Abstract
Aim
To obtain more insight into molecular mechanisms underlying oxidative stress in Balkan endemic nephropathy (BEN), biomarkers of oxidative stress and antioxidant enzyme activities were studied in 38 pre-dialysis BEN patients, 21 healthy BEN family members and 36 healthy subjects from non-endemic areas.
Methods
Protein thiol groups (P-SH), antioxidant enzyme activities [superoxide dismutase (SOD) and glutathione peroxidase (GPX)], were determined in plasma spectrophotometrically, while malondialdehyde adducts (MDA) by enzyme immunoassay.
Results
BEN patients had significantly lower plasma GPX activity in comparison with values for both control groups (p = 0.016), gradually decreasing with kidney function impairment estimated by glomerular filtration rate (r = 0.53, p = 0.002). GPX activity was inversely correlated with serum urea (r = −0.627, p < 0.001), creatinine (r = −0.53, p < 0.05), urinary excretion of protein and α1-microglobulin (r = −0.44, p = 0.012; r = −0.50, p < 0.007). Significant upregulation of SOD activity was observed in healthy BEN family members (p < 0.05). While the concentration of MDA adducts was similar in all three groups, BEN patients and healthy BEN family members exhibited increased protein damage, based on fewer P-SH groups in comparison with subjects from non-BEN areas (p = 0.085; p = 0.014, respectively).
Conclusions
Based on our results on increased oxidative protein damage in both pre-dialysis BEN patients and healthy BEN family members, it can be speculated that individuals from BEN areas, in general, are chronically exposed to some prooxidant environmental compounds. Moreover, decrease in plasma GPX activity, as a consequence of impaired kidney function, could further affect oxidative status in BEN patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 60 years ago, Balkan endemic nephropathy (BEN) was recognized as a familial chronic tubulointerstitial disease in certain Balkan rural areas [1, 2]. Previous studies clearly implied that the etiology of BEN is multifactorial, involving combined effects of genetic factors and exposure to environmental toxic and carcinogenic compounds [3–5]. So far, among various environmental factors examined, molecular epidemiological investigations have provided evidence for a causative association between chronic aristolochic acid intoxication and development of upper urinary tract tumors in BEN patients [6]. Nevertheless, two other hypotheses on BEN etiology are still attracting attention and have neither been proved nor denied: the possible etiologic role of the mycotoxin, ochratoxin A (OTA) and Pliocene lignite [4, 7, 8].
Oxidative stress represents one of the underlying mechanisms in the pathogenesis and progression of chronic kidney disease (CKD), due to both increased production of reactive oxygen species (ROS) and downregulation of antioxidant enzymes [9–11]. Moreover, hemodialysis per se further worsens the disturbed redox balance in end-stage renal disease, by inducing inflammation and ROS production [12, 13]. Although the role of oxidative stress in CKD has been widely investigated [9–15], data on oxidative stress parameters in BEN are still scarce [16–18]. Namely, only one study showed decreased activity of erythrocyte glutathione peroxidase (GPX1) in BEN patients [16]. Interestingly, recent findings suggested that oxidative stress parameters were significantly higher in healthy residents of BEN villages than in controls from non-BEN regions [18]. However, there are no data on oxidative stress parameters in pre-dialyzed BEN patients. Since it has been shown that all potential BEN environmental risk factors might cause increased ROS production, it is important to evaluate biomarkers of oxidative stress in the course of BEN progression. Thus, among OTA metabolites, OTA hydroquinone exerts strong pro-oxidant activity and after oxidation generates superoxide and the quinone electrophile. Increased ROS production and consequently, greater oxidative DNA damage were observed after OTA treatment of a human renal proximal tubular epithelial cell line (HK-2) [19]. A recent study in vitro also showed that aristolochic acid can cause oxidative stress-related DNA damage and apoptosis through GSH depletion [20], while ROS-mediated toxicity of some organophosphate insecticides and metals [21, 22] has also been suggested.
Having all that in mind, we conducted a study involving pre-dialysis BEN patients in different stages of the disease, healthy members of BEN families and control subjects living in non-endemic areas. In order to compare the antioxidant capacity and oxidative status of these groups, we determined plasma activities of two key antioxidant enzymes, glutathione peroxidase (GPX) and superoxide dismutase (SOD), together with two biomarkers of oxidative damage, malondialdehyde adducts (MDA) and protein thiol groups (P-SH).
Methods
Study subjects
The study included 38 patients with BEN, 21 healthy members of BEN families and 36 healthy persons living in non-endemic areas. BEN was diagnosed using the following criteria: (1) residents of endemic foci; (2) positive family history of BEN; (3) increased urinary excretion of low molecular weight (LMW) proteins; (4) mild proteinuria; (5) impaired renal function; (6) anemia; and (7) symmetrically reduced kidneys [23, 24]. Diagnosis of BEN was made in patients who, in addition to the first two criteria, had either LMW proteinuria or proteinuria and at least one of the remaining criteria but after exclusion of other kidney diseases. Increased low molecular weight proteinuria was defined as urinary excretion of α1-microglobulin (α1-MG) above 1.5 mg/mmol creatinine [23]. Mild proteinuria was defined as proteinuria between 20 and 50 mg/mmol creatinine. Impaired renal function was considered as a glomerular filtration rate (eGFR), estimated by the MDRD formula [25], less than 60 mL/min/1.73 m2 [26]. Anemia was defined as a hemoglobin concentration less than 130 g/L in men and postmenopausal women and less than 120 g/L in women before menopause [27]. Reduced kidney length was considered if the craniocaudal length of each kidney measured by sonography was less than 10 cm.
Healthy members from BEN families were persons selected during previous screening studies. All healthy persons had a negative medical history for kidney disease and hypertension and took no drug, and no pathological finding was detected by objective, laboratory and ultrasound examination at the time of entering the study. Healthy controls living in non-endemic areas had previously been treated for kidney stone in the Department of Urology and Nephrology, Clinical Center of Serbia. At the control outpatient examination, performed at the time of inclusion in the study, no sign of kidney and urinary tract disease was found.
The study was approved by the Ethical Committee of the Faculty of Medicine, Foča. Informed consent was obtained from all examined persons.
Biochemical analysis
Serum levels of creatinine, urea, glucose, cholesterol and triglycerides were measured using routine laboratory techniques. Urine protein was determined colorimetrically with pyrogallol red and urine α1-MG by an immunonephelometric assay (BN II nephelometer, Dade Behring). Superoxide dismutase (SOD) activity in plasma was measured by the method of Misra and Fridovich [28], based on the ability of SOD to inhibit auto-oxidation of epinephrine at an alkaline pH (pH 10.2). Plasma glutathione peroxidase (GPX) activity was assayed according to Gunzler et al. [29] with organic hydroperoxide as the substrate. Plasma protein thiol groups (P-SH) were determined using the method of Jocelyn [30] and expressed as mmol/g of protein. Plasma MDA adducts were measured by ELISA using the Oxiselect MDA adduct ELISA kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s protocol. MDA was expressed as pmol/mg of protein.
Statistical analysis
All analyses were performed using the SPSS statistical software package (version 10; SPSS. IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp). Data are presented as frequency and as mean and standard deviation, as appropriate. Comparison of the variables among the three groups was made with one-way analysis of variance (ANOVA) and the Kruskal–Wallis test, as appropriate. Differences between individual groups were evaluated by Fisher’s least significant difference (LSD) post hoc tests. The statistical significance of differences between group frequencies was determined using the Chi-square test. Correlation between variables was examined using Pearson’s correlation coefficient. A probability value of p < 0.05 was considered significant.
Results
Baseline characteristics of the BEN patients and controls from BEN and non-BEN areas are presented in Table 1. Patients with BEN were significantly older and had significantly lower eGFR in comparison with the control groups. As expected, all biochemical parameters of renal function in BEN patients differed significantly from those for the controls, including increased urinary excretion of α1-MG and proteins.
Table 2 presents activities of the key antioxidant enzymes, glutathione peroxidase (GPX) and superoxide dismutase (SOD), together with two biomarkers of oxidative damage in the three groups examined. Patients with BEN had significantly lower plasma GPX activity than healthy persons from both BEN (p = 0.008) and non-BEN (p = 0.031) areas. On the other hand, no significant difference was found for SOD activity between BEN patients and control subjects. However, members of BEN families had significantly higher plasma SOD activity than individuals living in non-BEN areas (p = 0.012).
It is important to note that MDA, a commonly used biomarker of lipid oxidative damage, exists both free and bound to proteins, nucleic acids and lipoproteins, which are designated as MDA adducts. Decreased P-SH content is a useful biomarker of oxidative damage of proteins, since sulfhydryl groups of plasma proteins have been suggested to be a ‘‘sacrificial’’ antioxidant in plasma and extravascular spaces. Our results showed that the mean plasma level of MDA adducts was similar in all three groups examined. However, the lower level of P-SH groups in BEN patients and controls from BEN areas in comparison with that for controls from non-BEN areas (p = 0.085 and p = 0.014, respectively) indicated increased protein oxidative damage.
We examined correlations of the activity of antioxidant enzymes and biomarkers of oxidative damage of proteins and lipids with the demographic and laboratory variables registered in BEN patients (Table 3). Only variables significantly (p < 0.05) associated with at least one of the oxidative stress parameter are presented. We found that the decreased activity of GPX observed in BEN patients was inversely correlated with serum urea (r = −0.63, p < 0.001) and creatinine (r = −0.53, p < 0.05), as well as urinary excretion of protein and α1-MG, as biochemical parameters of kidney damage (r = −0.44, p = 0.012; r = −0.50, p < 0.007). A similar association was also observed for P-SH content and serum urea. Significant positive correlations were obtained between GPX and eGFR and cholesterol. No significant correlations were found between SOD, MDA and the other examined variables.
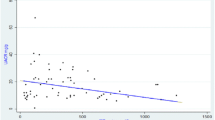
Plasma GPX activity in BEN patients at different stages of chronic kidney disease defined according to the KDOQI guidelines [26], together with values for both control groups, is presented in Fig. 1. Plasma GPX activity in BEN patients in the first stage of CKD, with normal eGFR, was similar (456.89 ± 150.56 U/L) to that for controls from BEN (450.87 ± 109.21 U/L) and non-BEN regions (425.71 ± 82.50 U/L). All other BEN patients had lower GPX activity than healthy controls, and the difference was significant for BEN patients with eGFR 30–60 mL/min/1.73 m2 (291.02 ± 84.08 U/L) and those with eGFR <30 mL/min/1.73 m2 (220.89 ± 143.24 U/L; p < 0.01). At the time of the study, eGFR calculated by MDRD equation and classification of chronic kidney disease proposed by KDOQI guideline was mostly used in our institutions and therefore presented here. With respect to the range of serum creatinine levels in BEN group, the difference in eGFR calculated by MDRD Eq. (65.17 ± 28.76) differed insignificantly from eGFR calculated by CKD-EPI Eq. (66.01 ± 29.31; p = 0.887) [31] recommended for eGFR calculation by recent KDIGO guideline [32]. Also, due to the small number of patients in certain stages of the disease, we considered the chronic kidney disease classification according KDOQI guideline convenient.
Discussion
To our knowledge, this is the first study that evaluated the activities of key antioxidant enzymes and parameters of oxidative damage in pre-dialysis BEN patients in different stages of the disease, in comparison with healthy members of BEN families and healthy controls from non-endemic areas. In BEN patients, the gradual decrease in plasma GPX activity, as a consequence of kidney function impairment, was accompanied by a moderate increase in oxidative protein damage. Interestingly, we observed preserved GPX activity in healthy BEN family members, but significant upregulation of SOD activity, which was accompanied with increased protein oxidative damage in comparison with healthy controls living in non-endemic areas.
Oxidative stress in CKD has a multifactorial origin, including effects of low molecular weight uremic toxins, nutritional inadequacy, elevated homocysteine level, chronic inflammation, anemia and decreased expression of glutathione peroxidase [9–14, 33–36]. Findings concerning oxidative stress in CKD have not always been consistent but usually documented declining antioxidant GPX activity [10, 11, 35, 36] and plasma levels of the sacrificial antioxidants, thiol (P-SH) groups [10, 14], with a rise in plasma MDA, as a biomarker of lipid oxidative damage [10, 12, 35]. It is important to note that, in contrast to erythrocyte GPX1 activity, the gradual decrease in plasma GPX activity in CKD patients is due to the fact that kidney is the main source of GPX3 isoenzyme in plasma. Thus, functional impairment of this organ leads to lower circulating enzyme activity [11, 33]. Indeed, our results showed that BEN patients had significantly lower plasma GPX activity than healthy persons from both control groups and this correlated with parameters of kidney function disorders, such as eGFR, urinary protein excretion. Moreover, our detection of a significant correlation between GPX activity and urinary excretion of α1-MG, a biomarker of proximal tubule damage, supports the finding that renal proximal tubule cells are the main source of plasma GPX [11, 33, 37].
On the contrary, plasma SOD activity in BEN patients did not differ from that in healthy controls and did not change significantly with deterioration of kidney function (no significant correlation with eGFR). This confirms the results of Atamer et al. [35] in CKD patients of non-BEN etiology. Similarly, Mimic-Oka et al. [10] found no significant difference in plasma SOD activity in CKD patients with creatinine clearance above 20 ml/min and that for healthy controls, but patients with creatinine clearance below 20 ml/min and those on hemodialysis had significantly higher plasma SOD activity than controls. However, several other groups found markedly lower plasma SOD activity in CKD patients when compared to values for controls [12, 15]. It can be hypothesized that, in the case of preserved SOD activity determined in our group of BEN patients, defective neutralization of reactive superoxide anions, as a consequence of decreased GPX activity, might contribute to increased ROS production.
Interestingly, healthy members of BEN families had significantly higher plasma SOD activity than healthy controls living in a non-endemic region. Considering the lower thiol-group levels with slightly increased MDA adducts, as biomarkers of oxidative damage, it seems that these healthy persons from BEN regions are chronically exposed to some prooxidant environmental compounds, with consequent upregulation of SOD activity, as a compensatory mechanism. As mentioned previously, experiments in vitro and in vivo have shown that ochratoxin A, a putative cause of BEN, induces increased ROS production followed by depletion of glutathione [38, 39]. Since kidney proximal tubular cells are the main target of ochratoxin A accumulation in the organism and considering the finding that these cells are also the dominant source of plasma GPX, the question that arises is why GPX is not upregulated in these subjects. One possible explanation lies in transcriptional regulation of GPX. Namely, nuclear factor-erythroid-2-related factor 2 (Nrf2) is a key regulator of transcription of a battery of stress response genes critical in mounting cellular defense against electrophiles or ROS, including antioxidant enzymes, the key enzymes responsible for glutathione synthesis and the major detoxifying enzymes. Until now, several studies have demonstrated that OTA can inhibit the Nrf2 oxidative stress response pathway, rendering cells and tissue more vulnerable to oxidative stress [39]. Finally, the importance of this transcription factor was confirmed in animal models of CKD, in which a continuous decline of Nrf2 activity during disease progression was observed [40].
In conclusions, healthy BEN family members exhibit increased protein damage, based on the decreased levels of protein thiol groups, as important “sacrificial’’ antioxidants in plasma. Oxidative modifications of proteins are accompanied by significant upregulation of SOD activity. In this line are the results of Domijan et al. [18], who recently suggested that urinary concentrations of MDA and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), as biomarkers of oxidative stress were increased in healthy residents of BEN villages.
Determination of byproducts of lipid and protein oxidative damage showed no differences in plasma MDA adducts among the three groups examined. Unfortunately, MDA adducts could not be measured in the plasma of all subjects involved in the study and the number of examined samples was small. Although the distribution of values obtained for MDA adducts had low-variance in all three groups (coefficient of variation less than 0.2) indicating the validity of the results, it is difficult to compare our results with those of other authors, who reported increased plasma MDA levels but most often measured in patients in end-stage renal disease on dialysis [10, 12, 35]. Nevertheless, our finding of a moderate increase of MDA adducts in both BEN patients and healthy BEN family members is in accordance with the results of Domijan et al. [18], who recorded significantly higher MDA urinary excretion in residents from BEN villages than in those from non-BEN villages.
Healthy controls from non-BEN area were selected from persons regularly controlled in our outpatient unit for metabolic stone evaluation and recurrence prevention. All these persons were subjected to urologic treatment of kidney stone one to 3 years before enrolment in the study and none of them had recurrent stone after treatment. At the time of enrolment their objective status was normal, laboratory findings, including kidney functions (eGFR, tubular functions, urinary proteins and sediment), were in the normal range, urine culture was negative, and ultrasound images of the kidneys were normal. All these tests are being carried out regularly in these patients once a year, which has enabled us to rule out kidney disease in these otherwise healthy individuals.
BEN patients included in the study were significantly older than healthy persons from both control groups. This could be expected because it has been known that the age of patients with manifested BEN has shifted to the older ages [41, 42]. Moreover, the effect of age on oxidative stress biomarkers must be taken into consideration. Namely, numerous evidence support the hypothesis that with age a rise of oxidative damage may be accompanied with affected ability to increase antioxidant defense enzymes under stress [43]. Based on the preserved SOD activity, accompanied with moderate increase in oxidative protein damage determined in our cohort of pre-dialysis BEN patients in comparison with much younger controls, we proposed that the this difference did not have significant impact on oxidative stress markers.
The main limitation of our study is the small sample size. However, this is the first evaluation of antioxidant enzymes and lipid and protein oxidation products in pre-dialysis BEN patients at different stages of the disease. In previous studies, parameters of oxidative stress were examined either in BEN patients with end-stage renal disease [16] or in residents of BEN settlements regardless of whether they were from BEN-affected or non-BEN families and whether they had BEN or were healthy [18]. The low dispersion of the obtained results indicates that the observed differences between the groups are worth further investigation.
In conclusion, based on our results it can be speculated that individuals from BEN areas, in general, are chronically exposed to some prooxidant environmental compounds. Data on increased protein oxidative damage, accompanied by significant upregulation of SOD activity in healthy BEN family members, confirm the existence of oxidative stress in these individuals. As a consequence of impaired kidney function, decrease of GPX activity in plasma could further affect oxidative status in pre-dialysis BEN patients.
References
Tancev I, Evstatijev P, Dorosiev D, Penceva Z, Cvetkov G (1956) Proucvanija na nefritite v’v Vracanska okolija. S’vr med 7(9):14–29
Danilovic V, Djurisic M, Mokranjac M, Stojimirovic B, Zivojinovic J, Stojakovic P (1957) Néphrites chroniques provoquées par l’intoxication au plomb par voie digestive (farine). Presse méd 65(90):2039–2040
Djukanovic L, Radovanovic Z (2008) Balkan nephropathy. In: De Broe ME, Porter GA (eds) Clinical nephrotoxins. Renal injury from drugs and chemicals, 3rd edn. Springer, New York, pp 843–858
Stefanovic V, Toncheva D, Polenakovic M (2015) Balkan nephropathy. Clin Nephrol 83(7 Suppl 1):64–69
Reljic Z, Zlatovic M, Savic-Radojevic A, Pekmezovic T, Djukanovic L, Matic M, Pljesa-Ercegovac M, Mimic-Oka J, Opsenica D, Simic T (2014) Is increased susceptibility to Balkan endemic nephropathy in carriers of common GSTA1 (*A/*B) polymorphism linked with the catalytic role of GSTA1 in ochratoxin a biotransformation? Serbian case control study and in silico analysis. Toxins (Basel) 6(8):2348–2362
Grollman AP, Shibutani S, Moriya M et al (2007) Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA 104(29):12129–12134
Pfohl-Leszkowicz A, Tozlovanu M, Manderville R, Peraica M, Castegnaro M, Stefanovic V (2007) New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in human nephropathy and urinary tract tumor. Mol Nutr Food Res 51:1131–1146
Maharaj SVM, Orem WH, Tatu CA, Lerch HE, Szilagyi DN (2014) Organic compounds in water extracts of coal: links to Balkan endemic nephropathy. Environ Geochem Health 36(1):1–17
Massy ZA, Stenvinkel P, Drueke TB (2009) The role of oxidative stress in chronic kidney disease. Sem Dial 4:405–408
Mimić-Oka J, Simić T, Djukanović L, Reljić Z, Davicević Z (1999) Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin Nephrol 51(4):233–241
Zachara BA, Adamowicz A, Trafikowska U, Pilecki A, Manitius J (2000) Decreased plasma glutathione peroxidase activity in uremic patients. Nephron 84:278–279
Günal SY, Ustündağ B, Günal Aİ (2013) The assessment of oxidative stress on patients with chronic renal failure at different stages and on dialysis patients receiving different hypertensive treatment. Indian J Clin Biochem 28(4):390–395
Yang CC, Hsu SP, Wu MS, Hsu SM, Chien CT (2006) Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int 69(4):706–714
Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J (2004) Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65(3):1009–1016
Singh DK, Winocour P, Farrington K (2011) Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7(3):176–184
Grubor-Lajsic G, Djordjević VB, Jovanovic-Galović A, Lecić N, Djordjević V, Spasić M (1998) Selenium-dependent and selenium-non-dependent glutathione peroxidase in patients with Balkan endemic nephropathy. J Environ Pathol Toxicol Oncol 17(3–4):321–324
Gluhovschi C, Modilcă M, Margineanu M, Gluhovschi G, Velciov S, Petrica L, Barzuca E, Gădălean F, Ivascu S, Kaycsa A, Ghiocel C (2014) Surprising good antioxidant status in patients with Balkan Endemic Nephropathy on hemodialysis undergoing vitamin C therapy. A pilot study. Rom J Intern Med 52(3):158–161
Domijan AM, Miletić-Medved M, Peraica M, Loft S (2013) Malondialdehyde and 8-oxo-7.8-dihydro-2′deoxyguanosine in the urine of residents from Balkan endemic nephropathy area in Croatia—a pilot study. Coll Antropol 37(4):1195–1198
Arbillaga L, Azqueta A, Ezpeleta O, López de Cerain A (2007) Oxidative DNA damage induced by Ochratoxin A in the HK-2 human kidney cell line: evidence of the relationship with cytotoxicity. Mutagenesis 22:35–42
Yu FY, Wu TS, Chen TW, Liu BH (2011) Aristolochic acid I induced oxidative DNA damage associated with glutathione depletion and ERK1/2 activation in human cells. Toxicol In Vitro 25(4):810–816
Wang L, Cao J, Chen D, Liu X, Lu H, Liu Z (2009) Role of oxidative stress, apoptosis, and intracellular homeostasis in primary cultures of rat proximal tubular cells exposed to cadmium. Biol Trace Elem Res 127(1):53–68
Shah MD, Iqbal M (2010) Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem Toxicol 48(12):3345–3353
Djukanović L, Marinković J, Marić I, Lezaić V, Dajak M, Petronić D, Matić M, Bukvić D (2008) Contribution to the definition of diagnostic criteria for Balkan endemic nephropathy. Nephrol Dial Transplant 23:3932–3938
Jelaković B, Nikolić J, Radovanović Z et al (2014) Consensus statement on screening, diagnosis, classification and treatment of endemic (Balkan) nephropathy. Nephrol Dial Transplant 29(11):2020–2027
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 39(2 Suppl 1):S1–S266
(1999) European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 14(Suppl 5):1–50
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Gunzler WA, Kremers H, Flohe L (1974) An improved coupled test procedure for glutathione peroxidase in blood. Zeitschrift Fuer Klinische Chemie Und Klinische Biochemie 12:444–448
Jocelyn PC (1987) Spectrophotometric assay of thiols. Methods Enzymol 143:44–61
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
KDIGO 2012 (2013) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3(1):1–150
Yoshimura S, Suemizu H, Nomoto Y, Sakai H, Katsuoka Y, Kawamura N, Moriuchi T (1996) Plasma glutathione peroxidase deficiency caused by renal dysfunction. Nephron 73:207–211
Mimić-Oka J, Savić-Radojević A, Pljesa-Ercegovac M, Opacić M, Simić T, Dimković N, Simić DV (2005) Evaluation of oxidative stress after repeated intravenous iron supplementation. Ren Fail 27(3):345–351
Atamer A, Kocyigit Y, Ecder SA, Selek S, Ilhan N, Ecder T, Atamer Y (2008) Effect of oxidative stress on antioxidant enzyme activities, homocysteine and lipoproteins in chronic kidney disease. J Nephrol 21(6):924–930
Mimic-Oka J, Simic T, Djukanovic L, Stefanovski J, Ramic Z (1992) Glutathione and its associated enzymes in peripheral blood cells in different stages of chronic renal insufficiency. Amino Acids 2(3):215–224
Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ (1994) Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol (Cell Physiol) 266:C367–C375
Schaaf GJ, Nijmeijer SM, Maas RF, Roestenberg P, de Groene EM, Fink-Gremmels J (2002) The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. Biochim Biophys Acta 1588(2):149–158
Limonciel A, Jennings P (2014) A review of the evidence that ochratoxin A is an Nrf2 inhibitor: implications for nephrotoxicity and renal carcinogenicity. Toxins (Basel) 6(1):371–379
Zoja C, Benigni A, Remuzzi G (2014) The Nrf2 pathway in the progression of renal disease. Nephrol Dial Transplant 29(Suppl 1):i19–i24
Janković S, Bukvić D, Marinković J, Janković J, Marić I, Djukanović L (2011) Time trends in Balkan endemic nephropathy incidence in the most affected region in Serbia, 1977–2009: the disease has not yet disappeared. Nephron Dial Transplant. 26:3171–3176
Cvitković A, Vuković-Lela I, Edwards KL, Karanović S, Jurić D, Cvorišćec D, Fuček M, Jelaković B (2012) Could disappearance of endemic (Balkan) nephropathy be expected in forthcoming decades? Kidney Blood Press Res 35(3):147–152
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine. Oxford University Press, New York
Acknowledgments
This work was supported by the Grant 175052 from the Serbian Ministry of Education, Science and Technological Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pavlović, D., Savić-Radojević, A., Plješa-Ercegovac, M. et al. Biomarkers of oxidative damage and antioxidant enzyme activities in pre-dialysis Balkan endemic nephropathy patients. Int Urol Nephrol 48, 257–263 (2016). https://doi.org/10.1007/s11255-015-1192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1192-9