
Measuring maximum HU (Fig. 1b) is easier than measuring mean HU (Fig. 1a). Our method is even more efficient when evaluating multiple slices of a thick stone. This series of tasks (measuring the size of the stone and setting the ROI) can be done conveniently in front of patients and provides essential information for evaluating treatment options. Since we have a high volume of urolithiasis patients, this level of accuracy and efficiency is essential for evaluating calculus images before treatment.
In this study, it became clear that CT attenuation value and size are predictors of treatment success. When evaluating stone size, many studies use major diameter as a predictor [3, 7, 14, 15]. We found that major diameter had the same degree of accuracy as volume. This is because minor diameter, which often coincides with ureteral diameter, does not vary significantly from patient to patient, hence the volume of a ureteral stone strongly depends on the major rather than the minor diameter. Therefore, it is only necessary to measure the major diameter without measuring minor diameter or calculating volume. However, in kidney stones, the minor diameter appears to be more important, and volume may be a better predictor for SWL outcome than major diameter.
SRP using a logistic regression model is easy to understand because it can directly calculate the probability of treatment failure, but it is based on a complicated equation. MMI is a simpler alternative that is calculated by multiplying the maximum CT attenuation value (HU) with the major diameter (mm). The cutoff value of MMI should be set for each facility. We identified a cutoff value of 12,000, at which the positive predictive value was 50% (sensitivity 0.41, specificity 0.83). That is, if MMI is 12,000 or more, two or more sessions of SWL will be required with a probability of 50% or more.
This study has several limitations. First, this was a retrospective study at a single institution. Larger prospective studies will determine whether similar results can be obtained in other facilities using different devices. As an additional application, our technique might be used to predict outcome of SWL for renal stones.
Conclusions
We examined predictors of successful SWL for ureteral stones and concluded that CT attenuation value and major diameter are useful for predicting treatment effect. Previously, mean HU was used to predict successful SWL, but we found that maximum HU is a better predictor than mean HU. We also examined novel indicators such as SRP and MMI, suggesting their usefulness.
References
Tran TY, McGillen K, Cone EB, Pareek G (2015) Triple D score is a reportable predictor of shockwave lithotripsy stone-free rates. J Endourol 29:226–230
Perks AE, Schuler TD, Lee J et al (2008) Stone attenuation and skin-to-stone distance on computer tomography predicts for stone fragmentation by shock wave lithotripsy. Urology 72(4):765–769
Badran YA, Abdelaziz AS, Shehab MA et al (2016) Is scoring system of computed tomography based metric parameters can accurately predicts shock wave lithotripsy stone-free rates and aid in the development of treatment strategies? Urol Ann 8:197–202
Niwa N, Matsumoto K, Miyahara M et al (2017) Simple and practical nomograms for predicting the stone-free rate after shock wave lithotripsy in patients with a solitary upper ureteral stone. World J Urol 35(9):1455–1461
Massoud AM, Abdelbary AM, Al-Dessoukey AA et al (2014) The success of extracorporeal shock-wave lithotripsy based on the stone-attenuation value from non-contrast computed tomography. AJU 12:155–161
Yazici O, Tuncer M, Sahin C et al (2015) Shock wave lithotripsy in ureteral stones: evaluation of patient and stone related predictive factors. Int Braz J Urol 41(4):676–682
Lee JY, Kim JH, Kang DH et al (2016) Stone heterogeneity index as the standard deviation of Hounsfield units. A novel predictor for shock-wave lithotripsy outcomes in ureter calculi. Sci Rep. 6:23988. https://doi.org/10.1038/srep23988
Yamashita S, Kohjimoto Y, Iguchi T et al (2017) Variation coefficient of stone density: a novel predictor of extracorporeal shockwave lithotripsy. J Endourol 31(4):384–390
Tanaka M, Yokota E, Toyonaga Y et al (2013) Stone attenuation value and cross-sectional area on computed tomography predict the success of shock wave lithotripsy. KJU 54:454–459
Mullhaupt G, Engeler DS, Schmid HP, Abt D (2015) How do stone attenuation and skin-to-stone distance in computed tomography influence the performance of shock wave lithotripsy in ureteral stone disease? BMC Urol 15:72
Ouzaid I, Al-qahtani S, Dominique S et al (2012) A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int 110:E438–E442
Niewada EB, Dybowski B, Radziszewski P (2014) Predicting stone composition before treatment -can it really drive clinical decisions? Cent Euro J Urol 67(4):392–396
Khalifa BB, Naouar S, Gazzah W, Salem B, Kamel RE (2016) Predictive factors of extracorporeal shock wave lithotripsy success for urinary stones. Latunisie Medicale 94:397–400
Geng JH, Tu HP, Shih PMC et al (2015) Noncontrast computed tomography can predict the outcome of shockwave lithotripsy via accurate stone measurement and abdominal fat distribuition determination. KJMS 31:34–41
Bandi G, Meiners RJ, Pickhardt PJ et al (2008) Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int 103:524–528
Gupta NP, Ansari MS, Kesarvani P, Kapoor A, Mukhopadhyay S (2005) Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int 95:1285–1288
Kuroda S, Ito H, Sakamaki K et al (2018) A new prediction model for operative time of flexible ureteroscopy with lithotripsy for the treatment of renal stones. PLoS One 13(2):e0192597
Baron RL, Rohmann CA, Lee SP, Shuman WP, Teefey SA (1988) CT evaluation of gallstones in vitro. Correlation with chemical analysis. AJR 151:1123–1128
Acknowledgements
The authors would like to thank Dr. Tomomi Yamada and appreciate her support for the statistical analyses of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugino, Y., Kato, T., Furuya, S. et al. The usefulness of the maximum Hounsfield units (HU) in predicting the shockwave lithotripsy outcome for ureteral stones and the proposal of novel indicators using the maximum HU. Urolithiasis 48, 85–91 (2020). https://doi.org/10.1007/s00240-019-01123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-019-01123-3