Abstract
Purpose
To investigate the association between tumor size and clinicopathologic factors and outcomes of upper urinary tract urothelial carcinoma (UTUC) in patients treated surgically for UTUC.
Methods
A single-center series of 235 consecutive patients who were treated surgically for UTUC between January 1999 and December 2011 was evaluated. Patients with a history of muscle-invasive urothelial carcinoma of the urinary bladder, those who received neoadjuvant therapies, and those with previous contralateral UTUC were excluded. Bladder-only recurrence, any recurrence, and cancer-specific mortality after surgery were analyzed. Recurrence-free probabilities and cancer-specific survival (CSS) were estimated using the Kaplan–Meier method and Cox regression analyses.
Results
Tumor size was significantly associated with age of the patient (P = 0.001), tumor location (P < 0.0001), tumor multifocality (P = 0.005), higher tumor stage (P < 0.0001), higher tumor grade (P = 0.038), lymphovascular invasion (P = 0.002), and mode of operation (P = 0.001). Tumor size was not associated with bladder-only recurrence (HR 0.91; 95 % CI 0.46–1.80; P = 0.79). The Kaplan–Meier method showed that tumor size >3 cm was significantly associated with worse CSS (P = 0.006, log rank). The 5-year CSS for patients with tumor size ≤3 cm was 70.1 % and for patients with tumor size >3 cm was 56.1 %. Tumor size was not associated with cancer-specific survival in multivariable analysis (HR 1.53; 95 % CI 0.89–2.61; P = 0.12).
Conclusions
Tumor size >3 cm was associated with a lower 5-year CSS at Kaplan–Meier analysis, but was not an independent predictor of CSS, bladder-only recurrence, and any recurrence-free survival at multivariable analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urothelial carcinomas are the fourth most common tumors after prostate (or breast) cancer, lung cancer, and colorectal cancer [1, 2]. They can be located in the lower urinary tract (bladder and urethra) or the upper urinary tract (renal pelvic and ureter). Upper urinary tract urothelial carcinoma (UTUC) is uncommon and accounts for only 5–10 % of all urothelial carcinomas [3].
In the past few years, several prognostic factors have been identified to help clinicians dealing with patients with UTUC in the decision-making process. Initial tumor stage, grade, multifocality, tumor architecture, presence of hydronephrosis, and extent of surgery are documented as major prognostic factors in patients with UTUC [3–7].
Tumor size is an established predictor of cancer-related outcomes in several malignancies. The prognostic impact of tumor size in patients with UTUC has not been fully assessed. It was reported that tumor size is an independent predictor of metastasis-free survival and cancer-specific survival after radical nephroureterectomy (RNU) [8]. Pieras et al. [9] observed that patients with a tumor diameter >4 cm had a higher risk of developing a bladder tumor recurrence. According to the TNM classification, size is not mentioned specifically as a prognostic factor in UTUC [10].
The aim of this study is to investigate the association between tumor size and clinicopathologic factors and outcomes of UTUC in patients treated surgically for UTUC.
Materials and methods
The present study cohort represents 235 patients who were surgically treated for UTUC between January 1999 and December 2011. Patients with a history of muscle-invasive urothelial carcinoma of the urinary bladder, those who received neoadjuvant therapies, and those with previous contralateral UTUC were excluded. In total, 203 patients were then available for evaluation. Hospital medical records from these 203 patients were retrospectively reviewed to assess the significance of several clinicopathologic factors stratified by tumor size (Table 1).
Diagnoses of UTUC were established by CT, excretory urography, retrograde ureteropyelogram, and/or ureteroscopy with tissue biopsies. Tumors were staged according to the TNM classification [10] and graded using the 1998 WHO classification [11]. Criteria for areas of Balkan endemic nephropathy (BEN) were the same as those used in previous studies [12, 13]. The settlements were designated as endemic when three and more cases of BEN were published or reported to the registry of BEN and non-endemic when no autochthonous cases of BEN were previously established. Included in the analysis were patients with permanent residence in BEN or non-endemic areas from their birth to the end of follow-up. Tumor multifocality was defined as the synchronous presence of two or more pathologically confirmed tumors in any upper urinary tract location. For tumor size, the maximum diameter of tumor was examined and recorded macroscopically as accurate as possible. The size of tumors was taken based on pathology report. Based on previous studies [14–16], the tumor size was stratified into two subgroups (≤3, >3).
Initial treatment of all patients was surgical. One hundred and seventy-five patients were operated by radical nephroureterectomy while 28 patients were treated by open conservative surgery including distal ureterectomy with reimplantation to the bladder and segmental ureterectomy with termino-terminal anastomosis (T–T anastomosis). Patients were selected for conservative procedures using criteria such as diminished renal function related to Balkan nephropathy, bilateral tumors, solitary kidney, or other serious comorbidities. Regional lymph node dissection was performed in patients with clinically apparent lymphadenopathy on a preoperative radiologic imaging or in those who were suspected of having enlarged lymph nodes intraoperatively. Adjuvant cisplatin-based combination chemotherapy was administered in patients with disease pT3 or pT4 and/or nodal involvement.
The median follow-up after surgery was 36 months (range 1–154 months). Patient follow-up was relatively uniform and consisted of physical examination and cystoscopy every 3 months during the first year and every 6–12 months thereafter. Chest radiography, abdominal ultrasonography, computed tomography (CT), and excretory urography were performed annually, with frequency, which was in concordance with European Association of Urology guideline. Thus, we performed CT urography every 6 months during the 2 years after RNU and then annually, and for patients with conservative management, CT urography was done 3 and 6 months after surgery and then annually. In conservative management, ureteroscopy of ipsilateral upper urinary tract was also done. Patients who were identified as having died from UTUC had progressive, widely disseminated metastases at the time of death.
Demographic and clinicopathologic factors were analyzed using the chi-square test or an unpaired t test. Recurrence-free probabilities and cancer-specific survival were estimated using the Kaplan–Meier method, and the log-rank test was used for the statistical differences. We defined the time of surgery as time zero. Univariable and multivariable Cox proportional hazards regression models were used to evaluate the association between various clinicopathologic factors and bladder-only recurrence, any recurrence, as well as cancer-specific mortality after surgery. In all tests, P < 0.05 (two-sided) was considered statistically significant.
Results
The median age was 66 years (range 36–88 years). One hundred and sixteen patients (57.1 %) had tumors larger than 3 cm. The association between tumor size and clinicopathologic features in all patients is shown in Table 1. Tumor size was significantly associated with age of the patient (P = 0.001), tumor location (P < 0.0001), tumor multifocality (P = 0.005), higher tumor stage (P < 0.0001), higher tumor grade (P = 0.038), lymphovascular invasion (P = 0.002), and mode of operation (P = 0.001). In patients with tumor size ≤3 cm, multifocal tumors were found in 43 % of patients, whereas in patients with tumor size >3 cm, multifocal tumors were found in 25 % of patients, which was statistically significant (P = 0.005). The distribution of UTUC pathologic stage in a cohort of patients with a tumor size ≤3 cm was pTa (10 %), pT1 (12 %), pT2 (39 %), pT3 (37 %), and pT4 (2 %). Specifically, patients with tumor size >3 cm had a greater probability (69.8 vs. 39.1 %) of having higher tumor stages (pT3 or greater). Twenty of the 28 (71.4 %) patients treated with conservative surgery had tumors smaller than 3 cm, which was statistically significant (P = 0.001).
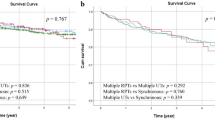
Kaplan–Meier analysis showed no difference in bladder-only recurrence (P = 0.51, log rank) between patients with tumor size ≤3 cm and those with tumor size >3 cm (Fig. 1). The 5-year bladder-only recurrence-free survival for patients with tumor size ≤3 cm was 73.2 % and for patients with tumor size >3 cm was 79.8 %. Bladder-only recurrence developed in 27.5 % of patients with tumor size ≤3 cm, whereas it occurred in 20.6 % of patients with tumor size >3 cm, which was not statistically significant (P = 0.25). Tumor size was not associated with bladder-only recurrence in any of analyses (univariable: HR 0.86; 95 % CI 0.49–1.53; P = 0.61, and multivariable: HR 0.91; 95 % CI 0.46–1.80; P = 0.79) (Table 2).
Using univariable analyses, demographic characteristic (HR 1.76 for areas of BEN vs. non-BEN areas 95 % CI 1.01–3.09; P = 0.04), history of bladder cancer (HR 2.05; 95 % CI 1.24–3.38; P = 0.005), tumor multifocality (HR 2.25; 95 % CI 1.39–3.63; P = 0.001), pathologic stage (HR 1.75; 95 % CI 1.06–2.89; P = 0.03), and lymphovascular invasion (HR 1.67; 95 % CI 1.01–2.80; P = 0.048) were associated with disease recurrence (any recurrence) (Table 3). Using multivariable analysis, demographic characteristics (HR 1.98; 95 % CI 1.12–3.48; P = 0.018) and tumor multifocality (HR 1.93; 95 % CI 1.13–3.30; P = 0.015) were the only independent predictors associated with worse disease recurrence survival (any recurrence). Kaplan–Meier analysis showed that any recurrence-free survival after surgery for UTUC is not related to tumor size (P = 0.18, log rank) (Fig. 2).
The Kaplan–Meier method showed that tumor size >3 cm was significantly associated with worse cancer-specific survival (P = 0.006, log rank, mean cancer-specific survival for patients with tumor size ≤3 cm 91.9 ± 8.6 months versus mean cancer-specific survival for patients with tumor size >3 cm 54.6 ± 4.9 months) (Fig. 3). The 5-year cancer-specific survival for patients with tumor size ≤3 cm was 70.1 % and for patients with tumor size >3 cm was 56.1 %. Using univariable analyses, age of the patient (HR 1.82; 95 % CI 1.02–3.24; P = 0.04), history of bladder cancer (HR 1.87; 95 % CI 1.19–2.94; P = 0.007), tumor size (HR 1.87; 95 % CI 1.18–2.95; P = 0.007), tumor stage (HR 3.10; 95 % CI 1.89–5.08; P = 0.001), lymphovascular invasion (HR 1.86; 95 % CI 1.16–2.98; P = 0.01), and lymph node status (HR 6.76; 95 % CI 3.03–15.1; P = 0.001) were associated with cancer-specific survival (Table 4). Using multivariable analysis, tumor stage (HR 2.59; 95 % CI 1.37–4.89; P = 0.003) and lymph node status (HR 4.98, 95 % CI 2.04–12.1; P = 0.001) were the only independent predictors associated with worse cancer-specific survival (Table 4). Tumor size was not associated with cancer-specific survival in multivariable analysis (HR 1.53; 95 % CI 0.89–2.61; P = 0.12) (Table 4).
When we took tumor size of 4 cm as cutoff value, there was no statistically significant difference in bladder-only recurrence and any recurrence-free survival, as well as cancer-specific mortality.
Discussion
We found that tumor size is associated with established features of biologically aggressive UTUC, such as tumor stage, grade, and lymphovascular invasion. Also, we found that tumor size is associated with age of the patient, tumor location, tumor multifocality, and mode of operation. Moreover, in univariable analyses, tumor size was associated with worse cancer-specific mortality, but had no effect on bladder-only recurrence and any recurrence-free survival. The Kaplan–Meier method showed that tumor size was significantly associated with cancer-specific survival.
In this study, patients with tumor size ≤3 cm had a greater probability (43 vs. 25 %) of having multifocal UTUC. Tumor multifocality is an independent prognosticator of disease progression and cancer-specific mortality in patients with organ-confined UTUC treated with RNU [17]. Also, the multiplicity of the UTUC is an independent risk factor for the occurrence of bladder cancer [13]. Despite these data, in this cohort study, tumor size had no effect on bladder-only recurrence and any recurrence.
Only a few studies to date have focused on the prognostic impact of tumor size in patients with UTUC. Simone et al. [14] investigated the relationship between tumor diameter and metastasis-free survival in patients with UTUC. In their study, no metastases were noted in patients presenting with a tumor diameter <3 cm, whereas patients with a tumor diameter ≥3 cm had a 5-year estimated metastasis-free survival of 67 %. Also, tumor diameter was an independent predictor of metastasis-free survival and disease-free survival and was the strongest prognostic indicator of the variables analyzed [14]. In our study, we have been able to show no significant difference in disease recurrence-free survival between patients with tumor size ≤3 cm and those patients with tumor size >3 cm. Tumor multifocality and demographic characteristics were the only independent predictors associated with worse disease recurrence survival, which is in accordance with our previous studies [18, 19].
In the present study, we confirmed that tumor size is unable to predict bladder-only recurrence-free survival in a single-center series of consecutive patients treated surgically for UTUC. This is not in agreement with several earlier studies [9, 21]. Pieras et al. [9] observed that patients with a tumor diameter >4 cm had a higher risk of developing a bladder tumor recurrence. Matsui et al. [21] reported that smaller tumors increase the risk of intravesical recurrence.
The 5-year cancer-specific survival for patients with tumor size ≤3 cm was 70.1 % and for patients with tumor size >3 cm was 56.1 %. In accordance with previous studies, we found that pathologic stage and lymph node metastasis are the strongest predictors of survival in UTUC cases [17–20]. In this study, tumor size was identified as a significant predictor of cancer-specific mortality in patients treated surgically for UTUC.
In this cohort of patients, 71.4 % of patients treated with conservative surgery had tumors smaller than 3 cm. Since patients from BEN regions have a higher risk of both bilateral disease and renal damage, experience with open conservative surgery of UTUC in our country is much greater than in other regions of Europe [22]. BEN is a tubulointerstitial kidney disease that is usually considered non-inflammatory [23]. An unusually high incidence of UTUC has been reported in Balkan countries, especially in areas of Balkan endemic nephropathy [12]. In a review of published reports, about two-thirds of patients with UTUC in Serbia were from BEN areas [12]. However, the incidence of BEN and UTUC in BEN regions of Serbia appears to have decreased over the last decade [24]. Interestingly, there is no difference in tumor size when we compared patients from BEN areas with those from non-BEN areas.
This study is inherently limited by biases associated with its retrospective design. Also, our results are subject to the inherent biases associated with high-volume tertiary care centers. Adjuvant treatments administered to patients with pT3 or pT4 disease could induce a bias, but these patients had the worst outcomes. In this cohort of patients, none had received neoadjuvant chemotherapy, which can be limitation of the study. We excluded from this analysis patients for whom we could not obtain complete information, possibly creating a selection bias. The results also are potentially limited by a relatively short median follow-up time as a result of the small number of patients followed for 5 years. Despite these limitations, our study has strengths, such as a centralized pathologic review and standardized follow-up.
Conclusions
Tumor size >3 cm was associated with a lower 5-year CSS at Kaplan–Meier analysis, but was not an independent predictor of CSS at multivariate analysis. We did not find any difference in bladder-only recurrence and any recurrence-free survival between patients with tumor size ≤3 cm and patients with tumor size >3 cm in a single-institution cohort of patients treated surgically for UTUC. However, well-designed multi-institutional studies are still needed to provide stronger evidence and to promote the use of tumor size in clinical practice.
References
Munoz JJ, Ellison LM (2000) Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 164:1523–1525
Ploeg M, Aben KK, Kiemeney LA (2009) The present and future burden of urinary bladder cancer in the world. World J Urol 27:289–293
Hall MC, Womack S, Sagalowsky AI et al (1998) Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology 52:594–601
Roscigno M, Cozzarini C, Bertini R et al (2008) Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol 53:794–802
Akdogan B, Dogan HS, Eskicorapci SY et al (2006) Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol 176(1):48–52
Kikuchi E, Margulis V, Karakiewicz PI et al (2009) Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 27:612–618
Favaretto RL, Shariat SF, Chade DC et al (2010) The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol 58:574–580
Margulis V, Shariat SF, Matin SF et al (2009) Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115:1224–1233
Pieras E, Frontera G, Ruiz X et al (2010) Concomitant carcinoma in situ and tumor size are prognostic factors for bladder recurrence after nephroureterectomy for upper tract transitional cell carcinoma. BJU Int 106:1319–1323
Fleming ID, Cooper JS, Henson DE et al (1997) Genitourinary site. In: Touhey R (ed) AJCC cancer staging manual, 5th edn. Lippincott-Raven, Philadelphia, pp 231–246
Epstein JI, Amin MB, Reuter VR et al (1998) The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 22:1435–1448
Djokic M, Hadzi-Djokic J, Nikolic J et al (1999) Comparison of upper urinary tract tumors in the region of Balkan nephropathy with those of other regions of Yugoslavia. Prog Urol 9:61–68
Milojevic B, Djokic M, Sipetic-Grujicic S et al (2011) Bladder cancer after managing upper urinary tract transitional cell carcinoma: risk factors and survival. Int Urol Nephrol 43(3):729–735
Simone G, Papalia R, Loreto A et al (2009) Independent prognostic value of tumor diameter and tumor necrosis in upper urinary tract urothelial carcinoma. BJU Int 103:1052–1057
Dragicevic D, Djokic M, Pekmezovic T et al (2007) Survival of patients with transitional cell carcinoma of the ureter and pelvis in Balkan endemic nephropathy and nonendemic areas of Serbia. BJU Int 99(6):1357–1362
Hisataki T, Miyao N, Masumori N et al (2000) Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology 55:663–667
Chromecki TF, Cha EK, Fajkovic H et al (2012) The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol 61(2):245–253
Milojevic B, Djokic M, Sipetic-Grujicic S et al (2012) Upper urinary tract transitional cell carcinoma: location is not correlated with prognosis. BJU Int 109:1037–1042
Raman JD, Ng CK, Scherr DS et al (2010) Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol 57(6):1072–1079
Milojevic B, Djokic M, Sipetic-Grujicic S et al (2012) Prognostic significance of non-muscle-invasive bladder tumor history in patients with upper urinary tract urothelial carcinoma. Urol Oncol. doi:10.1016/j.urolonc.2012.03.004
Matsui Y, Utsunomiya N, Ichioka K et al (2005) Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract. Urology 65:279–283
Dragicevic D, Djokic M, Pekmezovic T et al (2009) Comparison of open nephroureterectomy and open conservative management of upper urinary tract transitional cell carcinoma. Urol Int 82(3):335–340
Vukelic M, Sostaric B, Belicza M (1992) Pathomorphology of Balkan endemic nephropathy. Food Chem Toxicol 30:193–200
Markovic N, Ignjatovic I, Cukuranovic R (2005) Decreasing incidence of urothelial cancer in a Balkan endemic nephropathy region in Serbia. A surgery based study from 1969 to 1998. Pathol Biol 53:26–29
Acknowledgments
This work was supported by the Ministry for Science and Technology of the Republic of Serbia, through Contact No. 175042 (2011–2014).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milenkovic-Petronic, D., Milojevic, B., Djokic, M. et al. The impact of tumor size on outcomes in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol 46, 563–569 (2014). https://doi.org/10.1007/s11255-013-0533-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0533-9