Abstract
Purpose
To evaluate the outcome of the retrourethral transobturator sling (RTS) by functional magnetic resonance imaging (MRI) and to identify parameters associated with sling failure.
Methods
Of thirty recruited men with postprostatectomy stress urinary incontinence (SUI), 26 consecutively underwent functional MRI before sling procedure and 12 months thereafter in a prospective clinical cohort observational study. Periurethral/urethral fibrosis and sling visualization were evaluated on static sequences. The angle of the membranous urethra, position of the bladder neck and external urethral sphincter were assessed during Valsalva’s maneuver and voiding. Sling success was defined as no or one dry “security” pad.
Results
The success and failure rates were 58 % (15/26 patients) and 42 % (11/26 patients), respectively. The sling leads to reduction in the membranous urethra angle during Valsalva’s maneuver (39.55° vs. 36.82°, p = 0.025) and voiding (38.25° vs. 34.83°, p = 0.001) and elevation of the external urethral sphincter (2.9 vs. 4.8 mm, p = 0.017). Preoperative wider angle of the membranous urethra was significantly correlated with severe preoperative incontinence. Sling failure (p = 0.001) and severe preoperative incontinence (p = 0.001) were significantly related to only small changes of the membranous urethra angle. The interrater and intrarater reliability for membranous urethra angle was excellent (intraclass correlation coefficient ≥0.75).
Conclusions
The RTS leads to reduction in the membranous urethra angle. The extent of the changes in the membranous urethra angle is associated with RTS outcome. Functional MRI is a reliable noninvasive visualization tool of interactions between the sling and pelvic floor for further research on the complex nature of postprostatectomy SUI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The retrourethral transobturator sling (RTS) (AdVance®, American Medical Systems, Minnetonka, MN, USA) is widely used for treating postprostatectomy stress urinary incontinence (SUI). RTS was reported to relocate the urethral sphincter (EUS) complex and its supporting structures into a preprostatectomy position [1]. Recent long-term studies revealed acceptable and durable RTS success rates [2, 3]. To increase RTS efficacy, patients with mild or moderate SUI should be selected [4, 5]. However, RTS failure rate of 20–45 % [6] still remains high. This is due, on the one hand, to different definitions of the success rates reported [7] and, on the other hand, to a lack of the understanding of how RTS and pelvic floor (PF) structures interact. Imaging studies using real-time visualization of dynamic PF processes may help to understand the factors contributing to RTS failure.
The aim of this study was to evaluate RTS outcome using functional magnetic resonance imaging (MRI) for identifying parameters contributing to RTS failure and to assess the reliability of these measurements.
Materials and methods
Patients
In a prospective clinical cohort observational MRI study from December 2007 to January 2011, patients with persistent SUI intended to be treated with RTS were consecutively recruited from our outpatient clinic and followed up prospectively. All patients signed the informed consent form after ethical approval by the local institutional review board. Inclusion criteria were SUI persisting at least 1 year after radical prostatectomy (RP), residual EUS function confirmed by the repositioning test [2, 3, 8] and the intention to participate at one pre- and postoperative MRI examination in addition to the standard preoperative RTS diagnostics. All enrolled patients met the previously reported criteria [9] for RTS implantation. Known contraindications to MRI examinations such as claustrophobia, pacemaker, any metal implant and nonconservative SUI treatment before or after RTS placement, severe complications [10], history of adjuvant pelvic radiotherapy, anastomotic or urethral strictures at the time of recruitment were considered as exclusion criteria. The RTS preoperative workup included demographics, evaluation of medical history, uroflowmetry, postvoid residual urine (PVR), daily pad use, 1-h pad test, urethrocystoscopy and urodynamics to exclude detrusor overactivity and intrinsic sphincter deficiency through the abdominal leak point pressure (ALPP) measurements. Incontinence severity was evaluated by the number of pads used per day [9], 1-h pad test and the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF; range 0–21, minimum to maximum symptoms).
RTS was implanted by a well-trained surgeon using the surgical technique previously reported [1, 9].
For follow-up, daily pad use, 1-h pad test, ICIQ-SF, uroflowmetry and PVR were re-evaluated 6 and 12 months postoperatively. Twelve months after RTS, the postoperative MRI took place. RTS success was defined as no or one dry “security” pad.
MRI examination technique
MRI was performed on a 1.5 tesla scanner (Sonata, Siemens Medical Solutions, Erlangen, Germany) using phased body array coils covering the pelvis lying in the supine position. One hour before MRI, all patients had to empty the bladder and then to drink 200 mL water in order to provide a consistently full bladder during imaging to achieve some kind of standardization. To avoid any influence on the PF, all patients were administered an enema containing 100 mL of a solution of sodium phosphate into the rectum 30–45 min before MRI. Before the examination, patients were instructed to follow the examination protocol and to perform a Valsalva maneuver by attempting to maximally push down the PF and then to urinate in a urine bottle. The examination was started when patients had the first desire to void.
Static T2-weighted turbo spin echo (TSE) sequences [TR5720ms; TE107ms; slice thickness 3 mm; FieldOfView (FOV) 280/75 mm] in axial and sagittal orientation were acquired for evaluation of anatomical PF structures and planning of the dynamic examination. Functional dynamic real-time MRI was acquired by single slice TrueFISP (Fast Imaging with Steady State Precession) sagittal sequences (TR4.57ms; TE2.29ms; slice thickness 7 mm; FOV 270 mm) with a frequency of one image/s during Valsalva’s maneuver and voiding.
MRI data analysis
The pre- and postoperative MRI were independently analyzed for interrater reliability of the measurement technique on a PACS workstation by radiologist and urologist with expertise on abdominal and pelvic imaging. Additionally, the same readers re-evaluated MRI 6 weeks after the first analysis to assess the intrarater reliability of the measurements. The readers were blinded to the patients’ name, demographics, clinical examinations’ results and outcome.
On static images, PF morphology, severity of periurethral/urethral fibrosis, RTS visualization and position were qualitatively assessed before and after RTS as previously reported [11, 12].
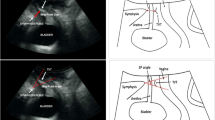
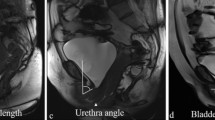
For the assessment of the parameters on dynamic MRI, the pubo-coccygeal line (PCL) served as a reference line connecting the inferior symphyseal border with the last coccygeal joint [13] (Fig. 1a–d). Dynamic images were quantitatively analyzed for the distance between the PCL and the bladder neck (BN) as well as for the distance between the PCL and the EUS (Fig. 1b, d). To quantitatively assess the position of the membranous urethra, the angle between the bladder neck line (BNL) and perpendicular line to the PCL in the BNL area was additionally measured on dynamic MRI and labeled as the angle of the membranous urethra (AMU) (Fig. 1a, c). The BNL was defined as connection of the BN centerline and the membranous urethra. All above-mentioned parameters on dynamic MRI were assessed pre- and postoperatively during a maximum effort Valsalva’s maneuver and voiding.
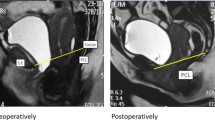
Cine-MRT (single slice TrueFISP) during a maximum of voiding before RTS (a, b) and thereafter (c, d) of patient with preoperative stress urinary incontinence and successful outcome after sling implantation. The distance between the external urinary sphincter (EUS) (arrow) and the pubo-coccygeal reference line (PCL) and the distance between the bladder neck (asterisk) and the PCL are evaluated before (b) and after RTS (d). Membranous urethra angle (arrow) is reduced after RTS (c) compared to it before RTS (a). P stands for the line perpendicular to the PCL and crossing the bladder neck line (BNL), the arrow presents the angle of the membranous urethra. Sagittal (e) and axial (f) T2-weighted images with a visualization of the sling (arrows)
The change of the AMU after RTS was defined as primary outcome criterion. The secondary outcome of the study was to assess whether AMU and/or distances between the BN and PCL or between the EUS and PCL were associated with RTS failure.
Statistical analysis
Sample size was calculated using G*Power statistical power analysis program (v. 3.1.7, free software written by F. Faul, University of Kiel, Germany). Considering results of the previous work [14], the effect size of 2.73, a type 1 error (p < 0.001), and a power of 0.95 required 10 patients for each group. Success rate after RTS was defined as 55 %. Counting a missing value of 50 %, 30 patients needed to be enrolled in the study.
All analyses were performed on the Statistical Package for Social Sciences (SPSS), Version 21. For normal distributed data, paired t test or independent t test were used for differences within the groups or between the groups, respectively. Otherwise, the Wilcoxon signed-rank or the Mann–Whitney test was performed. The intrarater and interrater reliability was assessed using the intraclass correlation coefficient (ICC) and interpreted according to the Fleiss criteria [15]: ICC ≥ 0.75 (rating: excellent); 0.40 ≥ ICC < 0.75 (rating: fair to good); and ICC < 0.40 (rating: poor). A p value of <0.05 (two tailed) was considered as significant.
Results
Characteristics of patients and RTS outcome
Demographics and clinical characteristics of the patients are presented in Table 1. A total of 30 patients were enrolled in the study, with four of them had to be excluded since they were not available for the postoperative MRI examination. The success rate was 58 % (15/26 patients). Of 11 (42 %) patients regarded as RTS failure, 9 (35 %) patients had significant pad reduction (>50 % pad use) and 2 (8 %) patients were unchanged after surgery. Pad weight in the 1-h pad test (p = 0.001) and the number of daily pad use (p = 0.001) of the overall cohort decreased significantly postoperatively (Table 2). The maximal flow rate (p = 806) of the overall cohort did not change significantly (Table 2). The micturition volume of the overall cohort increased significantly (p = 0.022) after RTS (Table 2). The analysis of micturition volume in relation to sling outcome revealed that only patients with RTS success (289 mL ± 81 vs. 342 mL ± 64, p = 0.029) had significant postoperative increase of the micturition volume compared to patients with RTS failure (214 mL ± 23.2 vs. 221 mL ± 23.4, p = 0.443).
There were no significant differences in frequency of RTS failure (p = 0.683) between the patients with severe preoperative SUI (3/9 patients, 33 %) and those with mild or moderate SUI (8/17 patients, 47 %).
ALPP was significantly correlated with the preoperative number of daily pad use (r = −0.73, p = 0.001) and severity of preoperative incontinence (60.2 cmH2O ± 1.7 vs. 67.1 cmH2O ± 7.3, p = 0.001). There were no significant relations between ALPP and the preoperative AMU (r = 0.38, p = 0.069), AMU change (r = −0.33, p = 0.117) or RTS outcome (64.9 cmH2O ± 7.1 vs. 64.5 cmH2O ± 6.7, p = 0.838).
Morphological assessment of PF structures and RTS on static MRI
RTS was indirectly visualized in 11 (48 %) of 26 patients on axial T2-weighted MRI images and in 14 (54 %) of 26 patients on sagittal images (Fig. 1e, f). There were no significant relations between the RTS outcome and RTS visualization or urethral/periurethral fibrosis (Table 3).
PF structures and RTS on functional dynamic MRI
Compared with the preoperative dynamic MRI, there was an elevation of the BN and EUS position in all patients after RTS on qualitative analysis. The quantitative analysis revealed only significant elevation of the EUS (p = 0.017) and no changes in the BN position (p = 0.094) comparing preoperative versus postoperative dynamic MRI of the overall cohort (Table 2). The postoperative lowering of the BN and EUS below the PCL occurred in 4 (27 %) of 15 patients with RTS success and in 9 (82 %) of 11 patients with RTS failure on qualitative analysis. However, this difference was not exceeding the conventional level of statistical significance for the BN (p = 0.576) or EUS (p = 0.3) on quantitative analysis (Table 3).
All patients of the overall cohort had a significantly smaller AMU after RTS during Valsalva’s maneuver (p = 0.025) and voiding (p = 0.001) (Table 2). There were no significant differences in the preoperative AMU during Valsalva’s maneuver (p = 0.119) and voiding (p = 0.331) between the patients with RTS failure and those with RTS success (Table 3).
RTS failure was significantly associated with smaller AMU change after RTS during voiding (p = 0.001) (Table 3). This trend tailed to nonsignificance (p = 0.392) during Valsalva’s maneuver (Table 3). On receiving operating characteristic curve, the cutoff value for AMU changes to distinguish between the patients with RTS success and those with RTS failure was 2.8° (sensitivity = 80 % and 1-specificity = 20 %).
The patients with severe preoperative SUI (9/26 patients) had significantly wider preoperative AMU during Valsalva’s maneuver (43.54° vs. 37.55°, p = 0.044) and voiding (43.57° vs. 35.58°, p = 0.001), significant smaller AMU change (1.33° vs. 4.47°, p = 0.045) during voiding and nonsignificant smaller AMU change during Valsalva’s maneuver (1.56° vs. 3.31°, p = 0.653) compared to patients with moderate and mild preoperative SUI. Of 9 (35 %) patients with severe preoperative SUI, 6 (67 %) patients had success, and 3 (33 %) patients had failure after RTS. The analysis of patients with severe preoperative SUI in relation to sling outcome revealed no significant differences in preoperative AMU during Valsalva’s maneuver (34.5° vs. 38.6°, p = 0.473) and voiding (34.2° vs. 34.4°, p = 0.967), AMU change during Valsalva’s maneuver (0.5° vs. 2.52°, p = 0.786) and voiding (0.5° vs. 2.4°, p = 143) between the patients with RTS failure and those with RTS success.
For AMU, mean intrarater reliability ICCs were 0.96 (95 % CI 0.91, 0.98) pre- and 0.81 (95 % CI 0.57, 0.92) postoperative, respectively. Mean interrater reliability ICCs for AMU evaluated by two examiners were 0.88 (95 % CI 0.73, 0.95) pre- and 0.82 (95 % CI 0.58, 0.92) postoperative, respectively. Mean intrarater and interrater reliability ICCs for distances between PCL and BN or EUS ranged between 0.88 (95 % CI 0.72, 0.94) pre- and 0.93 (95 % CI 0.84, 0.97) postoperatively.
Discussion
Only a few studies have analyzed factors associated with RTS failure [6, 16]. Usable tools for the causal diagnosis of RTS failure are still limited. The present study was conducted to evaluate the RTS outcome and to identify parameters contributing to RTS failure using MRI.
EUS lowering is one of the identified factors contributing to SUI [14]. RTS leads to BN and EUS elevation on qualitative analysis [12]. The qualitative analysis of the present study revealed more frequent postoperative lowering of the BN and EUS in patients with RTS failure compared to those with RTS success. However, this difference did not reach statistical significance on quantitative analysis. Despite the excellent intrarater and interrater reliability of the MRI measurements, quantitative differences of these parameters seem to be very delicate to be related to RTS outcome. The divergent location of the starting point on the EUS to measure these distances may be essential to detect a difference between the RTS success and failure. For this reason, the elevation of the EUS seems to be only useful as parameter for qualitative comparisons between RTS success and failure. Our findings are consistent with those of Suskind et al. [17] describing only little differences without statistical significance in the “mobility” of the BN and urethra at rest and during Valsalva’s maneuver between continent and incontinent men after RP.
Periurethral fibrosis is known as a factor contributing to incontinence [11] due to an impaired sphincter function with loss of sphincter elasticity, closure and contractility. In the present study, no patients presented signs of severe periurethral fibrosis affecting the whole periurethral circumference. The occurrence of mild and moderate periurethral fibrosis was higher in RTS-failed patients. The small number of patients with signs of only mild or moderate periurethral fibrosis in compared groups might be a reason for nonsignificant relations between the periurethral fibrosis and RTS outcome. Apparently, patients without a negative impact of periurethral fibrosis on residual sphincter function may have a benefit from RTS.
In a recent study using 3.0 tesla MRI scanner, Pistolesi et al. [18] reported about a significant association between the sling visualization on sagittal MRI and continence after RTS. Indentation and a clearly visible urethral bulb were the MRI criteria for sling visualization. Considering reported criteria [18], we could clearly visualize RTS not only on sagittal, but also on axial MRI in 11 of 26 patients. We assume that RTS visualization may depend on fibrosis severity in the assessed area and not on the choice of MRI scanner. Since the sling is quite a large structure, it is not necessary to perform MRI on a high-field scanner of 3.0 tesla because sling visualization is also possible on a 1.5 tesla scanner. Furthermore, our data showed no significant association between RTS visualization and its outcome. We propose that sling visualization alone cannot serve for defining a RTS correct position in terms of sufficient relocation and repositioning of the EUS and bulbar urethra to recover continence after RTS.
Based on previous research [14], we evaluated the AMU in the present study as one of the possible functional parameters contributed to SUI. Apparently, the value of the AMU depends on the position of the BNL (Fig. 1a, c), which may be influenced by multiple factors ranging from different pre- and postoperative anatomical PF changes to sling placement characteristics. The current study revealed significant AMU reduction after RTS in all patients at Valsalva’s maneuver and voiding. However, RTS-failed patients presented a significant smaller AMU change during voiding compared to continent patients. The extent of AMU change to more than 2.8° correlated with continence after RTS. The measurement of AMU revealed excellent intrarater and interrater reliability even though the cutoff value of this parameter is rather small.
It has been reported that optimal patient selection for RTS should include mild or moderate SUI [3–5]. However, comparable success rates after RTS were also reported after inclusion of patients with severe SUI [2, 6, 9]. About one-third of patients (35 %) had severe SUI in the present study. However, the selection of patients in our study included no factors known to affect the RTS outcome such as adjuvant radiotherapy or history of nonconservative incontinence therapy. This may explain the consistent success rate of 58 % (15/26 patients) of the present study with the reported data [2, 3]. Severe preoperative incontinence was significantly related to a wider preoperative AMU and smaller postoperative AMU change. In patients suffered from severe preoperative SUI and experienced RTS failure, this trend failed to reach statistical significance, probably, due to a small number of patients in subgroups analyzed.
The change of the AMU could apparently be a sign to what extent the relocation of the membranous urethra due to RTS placement occurred. This factor seems to be one of the relevant factors for RTS success. Obviously, patients with severe preoperative SUI may need a greater length of proximal urethral relocation to regain continence compared to patients with mild or moderate SUI. In this context, the assessment of the preoperative AMU alone is not predictive of RTS failure, but it may be helpful for evaluation of the AMU change and its correlation with the RTS outcome in situations of suspected inadequate relocation of the proximal urethra due to RTS implantation. However, these assumptions are needed to be evaluated in future clinical studies.
The results of the present study should be interpreted considering the limitations. The study was powered only for AMU changes after RTS. The analysis of factors contributing to RTS failure might be underpowered due to a rather small sample size in compared subgroups despite excellent reliability. The drop-off rate of 13 % appears relatively high considering the study sample size. MRI is an expensive tool and not for each urologist available. The use of alternative tools such as voiding cystourethrogram and ultrasonography should be evaluated considering X-ray doses of radiation for patient and ability to visualize the parameters assessed in the present study.
The present study offers a first guide for urologists and radiologists regarding changes of the functional PF anatomy after RTS and factors that might be essential for RTS failure in correlation with clinical parameters.
In conclusion, RTS leads to an AMU reduction and EUS elevation, respectively. A wider preoperative AMU was significantly correlated with severe preoperative incontinence. RTS failure and severe preoperative incontinence were significantly related to only small AMU changes. The extent of the AMU change after RTS placement seems to be a relevant factor for RTS success. Future clinical studies are needed to evaluate whether the AMU change correlates with the length of the membranous urethra relocation during RTS and to assess whether patients with severe preoperative incontinence may have a benefit from a greater relocation of the proximal urethra. Functional MRI is a reliable noninvasive visualization tool of interactions between the RTS and PF for further research on the complex nature of postprostatectomy SUI.
References
Rehder P, Gozzi C (2007) Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol 52(3):860–866. doi:10.1016/j.eururo.2007.01.110
Zuckerman JM, Edwards B, Henderson K, Beydoun HA, McCammon KA (2014) Extended outcomes in the treatment of male stress urinary incontinence with a transobturator sling. Urology. doi:10.1016/j.urology.2013.10.065
Rehder P, Haab F, Cornu JN, Gozzi C, Bauer RM (2012) Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur Urol 62(1):140–145. doi:10.1016/j.eururo.2012.02.038
Rehder P, Webster G (2011) The AdVance® Male Sling: patient selection and workup. Eur Urol Suppl 10(4):390–394. doi:10.1016/j.eursup.2011.04.005
Cornu JN, Sebe P, Ciofu C, Peyrat L, Cussenot O, Haab F (2011) Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: focus on prognostic factors. BJU Int 108(2):236–240. doi:10.1111/j.1464-410X.2010.09765.x
Soljanik I, Gozzi C, Becker AJ, Stief CG, Bauer RM (2012) Risk factors of treatment failure after retrourethral transobturator male sling. World J Urol 30(2):201–206. doi:10.1007/s00345-011-0671-6
Bauer RM, Gozzi C, Hubner W, Nitti VW, Novara G, Peterson A, Sandhu JS, Stief CG (2011) Contemporary management of postprostatectomy incontinence. Eur Urol 59(6):985–996. doi:10.1016/j.eururo.2011.03.020
Bauer RM, Gozzi C, Roosen A, Khoder W, Trottmann M, Waidelich R, Stief CG, Soljanik I (2013) Impact of the ‘repositioning test’ on postoperative outcome of retroluminar transobturator male sling implantation. Urol Int 90(3):334–338. doi:10.1159/000347123
Bauer RM, Mayer ME, Gratzke C, Soljanik I, Buchner A, Bastian PJ, Stief CG, Gozzi C (2009) Prospective evaluation of the functional sling suspension for male postprostatectomy stress urinary incontinence: results after 1 year. Eur Urol 56(6):928–933. doi:10.1016/j.eururo.2009.07.028
Bauer RM, Mayer ME, May F, Gratzke C, Buchner A, Soljanik I, Bastian PJ, Stief CG, Gozzi C (2010) Complications of the AdVance transobturator male sling in the treatment of male stress urinary incontinence. Urology 75(6):1494–1498. doi:10.1016/j.urology.2009.12.012
Paparel P, Akin O, Sandhu JS, Otero JR, Serio AM, Scardino PT, Hricak H, Guillonneau B (2009) Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol 55(3):629–637. doi:10.1016/j.eururo.2008.08.057
Soljanik I, Bauer RM, Becker AJ, Stief CG, Gozzi C, Soljanik O, Kirchhoff SM (2013) Morphology and dynamics of the male pelvic floor before and after retrourethral transobturator sling placement: first insight using MRI. World J Urol 31(3):629–638. doi:10.1007/s00345-012-0884-3
Lienemann A, Sprenger D, Janssen U, Grosch E, Pellengahr C, Anthuber C (2004) Assessment of pelvic organ descent by use of functional cine-MRI: which reference line should be used? Neurourol Urodyn 23(1):33–37. doi:10.1002/nau.10170
Soljanik I, Bauer RM, Becker AJ, Stief CG, Gozzi C, Solyanik O, Brocker KA, Kirchhoff SM (2014) Is a wider angle of the membranous urethra associated with incontinence after radical prostatectomy? World J Urol. doi:10.1007/s00345-014-1241-5
Fleiss J (1981) Statistical methods for rates and proportions, 2nd edn. Wiley, New York
Warner JN, Grimsby GM, Tyson MD, Wolter CE (2012) Bladder capacity on preoperative urodynamics may impact outcomes on transobturator male slings. Neurourol Urodyn 31(7):1124–1127. doi:10.1002/nau.22233
Suskind AM, DeLancey JO, Hussain HK, Montgomery JS, Latini JM, Cameron AP (2014) Dynamic MRI evaluation of urethral hypermobility post-radical prostatectomy. Neurourol Urodyn 33(3):312–315. doi:10.1002/nau.22408
Pistolesi D, Zampa V, Gozzi C, Mariani C, Santarsieri M, Faggioni L, Bartolozzi C, Selli C (2014) Could the sling position influence the clinical outcome in male patients treated for urinary incontinence? A magnetic resonance imaging study with a 3 Tesla system. Urology 83(2):471–476. doi:10.1016/j.urology.2013.10.024
Conflict of interest
Ricarda M. Bauer and Christian Gozzi declare lectures, consultancy work and participation in clinical trials for AMS. All other authors declare that no funding or other agreement has limited their ability to fairly complete and publish this data, and they had full control of the primary data. There has been no extra-institutional funding for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soljanik, I., Solyanik, O., Stief, C.G. et al. The extent of changes in the membranous urethra angle is associated with the outcome of retrourethral transobturator sling procedure. Int Urol Nephrol 47, 249–255 (2015). https://doi.org/10.1007/s11255-014-0888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0888-6