Abstract
Objective
To identify the preoperative predictors of extraurothelial recurrence (EUR) after radical nephroureterectomy (RNU) in patients with upper tract urothelial carcinoma (UTUC).
Methods
A single-center series of 238 consecutive patients who were treated with RNU for UTUC was evaluated. Recurrence-free probabilities and cancer-specific survival (CSS) were estimated using the Kaplan–Meier method. Multivariate Cox proportional hazards regression models were used to evaluate the association between various clinicopathological factors and EUR.
Results
The median time to EUR was 17.6 months (range 3–73 months). EUR-free survival rates at 1, 3, 5, and 7 years were 87.8, 75.2, 73.5, and 72.6 %, respectively. In multivariate Cox regression analyses, tumor stage (HR 27.4; 95 % CI 7.83–95.8; p = 0.0001) and lymphovascular invasion (LVI) (HR 1.53; 95 % CI 1.22–3.12; p = 0.01) were independently associated with EUR. In patients with EUR, 5-year CSS estimate was 29.2 %. Tumor stage (HR 14.3; 95 % CI 4.55–45.2; p < 0.001) and EUR (HR 2.7; 95 % CI 1.54–4.73; p = 0.001) were the only independent predictors associated with worse CSS.
Conclusions
EUR significantly affected the prognosis in patients with UTUC managed by RNU. Patient with EUR had a greater probability of having higher tumor stages, higher tumor grades, and positive LVI. Tumor stage and LVI were independently associated with a worse EUR-free survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon disease, accounting for 5 % of all urothelial carcinomas with an incidence of two cases per 100,000 person-years [1]. Radical nephroureterectomy (RNU) constitutes the gold standard treatment for individuals with bulky, invasive, and/or high-grade UTUC [2]. However, despite definitive surgery, UTUC remains a malignancy with a high potential for local and distant recurrence, with reported recurrence rates of 22–66 % [3, 4].
Such outcomes indicate the importance of choosing adequate treatment plans and proper adjuvant therapy strategies for patients with higher risks of failure. Currently, no randomized trials that have investigated the role of adjuvant chemotherapy for UTUC exist. A recent meta-analysis showed that cisplatin-based adjuvant chemotherapy for UTUC was beneficial in terms of overall survival and disease-specific survival [5].
A multivariate analysis of patients with organ-confined UTUC treated by RNU showed cancer-specific mortality and progression-free survival to be significantly influenced by age, pathologic stage, lymphovascular invasion (LVI), and tumor multifocality [6]. Lymph node involvement at the time of RNU could be a reliable prognostic factor for metastatic relapse, but there is a lack of standardization in performing surgical lymph node removal in UTUCs, and the debate is ongoing around the appropriate area of dissection for the lymphadenectomy. Identification of prognostic factors allows the definition of high-risk groups of patients for whom specific therapy may be necessary.
The present study aimed to identify the preoperative predictors of extraurothelial recurrence (EUR) after RNU in patients with UTUC.
Patients and methods
A total of 317 patients with UTUC, who had undergone RNU between January 1999 and December 2013, were selected from the database. Patients who had undergone a previous or concurrent cystectomy, neoadjuvant chemotherapy, or had distant metastasis at diagnosis were excluded from the study. Patients for whom LVI status was not mentioned in the initial pathological report were also excluded from the study. In total, 238 patients (mean age 66.5 years, range 36–88) were then available for evaluation. Hospital medical records from these 238 patients were retrospectively reviewed to assess the significance of several clinicopathologic factors stratified by EUR (Table 1).
Tumors were staged according to the TNM classification [7] and graded using the 1998 WHO classification [8]. Tumor location was defined as either renal pelvic or ureteral based on the location of the dominant tumor. The dominant lesion was defined as that with the highest pathological tumor stage (pT). For multifocal tumors at the same stage, the higher grade was selected for main tumor location. Tumor multifocality was defined as the synchronous presence of two or more pathologically confirmed tumors in any upper urinary tract location. No immunohistochemistry techniques were used to determine the presence of LVI.
Cisplatin-based adjuvant chemotherapy was administered to some patients with pathologically confirmed lymph node metastasis (LNM) or with muscle-invasive disease (48 patients). Local recurrence and metastasis were considered to be EUR. Routine follow-up consisted of physical examination and cystoscopy every 3 months during the first year and every 6–12 months thereafter. Chest radiography, abdominal ultrasonography, computed tomography, and excretory urography were performed annually, depending on the clinical stage of the cancer in the upper urinary tract. Most patients who were identified as having died from UTUC had progressive, widely disseminated metastases at the time of death.
The initial treatment of all patients was open RNU. Lymph node dissection (LND) was performed in 49 patients, while 189 patients did not receive LND (pNx). Whether LND would be performed or not as well as the extent of LND when performed were determined by each surgeon during surgery. Mainly LND were performed in patient with palpably or visibly (intraoperatively or radiographically) suspicious lymph nodes.
Demographic and clinicopathological factors were analyzed using the Chi-square test or an unpaired t test. Recurrence-free probabilities and cancer-specific survival were estimated using the Kaplan–Meier method, and the log-rank test was used for the statistical differences. We defined the time of surgery as time zero. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the association between various clinicopathological factors and EUR, as well as cancer-specific mortality after surgery. In all tests, p < 0.05 (two-sided) was considered statistically significant. All statistical analyses were performed using SPSS© (version 17).
Results
Clinical and pathologic data
The patients’ demographic, clinical, and pathological profiles are listed in Table 1. The median follow-up after surgery was 34.5 months (range 1–154 months). Of the 238 patients included in the present study, 65 (27.3 %) patients had EUR. From the patients with EUR, 58.3 % had initial recurrence in lymph nodes, 35.5 % had recurrence in distant organs, and 6.2 % had recurrence in local area.
Patient with EUR had a greater probability (43.3 vs. 30.7 %) of having higher tumor stages (p = 0.001) and higher tumor grades (36.6 vs. 29.1), which was statistically significant (p = 0.001). Lymphovascular invasion (p = 0.03) was also associated with EUR.
Predictors of EUR
In univariate Cox regression analyses, tumor grade (HR 2.03; 95 % CI 1.21–3.42; p = 0.008), tumor stage (HR 22.6; 95 % CI 7.01–72.7; p = 0.001), and LVI (HR 1.85; 95 % CI 1.05–3.25; p = 0.03) were associated with EUR (Table 2). In multivariate Cox regression analyses (Table 2), tumor stage (HR 27.4; 95 % CI 7.83–95.8; p = 0.0001) and LVI (HR 1.53; 95 % CI 1.22–3.12; p = 0.01) were independently associated with EUR.
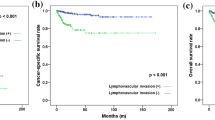
The median time to EUR was 17.6 months (range 3–73 months). EUR-free survival rates at 1, 3, 5, and 7 years were 87.8, 75.2, 73.5, and 72.6 %, respectively (Fig. 1). The EUR-free survival rates were significantly lower in patients with higher tumor stages (pT3 or greater, p = 0.001, log-rank) and higher tumor grades (G > 2, p = 0.006, log-rank). Also, EUR-free survival rates were lower in patients with LVI (p = 0.03, log-rank).
Outcomes of patients with EUR
The 5-year cancer-specific survival (CSS) estimate was 71 %. However, in patients with EUR, 5-year CSS estimate was 29.2 %. In multivariate Cox regression analyses, tumor stage (HR 14.3; 95 % CI 4.55–45.2; p < 0.001) and EUR (HR 2.7; 95 % CI 1.54–4.73; p = 0.001) were the only independent predictors associated with worse CSS. The CSS in patients with EUR was significantly lower compared with those without EUR (p < 0.001, log-rank, mean CSS for patients with EUR 37.5 ± 4.8 months vs. mean CSS for patients without EUR 119.9 ± 7.1 months). Also, overall survival in patients with EUR was significantly lower compared with those without EUR (p < 0.001, log-rank, mean overall survival for patients with EUR 33.1 ± 3.6 months vs. mean overall survival for patients without EUR 99.8 ± 7.1 months).
Lymph node dissection was performed in 21 % of patients. Lymph node metastases after LND are shown in 26 % of patients.
Discussion
To contribute to the ongoing discussions of UTUC predictive factors for recurrence, we analyzed the preoperative predictors of EUR after RNU. Pathological T stage, LVI, and C-reactive protein levels are documented as major prognostic factors for recurrence of UTUC [9–11]. In our study, the factors associated with a higher risk of EUR were pT3 or greater tumor stage and higher tumor grade (G3). In multivariate analysis, only tumor stage and LVI were independently associated with a worse oncologic outcome. LVI is the primary and essential step in the systemic dissemination of cancer cells [12]. Several studies have shown an association between the presence of LVI on the bladder biopsy specimen and extravesical disease at the time of cystectomy [13, 14]. Only a few publications have suggested that LVI has a negative impact on UTUC survival and have advocated the use of this factor to predict urothelial recurrence and metastatic spread [15–18]. We noted that EUR-free survival rates were significantly lower in patients with LVI.
In the present study, adjuvant cisplatin-based combination chemotherapy was only administered in patients with disease pT3 or pT4 and/or nodal involvement. Urothelial tumors are chemo-sensitive particularly to cisplatin-based regimens [19]. Recently, Raman et al. [20] showed a 15 % improvement in CSS for high-risk RNU patients who received adjuvant chemotherapy compared with those who did not. Kwak et al. [21] noted that the use of adjuvant chemotherapy and nodal status were associated with overall survival following UTUC surgery. Bamias et al. [22] prospectively studied a cohort of 36 patients who received adjuvant chemotherapy following UTUC surgery and reported a reduction in metastatic recurrences, although there was no difference in survival at 5 years. The use of adjuvant chemotherapy in the treatment of UTUC remains largely limited due to the decline in renal function following RNU.
The French Collaborative National Database on UTUC did not find any significant benefit in patient outcomes despite the use of adjuvant chemotherapy [23]. Only about 22 % of high-risk patients with a pathologic stage ≥pT3N0 and/or N+ and/or M+ disease received adjuvant chemotherapy, with only 50 % treated with a cisplatin-based regimen. The 5-year overall and CSS rates were 43 and 60 %, respectively, with no difference in outcomes compared with the group who did not receive adjuvant chemotherapy. It is again notable that patients offered adjuvant chemotherapy had a higher pathologic stage and a higher-grade disease. To date, there have been no randomized trials confirming the efficacy of postoperative chemotherapy or radiotherapy in patients with UTUC.
The 5-year cancer-specific survival for all patients was 70.1 % and for patients with EUR was 29.2 %. Overall survival in patients with EUR was significantly lower compared with those without EUR. In accordance with previous studies, tumor stage and EUR were the only independent predictors associated with worse CSS [3, 24]. Use of prognostic factors might help identify patients who could benefit from intensified therapy and monitoring. For example, prognostic factors could be used to identify patients for whom neoadjuvant or adjuvant chemotherapy is likely to be beneficial. Inclusion criteria for clinical trials based upon such prognostic factors might ensure an adequately selected study population to demonstrate a benefit for experimental therapies.
There are several limitations to the current study. First, it is inherently limited by biases associated with its retrospective design. Our results are subject to the inherent biases associated with high-volume tertiary care centers. Adjuvant treatments administered to patients with pT3 or pT4 disease could induce a bias, but these patients had the worst outcomes. In this cohort of patients, none had received neoadjuvant chemotherapy, which can be limitation of the study. In addition, patients were not randomized to receive LND or not, and the extent of LND was decided by each surgeon. Despite these limitations, our study has strengths, such as a centralized pathologic review and standardized follow-up.
Conclusions
EUR significantly affected the prognosis in patients with UTUC managed by RNU. Patient with EUR had a greater probability of having higher tumor stages, higher tumor grades, and positive LVI. Tumor stage and LVI were independently associated with a worse EUR-free survival. When a lymphadenectomy has not been achieved, the reporting of LVI status is crucial to identify those patients who are at higher risk of metastatic relapse. Tumor stage and EUR were the only independent predictors associated with worse CSS.
Determining prognostic factors of EUR can aid in the decision making in relation to management of patients with UTUC, consideration of adjuvant therapy, and selection of patients who would benefit from inclusion into clinical trials.
References
Jamal A, Siegel R, Ward E et al (2006) Cancer statistics, 2006. CA Cancer J Clin 56(2):106–130
Raman JD, Scherr DS (2007) Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol 4:432–443
Margulis V, Youssef RF, Karakiewicz PI, Upper Tract Urothelial Carcinoma Group et al (2010) Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 184:453–458
Favaretto RL, Shariat SF, Chade DC et al (2010) The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol 58:574–580
Leow JJ, Martin-Doyle W, Fay AP et al (2014) A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 66(3):529–541
Chromecki TF, Cha EK, Fajkovic H et al (2012) The impact of tumor multifocalityon outcomes in patients treated with radical nephroureterectomy. Eur Urol 61:245–253
Fleming ID, Cooper JS, Henson DE et al (1997) Genitourinary site. In: Touhey R (ed) AJCC cancer staging manual, 5th edn. Lippincott-Raven, Philadelphia, pp 231–246
Epstein JI, Amin MB, Reuter VR et al (1998) The World Health Organization/International Society of Urological pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 22:1435–1448
Saito K, Kawakami S, Ohtsuka Y et al (2007) The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int 100:269–273
Kikuchi E, Margulis V, Karakiewicz PI et al (2009) Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 27:612–618
Zigeuner R, Shariat SF, Margulis V et al (2010) Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 57:575–581
Sundar SS, Ganesan TS (2007) Role of lymphangiogenesis in cancer. J Clin Oncol 25:4298–4307
Millikan R, Dinney C, Swanson D et al (2001) Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 19:4005–4013
Kunju LP, You L, Zhang Y et al (2008) Lymphovascular invasion of urothelial cancer in matched transurethral bladder tumor resection and radical cystectomy specimens. J Urol 180:1928–1932
Ku JH, Byun SS, Jeong H et al (2013) Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer 49(12):2665–2680
Hurel S, Rouprêt M, Ouzzane A et al (2013) Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int 111(8):1199–1207
Novara G, Matsumoto K, Kassouf W et al (2010) Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 57(6):1064–1071
Milojevic B, Djokic M, Sipetic-Grujicic S et al (2013) Prognostic significance of non-muscle-invasive bladder tumor history in patients with upper urinary tract urothelial carcinoma. Urol Oncol 31(8):1615–1620
Yagoda A (1987) Chemotherapy of urothelial tumors. Cancer 60:574–585
Raman JD, Lin YK, Kaag M et al (2014) High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol 32(1):47.e9-14. doi:10.1016/j.urolonc.2013.06.015
Kwak C, Lee SE, Jeong IG et al (2006) Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology 68:53–57
Bamias A, Deliveliotis CH, Fountzilas G et al (2004) Adjuvant chemo-therapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J Clin Oncol 22(11):2150–2154
Vassilakopoulou M, de la Motte Rouge T, Colin P et al (2011) Outcomes after adjuvant chemotherapy in the treatment of high risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a Large Multicenter Collaborative Study. Cancer 117:5500–5508
Milenkovic-Petronic D, Milojevic B, Djokic M et al (2014) The impact of tumor size on outcomes in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol 46(3):563–569
Acknowledgments
This work was supported by the Ministry for Science and Technology of the Republic of Serbia, through Contact No. 175042 (2011–2014).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zoran Dzamic and Bogomir Milojevic have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dzamic, Z., Milojevic, B., Kajmakovic, B. et al. Extraurothelial recurrence after radical nephroureterectomy: preoperative predictors and survival. Int Urol Nephrol 47, 775–779 (2015). https://doi.org/10.1007/s11255-015-0946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0946-8