Abstract
Urbanisation affects not only large ecosystems but also small ones, such as ponds, through changes in environmental parameters. It consequently impacts the biodiversity of all organisms, including zooplankton. However, disturbances due to urbanisation may have different levels of impact on ecosystems. We therefore aimed to determine how different degrees of disturbance and environmental parameters affect zooplankton species diversity and which zooplankton species could indicate the disturbance degree and water quality of tropical urban ponds. We recorded 63 species, namely, 46 species of rotifers and 17 species of cladocerans. The overall species diversity tended to decrease from the low to the high disturbance areas. The level of disturbance, temperature, salinity, phycocyanin and vegetation affected the zooplankton species composition. More common species were found in the low disturbance areas, and among the few species in the highly disturbed areas, the distribution was more specific. We therefore propose that rotifer and cladoceran species be used as bioindicators to indicate water quality and the degree of disturbance. Our study provides significant insights into the relationship between zooplankton and environmental factors in oriental tropical regions and presents a framework for identifying crucial species associated with levels of disturbance, particularly in the habitats of Oriental Asia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Zooplankton diversity and species-environment variations in tropical urban freshwater ponds.
Introduction
Biodiversity is influenced by anthropogenic disturbances in several ways, and these events are a leading cause of biodiversity decline throughout the world (McMichael 2000). Such disturbances, especially in the form of urbanisation, have been growing due to the ever-increasing human population, with more than half of all people currently living in urban areas (Ritchie and Roser 2018). Such rapid urbanisation often results in habitat modification and/or destruction, a decrease in native flora and fauna biodiversity and/or a rise in non-native species numbers (McMichael 2000; McKinney 2002, 2006, 2008; Kondratyeva et al. 2020; Hou et al. 2023).
Freshwater ecosystems, such as lakes, ponds and rivers, constitute only 1% of the available fresh water yet harbour very high levels of biodiversity (Strayer and Dudgeon 2010). Urbanisation affects freshwater habitats by way of degradation, rising temperatures, acidification, eutrophication and overexploitation, to name a few impacts (Saulnier-Talbot 2016; Geist and Hawkins 2016; Cantonati et al. 2020). Research has shown that these factors contribute significantly to the decline in the taxonomic as well as the functional richness and diversity of various organisms across different taxa in all types of freshwater habitats (Moyle and Leidy 1992; Biswas and Mallik 2010 et al. 2019; Feisal et al. 2023). In many instances, rare species are lost following high levels of anthropogenic disturbance and are replaced by generalist and/or non-native species (Leita ̃o et al. 2016; Vincent et al. 2020).
Smaller lentic water bodies, such as ponds, are more numerous and widespread globally than lakes and reservoirs. Despite their small size, they contribute significantly to regional biodiversity (De Meester et al. 2005). However, these small water bodies tend to be more susceptible to disturbances than larger ones (Lepori and Hjerdt 2006). Due to their numbers, the higher connectivity of such small water bodies with the surrounding terrestrial ecosystem makes them particularly vulnerable to growing land use pressures and environmental change (Riley et al. 2018).
Zooplankton are an important component of freshwater ecosystems and play significant roles as primary consumers and/or secondary producers (Lomartire et al. 2021). They react to environmental variations via changes in their composition, richness, abundance and distribution. (Athira et al. 2022; Du et al. 2023). Substantial evidence has been generated to indicate the effects of urbanisation on zooplankton diversity patterns, especially in large water bodies like lakes, reservoirs and rivers (Pecorari et al. 2006; Razak and Sharip 2019; Shen et al. 2021). Kuczynska-Kippen (2020) showed that ponds with low levels of human disturbance generally harbour richer zooplankton communities than highly disturbed ones. Studies on temperate zooplankton communities have shown that urbanisation-driven temperature increases in ponds cause shifts in their composition, with larger species being filtered out. In addition, urban ponds tend to select for generalist species with widespread distributions, which suggests biotic homogenisation (Engelen et al. 2017).
Limited research exists on the effects of anthropogenic disturbances on freshwater diversity in the tropical regions of Asia (Bannister et al. 2019) even though these geographic regions are experiencing more extensive environmental changes than any others (Dudgeon 2000). Findings from the available literature suggest a decrease in the taxonomic and/or functional diversity of organisms such as zooplankton and aquatic insects in urban reservoir, urban rivers and rice fields due to increasing disturbance (Liu et al. 2020; Kulkarni and Padhye 2021; Padhye and Dahanukar 2015; Plangklang and Athibai 2021; Eriksen et al. 2021).
Given this background, we studied the effects of anthropogenic disturbances on zooplankton communities in tropical ponds. Specifically, we addressed the following questions: (1) How does species diversity (alpha and beta) change from ponds with low levels of disturbance to those that are highly disturbed? (2) What are the species–environment associations in these habitats, and which environmental variables are significantly correlated with species distribution? (3) Which species or groups of species have potential as local bioindicators of highly disturbed habitats? Our research contributes to a better understanding of the zooplankton ecology of ponds in the less studied oriental tropical regions and their feasibility as bioindicators of disturbance.
Materials & methods
Sampling sites and environmental parameters measurements
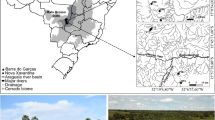
Samples were collected from eight freshwater ponds in an urban area of Nakhon Nayok Province, Central Thailand, every three months between July 2018 and July 2019. The environmental measurements taken in the field included water temperature (°C), conductivity (µs cm− 1), total dissolved solids (mg L− 1), salinity (ppt), dissolved oxygen (mg L− 1), chlorophyll a (µg L− 1), phycocyanin (mg L− 1), NO3-N (mg L− 1) and pH using a calibrated multiparameter Sonde (YSI EXO Multiparameter Sonde and YSI EXO Handheld Display 599,150). Each pond was categorised into three disturbance levels by disturbance score including low disturbance ponds (scored 2.40–3.00), moderate disturbance ponds (scored 1.70–2.30) and high disturbance ponds (scored 1.00–1.60). These disturbance scores were determined from the character of the substrates, sediment deposition, percent cover of vegetation, water fluctuation and number and intensity of human activities, according to the field data sheet provided by Resh and Giap (2010). The disturbance levels and environmental parameters of each sample are presented in supplementary Table 1. In addition, we performed a Principal Component Analysis (PCA) using the environmental variables to check the distribution of the ponds with respect to the environmental data. The analysis was carried out using ‘prcomp’ function in R (with scaling). Visualization of the plot was done using the ‘ggfortify’ package. The ponds were color coded as per their designated disturbance categories. It was observed that the first 2 PCA axes explained > 70% of the total variation and the ponds did separate out to a large extent based on the environmental data (Supplementary Tables 11–12; supplementary Fig. 4).
Sampling, sorting and examination
A total of 40 samples was quantitatively sampled by filtering 20 L of water through a 20 μm mesh-size plankton net. All the samples were immediately preserved with 95% ethyl alcohol. Rotifers and cladocerans were identified using complete and up-to-date publications relevant to each group (i.e. Koste and Shiel 1989, 1990; Nogrady et al. 1995; Segers 2007; Segers et al. 1996; Sanoamuang 2002; Shiel and Koste 1992; Shiel and Sanoamuang 1993 for rotifers and Korovchinsky 1992; Smirnov 1992,1996; Sinev 2016; Van Damme et al. 2011 for cladoceran.)
Data analysis
Alpha diversity
The Shannon diversity for each sample was calculated using the Hellinger-transformed species abundances of both rotifers and cladocerans in the ‘vegan’ package in R. We tested the effects of increasing disturbance (as the categorical independent fixed effects variable with three levels) on the alpha diversity (continuous response variable) using linear mixed effects models. The season was included as a random effect in the model, as it is known to influence zooplankton diversity (Harris et al. 2000). We also checked whether pond identity as a covariate would better explain the variations in the alpha diversity. The model comparison showed that pond identity was not significant (supplementary Table 2), and it was thus removed from further analyses. The models were fitted using the ‘lme4’ (Bates et al. 2015) package in R. The assumption of heterogeneity of variance was checked using Levene’s test (supplementary Table 3), while the model assumptions were assessed visually by histograms (distribution) of the residuals. The residual plots of the models (supplementary Figs. 2–3) for both groups showed deviations from normality. We therefore used a permutation-based approach to check the significance of the fixed effects using the ‘permanova.lmer’ function from the ‘predictmeans’ package (Luo et al. 2022). The models were run with 999 permutations, and the F value was estimated using Satterthwaite’s method (default). The significance of the random effect was checked via model comparison, and the best fit model was selected based on the Bayesian information criterion (BIC) values obtained using the ‘anova’ function from the ‘stats’ package in R.
Beta diversity
The overall beta diversity of both the rotifers and cladocerans between all the pond samples was calculated using the ‘abundance-based multiple-site dissimilarities’ on the Hellinger-transformed abundances from the βpart package in R (Baselga et al. 2012).
Permutation-based multivariate ANOVA (one‐way PERMANOVA) was carried out to determine any significant differences in both the rotifer and cladoceran species communities found in the three levels of disturbance. We used Bray–Curtis dissimilarities to obtain the distance matrix for the analysis. The significance was tested by running 5000 permutations with an alpha of 0.05. We used the function ‘adonis2’ from the vegan package in R for PERMANOVA. We used the sampled season as a block variable in the analysis. The homogeneity of dispersion was checked prior to actual test using the ‘betadisper’ function in the vegan package (supplementary Table 4).
Phi index of association
We used the Phi index of association to assess the unique associations of both the rotifers and cladocerans with the disturbance levels (De Cáceres and Legendre 2009) using the ‘multipatt’ function from the ‘indicspecies’ package in R. The ‘r.g’ value for the ‘func’ argument of the ‘multipatt’ function was used to account for the unequal sample size at each level of the disturbance level. A total of 4999 permutations were run to obtain the significance of association of each species with any of the three disturbance groups using an alpha of 0.05.
Species–environment associations
The species distributions with respect to the local environmental variables of all the pond samples from all three disturbance levels (supplementary Tables 5, 10) were assessed via canonical correspondence analysis (CCA) (Braak and Verdonschot 1995) using the vegan package in R. We removed highly collinear environmental variables (cutoff > 0.85) from the analysis using the ‘caret’ package in R (see supplementary Table 6 for the correlation values). The Hellinger-transformed species abundance data from all the samples along with the final environmental variables were used to build the model. The collinearity in the environmental variables was further assessed by observing the variance inflation criterion (vic) values using the ‘vic.cca’ function of the vegan package (cutoff > 5). The significance of (a) the overall model and (b) the individual CCA axes (supplementary Tables 7–8) was assessed using the ‘anova.cca’ function from the vegan package with 4999 permutations to obtain the p values. The correlation of each environmental variable with the first two CCA axes was also calculated using the scores function in vegan (supplementary Table 9).
Results
Faunistic summary
A total of 63 species were observed in the 40 samples collected from the studied region, with rotifers comprising 46 species and cladocerans 17. The overall zooplankton species richness decreased with increasing disturbance (low disturbance: 51 species; moderate disturbance: 44 species; high disturbance: 16 species). This pattern was also consistent for the individual zooplankton groups as well as the average species numbers per sample (rotifers – low disturbance: nine species, moderate disturbance: six species, high disturbance: four species; cladocerans – low disturbance: two species, moderate disturbance: one species, high disturbance: one species) (Fig. 1A). Lecane was the most diverse genus of rotifer (12 species), while Chydorus, Macrothrix and Diaphanosoma were the three most diverse genera comprising two species each. In addition, Lecane bulla, Polyarthra sp., Trochosphaera aequatorialis and Diaphanosoma excisum were found in every studied pond (Table 1).
The Shannon diversity similarly decreased from the low (rotifers: 1.59 ± 0.71; cladocerans: 0.49 ± 0.48) to moderate (rotifers: 1.40 ± 0.57; cladocerans: 0.10 ± 0.28) to high disturbance ponds (rotifers: 0.88 ± 0.59; cladocerans: 0.13 ± 0.30) (Fig. 1B). The differences in Shannon diversity of the rotifers were significant between the three types of ponds, whereas they were marginally significant in the case of the cladocerans (Table 2). The model comparison also showed that seasonality had no significant effect in explaining the variation in Shannon diversity (Table 2).
The overall beta diversity between the species communities was large for both the rotifers (0.98) and cladocerans (0.95). Species like Lecane bulla, Polyarthra sp., Trichocerca similis, Anthalona harti and Chydorus eurynotus were found in the low disturbance ponds, while Filinia longiseta, Brachionus forficula, Ceriodaphnia cornuta and Moina micrura were characteristically found in the high disturbance ponds. PERMANOVA revealed that these differences were significant in both the rotifer and cladoceran communities (Table 3).
The CCA described the significant variations in the species communities based on the local environmental variables (χ2 = 1.72, F = 1.39, p = .002). The first two axes explained 69% of the total variance (Fig. 2), with the first axes being significant (see supplementary Table 7 for the significance of the individual CCA axes). The low and high disturbance ponds could be clearly separated based on the environmental data, with moderately disturbed ponds lying roughly between the other two groups. Temperature, salinity and phycocyanin were important environmental variables associated with the first axis and had higher positive correlations with many of the high disturbance ponds, while aquatic vegetation was clearly associated with the ponds with low disturbance (supplementary Table 8). Many rotifer species and all the chydorids and macrothricids (scrapers) seemed to be associated with the low/moderate disturbance ponds that had aquatic vegetation (Figs. 3 and 4). Species like Filinia longiseta, F. novaezealandiae and Trochosphaera aequatorialis were positively associated with higher values of salinity, temperature and phycocyanin, while species like Platyias quadricornis, Lecane luna and L. papuana were negatively correlated with those environmental variables. Most members of Lecane (except L. luna and L. papuana ) were associated with lower values of salinity, temperature and phycocyanin as opposed to Filinia, for which most of the species (except F. opoliensis) occurred in ponds that had values of the same environmental variables. Meanwhile, the Brachionous species were seen on both the sides of this gradient. Cladoceran planktonic filterers, such as Ceriodaphnia cornuta, Moina micrura and Pseudosida bidentata, were seen in the high disturbance ponds. In contrast, their congeners, namely, Chydorus reticulaus and Moinodaphnia macleayi, were associated with the low disturbance ponds.
A total of four rotifers and three cladocerans each showed a significant association with a single level of disturbance. The correlations ranged from moderate to weak, with the highest value of 0.53 for Filinia novaezealandiae and 0.41 for Moina micrura (Table 4; Figs. 3 and 4).
Discussion
We observed a characteristic decrease in zooplankton richness and species diversity with increasing anthropogenic disturbance, with a significant difference in species communities between the low and high disturbance ponds. The effects of anthropogenic disturbance by way of the modification or destruction of habitats affects the diversity of organisms across different phyla (e.g. benthic communities – Dudgeon et al. 2006; macroinvertebrates – Nichols et al. 2016; nanoperiphytic algae – Dunck et al. 2019; insects – Husseini et al. 2019; benthic macroinvertebrates – Sripanya et al. 2022; Gecko –Martín et al. 2023), and our results are in line with the findings of studies in other regions of the world (e.g. Kuczynska-Kippen 2020; Qin et al. 2020; Shen et al. 2021). The species numbers in our study were also lower than those of other types of habitats in Thailand (streams – Sa-ardrit and Beamish 2005; swamps – Maiphae et al. 2008) as well as urban ponds in other (sub) tropics (Phan et al. 2021; Shen et al. 2021).
Species diversity differences in the cladocerans were not as apparent as those observed in the rotifers (Table 2). Occurrences of nearly 50% of the rotifers and 80% of the cladocerans were rare (occurrences of three samples and less compared to total samples). Nevertheless, the cladoceran communities in all three types of ponds commonly consisted of species like Moina micrura and Ceriodaphnia cornuta alongside the rare species and had relatively similar (and high) densities (150–1,866 individuals/L) in the moderate and high disturbance ponds, with lower densities in the low disturbance ponds. However, a clear difference in alpha diversity was seen in the rotifers due to the higher species numbers, larger differences in abundance between the species and the characteristic presence of some rare species (e.g. Lecane signifera, L. ludwigii and Manfredium sp.) only in the low disturbance ponds. Additionally, strongly competitive interactions among rotifers in their natural environments can control the coexistence or exclusion of species (DeMott and Kerfoot 1982; Negreiros et al. 2010). The food niches of rotifers are also more specialised than those of cladocerans (Bogdan and Gilbert 1982). These characteristics could further explain the apparent patterns of Shannon diversity in the rotifers in this study.
Changes in diversity determine community stability by influencing the response to disturbances and/or environmental fluctuations (Hughes 2010). The disappearance of rare species due to increasing disturbance affected the zooplankton species communities and was reflected in the high beta diversity values between the three pond types. Rare species are also sensitive to sudden changes in the local environment (Leita ̃o et al. 2016), and their loss with increasing disturbances is well documented in many organisms (Floren et al. 2001; Dudgeon 2006; Alroy 2017; Dunck et al. 2019; Sripanya et al. 2022). Anthropogenic disturbances modify freshwater ecosystems by way of physical (desiltation, the removal of vegetation), chemical (the addition of nutrients) and biological (invasive species) factors (Candolin and Rahman 2023). Biological assemblages are shaped by such habitat stressors (e.g. eutrophication) acting as templates (Townsend and Hildrew 1994), which can prevent the colonisation of species lacking some biological traits (e.g. morphological and physiological). We noticed a clear change in some of these factors between the three pond types, especially in the case of the highly disturbed ponds that had been affected by eutrophication and agricultural activities. Eco-evolutionary changes occurring at the morphological and physiological levels in response to anthropogenic changes may drive species/population selection even further (Alberti et al. 2017; Catullo et al. 2019). Studies have shown that certain cladocerans and rotifers adapt very rapidly to increases in water temperature by changing some life history and physiological traits (Brans et al. 2017; Wenjie et al. 2019). A significant correlation of Filinia novaezealandiae, Trochosphaera aequatorialis and Moina micrura to highly disturbed ponds suggests such changes occur as a response to additional disturbances in these species; however, we did not specifically study this phenomenon.
Most moderate and high disturbance ponds showed higher temperatures and phycocyanin and salinity values with no/less aquatic vegetation. Higher water temperatures directly affect the biotic and abiotic aspects of freshwater ecosystems, and an anthropogenic-mediated temperature rise can lead to decreases in freshwater biodiversity and influence the species distribution and survival probabilities of many freshwater organisms (Clarke 2009; Hieno et al. 2009; Ahmed et al. 2022). Thermal stress can also affect the physiological and biochemical processes of freshwater organisms and boost the bioaccumulation of chemicals in their body tissues, ultimately leading to higher chemical toxicity (Pajk et al. 2017). Increasing eutrophication can be proportional to rising temperatures, which can lead to rapid algal growth (Dunck et al. 2015; Gatti 2016; Schobben et al. 2016). The chances of cyanobacteria outcompeting other algae also increase with increasing temperatures (Ahmed et al. 2022). Moreover, nutrient enrichment causes harmful cyanobacterial blooms, which can lead to the release of toxins along with a decrease in primary producers due to decreased light penetration (Romanowska-Duda et al. 2002; Frumin and Gildeeva 2014; Lind et al. 2018). Although we did not measure the water transparency, the very high eutrophic water bodies were turbid with the presence of cyanobacteria and the absence of aquatic vegetation. Human-mediated disturbances can cause the salinisation of freshwater habitats (Velthuis et al. 2023). Salinity tolerance varies in aquatic organisms, but higher values can certainly affect freshwater biodiversity. Studies have even linked higher concentrations with algal blooms (which we also observed in the high disturbance ponds) (e.g. Conley et al. 2009; Paerl and Paul 2012; Lind et al. 2018). An increase in salinity can alter the food web interactions in freshwater systems at every level and negatively affect organisms like zooplankton (Lind et al. 2018). Higher salinity may significantly reduce fecundity and result in developmental delays as well as a decrease in the growth rate in cladocerans, especially in non-adapted daphniid populations (Goncalves et al. 2007). More than 70% of the cladoceran species in our study were non-daphniid (Table 1). In addition, salinity can impact aquatic vegetation, which is associated with increased species richness among zooplankton (Nielsen et al. 2003).
In this study, rotifer species like Lecane curvicornis and Trichocerca similis, all the scraper-cladocerans (cladoceran capable of ‘scraping’ the substrate for food), such as Coronatella monancantha, Chydorus eurynotus and C. reticulatus, and the benthic/meio-benthic Ilyocryptus thailandensis were associated with the low disturbance ponds with aquatic vegetation (and lower temperatures and phycocyanin values). Aquatic vegetation provides a more diverse environment and offers a rich food source for many zooplankton species, as well as an efficient refuge area from predators (Stansfield et al. 1997; Choedchim et al. 2017). Species like Ceriodaphnia cornuta, Moina micrura, and Filinia novaezealandiae were highly abundant, even in the ponds with high levels of disturbance. Species from the cladoceran genus Moina, rotifer genus Filinia and species like Trochosphaera aequatorialis and Ceriodaphnia cornuta are notably tolerant to water pollution (Edmondson 1959; Kumar and Kiran 2016; Sharaf et al. 2023). Moina micrura, in particular, is known to occur in habitats that (a) are eutrophic/disturbed and (b) have high conductivity (~ 8,899.53 µS/cm) and total dissolved solids values (~ 5,197 mg/L), while Ceriodaphnia cornuta is a dominant species in some water bodies with heavy cyanobacteria blooms (Kumar and Kiran 2016; Gu et al. 2020; Padhye 2020). M. micrura was found in the highly disturbed ponds with a conductivity of up to 3,895 µS/cm and total dissolved solids of up to 1,994.67 mg/L in our study. Some of these species showed a moderate yet significant correlation with low and high disturbance ponds, which highlights the need for more focused research on their role as local bioindicators.
Freshwater ecosystems in the tropics, in particular, differ from those in cooler climates. For example, they are more sensitive to increases in nutrient availability due to more efficient nutrient recycling combined with higher ambient temperatures and more solar radiation stability at or near the equator (Lewis 1996). Management strategies developed for temperate ecosystems cannot be directly transferred to tropical ecosystems. A study on tropical freshwater ecosystems is therefore necessary to understand the changes in organisms found in tropical water bodies.
Conclusions
Environmental factors and habitat disturbances, especially urbanisation, in tropical Asia tend to influence species biodiversity, as they do in temperate regions. In the present case, pH, salinity and the level of disturbance were the most important factors affecting species diversity and composition. Of these, species diversity tended to decrease from the low to the high disturbance habitats. In addition, the distribution of the majority of the species found in the high disturbance areas was restricted, whereas the majority of the species found in the low disturbance areas with highly vegetated areas were widely distributed. As a result, we posit that some species including Trichocerca similis, Lecane curvicornis, Filinia novaezealandiae, Trochosphaera aequatorialis, Chydorus eurynotus, Anthalona harti and Moina micrura have the potential to be bioindicators of water quality and the degree of disturbance. The results of our study provide important insights into the relationship between zooplankton and environmental conditions in oriental tropical areas and offer a framework for identifying key species related to disturbance levels, especially habitats in Oriental Asia. Further experiments on these species to determine their tolerance to environmental changes and different levels of disturbance are warranted.
References
Ahmed SF, Kumar PS, Kabir M, Zuhara FT, Mehjabin A, Tasannum N, Hoang AT, Kabir Z, Mofijur M (2022) Threats, challenges and sustainable conservation strategies for freshwater biodiversity. Environ Res 214:113808. https://doi.org/10.1016/j.envres.2022.113808
Alberti M, Marzluff J, Hunt VM (2017) Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos Trans R Soc 372:20160029. https://doi.org/10.1098/rstb.2016.0029
Alroy J (2017) Effects of habitat disturbance on tropical forest biodiversity. PNAS 114:6056–6061. https://doi.org/10.1073/pnas.1611855114
Athira TR, Nefla A, Shifa CT, Shamna H, AlMaarofi SS, Rashiba PA, Reshi OR, Jobiraj T, Thejass P, Muzaffar BS (2022) The impact of long-term environmental change on zooplankton along the southwestern coast of India. Environ Monit Assess 194:1–15. https://doi.org/10.1007/s10661-022-09921-w
Bannister W, McGowan S, Adelina C, Quak SBJ, Fong SL, Mendoza M, Papa SDR, Taylor D (2019) Potential anthropogenic regime shifts in three freshwater lakes in Tropical East Asia. Freshw Biol 64:708–722. https://doi.org/10.1111/fwb.13256
Baselga A, David C, Orme L (2012) Betapart: an R package for the study of beta diversity methods Ecol. Evol 3(5). https://doi.org/10.1111/j.2041-210X.2012.00224.x
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Biswas SR, Mallik AU (2010) Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecol 91:28–35. https://doi.org/10.1890/08-0887.1
Bogdan KG, Gilbert JJ (1982) Seasonal patterns of feeding by natural populations of Keratella, Polyarthra and Bosmina. Clearance rates, selectivity and contributions to community grazing. Limnol Oceanogr 27:918–934. https://doi.org/10.4319/lo.1982.27.5.0918
Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and relate multivariate methods in aquatic ecology. Aquat Sci 57:256–289. https://doi.org/10.1007/BF00877430
Brans KI, Govaert L, Engelen JMT, Gianuca AT, Souffreau C, De Meester L (2017) Eco-evolutionary dynamics in urbanized landscapes: evolution, species sorting and the change in zooplankton body size along urbanization gradients. Royal Soc 372:20160030. https://doi.org/10.1098/rstb.2016.0030
Candolin U, Rahman T (2023) Behavioural responses of fishes to anthropogenic disturbances: adaptive value and ecological consequences. J Fish Biol 1–11. https://doi.org/10.1111/jfb.15322
Cantonati M, Poikane S, Pringle CM, Stevens LE, Turak E, Heino J, Richardson JS, Bolpagni R, Borrini A, Cid N, Iková MC, Galassi DMP, Hájek M, Hawes I, Levkov Z, Flores LN, Saber AA, Cicco MD, Fiasca B, Hamilton PB, Cka JK, Segadelli S, Znachor P (2020) Characteristics, main impacts, and stewardship of Natural and Artificial Freshwater environments: Biodivers. Conserv Water 12:260. https://doi.org/10.3390/w12010260
Catullo RA, Llewelyn J, Phillips BL, Moritz CC (2019) The potential for Rapid Evolution under Anthropogenic Climate Change. Curr Biol 29(19):R996–R1007. https://doi.org/10.1016/j.cub.2019.08.028
Choedchim W, Van Damme K, Maiphae S (2017) Spatial and temporal variation of Cladocera in a tropical shallow lake. J Limnol 53:233–252. https://doi.org/10.1051/limn/2017006
Clarke SJ (2009) Adapting to climate change: implications for freshwater biodiversity and management in the UK. Rev Aquac 2(1):51–64. https://doi.org/10.1608/FRJ-2.1.3
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens C GE (2009) Controlling eutrophication: nitrogen and phosphorus. sci 323:1014–1015. https://doi.org/10.1126/science.1167755
De Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecol 90:3566–3574. https://doi.org/10.1890/08-1823.1
De Meester L, Declerck S, Stoks R, Van De Louette G, De Bie T, Michels E, Brendonck L (2005) Ponds and pools as model systems in conservation biology,ecology and evolutionary biology. Aquat Conserv 15:715–725. https://doi.org/10.1002/aqc.748
Demott WR, Kerfoot WC (1982) Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecol 63:1949–1966. https://doi.org/10.2307/1940132
Du C, Zhao F, Shang G, Wang L, Jeppesen E, Zhang L, Zhang W, Fang X (2023) Ammonia influences the Zooplankton assemblage and Beta diversity patterns in complicated Urban River ecosystems. Water 15:1449. https://doi.org/10.3390/w15081449
Dudgeon D (2000) Conservation of freshwater biodiversity in oriental Asia: constraints, conflicts, and challenges to science and sustainability. Limnol 1:237–243. https://doi.org/10.1007/s102010070012
Dudgeon D (2006) The impacts of human disturbance on stream benthic invertebrates and their drift in North Sulawesi. Indonesia Freshw Biol 51:1710–1729. https://doi.org/10.1111/j.1365-2427.2006.01596.x
Dunck B, Lima-Fernandes E, Cássio F, Cunha A, Rodrigues L, Pascoal C (2015) Responses of primary production, leaf litter decomposition and associated communities to stream eutrophication. Environ Pollut 202:32–40. https://doi.org/10.1016/j.envpol.2015.03.014
Dunck B, Felisberto SA, Nogueira IS (2019) Effects of freshwater eutrophication on species and functional beta diversity of periphytic algae. Hydrobiologia 837:195–204. https://doi.org/10.1007/s10750-019-03971-x
Edmondson WT, Ward HB (1959) Fresh-water Biology. Wiley, New York
Engelen J, De Meester L, Brendonck L (2017) The impact of urbanization on ponds and their zooplankton communities. PhD thesis. Leuven University
Eriksen TE, Friberg N, Brittain JE, Søli G, Ballot A, Årstein-Eriksen E, Blakseth TA, Braaten HFV (2021) Ecological condition, biodiversity and major environmental challenges in a tropical river network in the Bago District in South-central Myanmar: first insights to the unknown. Limnologica 86:125835. https://doi.org/10.1016/j.limno.2020.125835
Feisal NAF, Kamaludin NH, Sani MFA, Ahmad AKD, Ahmad AM, Razak NFA, Ibrahim TNBT (2023) Anthropogenic disturbance of aquatic biodiversity and water quality of an urban river in Penang, Malaysia. Water Sci Eng 1–9. https://doi.org/10.1016/j.wse.2023.01.003
Floren A, Freking A, Biehl M, Linsenmair KE (2001) Anthropogenic disturbance changes the structure of arboreal tropical ant communities. Ecography 24(5):547–554. https://doi.org/10.1111/j.1600-0587.2001.tb00489.x
Frumin GT, Gildeeva IM (2014) Eutrophication of water bodies—A global environmental problem. J Gen Chem 84:2483–2488. https://doi.org/10.1134/S1070363214130015
Gál B, Sziváka I, Heinoc J, Schmera D (2019) The effect of urbanization on freshwater macroinvertebrates – knowledge gaps and future research directions. Ecol Indic 104:357–364. https://doi.org/10.1016/j.ecolind.2019.05.012
Gatti RC (2016) Freshwater biodiversity: a review of local and global threats. Int J Environ Stud 73:1–18. https://doi.org/10.1080/00207233.2016.1204133
Geist J, Hawkins SJ (2016) Habitat recovery and restoration in aquatic ecosystems: current progress and future challenges. Aquat Conserv : Mar Freshw 26:942–962. https://doi.org/10.1002/aqc.2702
Goncalves AMM, Castro BB, Pardal MA, Gonc¸alves F (2007) Salinity effects on survival and life history of two freshwater cladocerans (Daphnia magna and Daphnia longispina). Limnol 43:13–20. https://doi.org/10.1051/limn/2007022
Gu L, Qin S, Lu NA, Zhao Y, Zhou Q, Zhang LU, Sun Y, Huang Y, Lyu K, Yang Z (2020) Daphnia mitsukuri traits responding to predation cues alter its population dynamics Ecol. Indic 117:106587. https://doi.org/10.1016/j.ecolind.2020.106587
Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (2000) ICES Zooplankton Methodology Manual. Academic press
Hou Y, Li J, Li G, Qi W (2023) Negative effects of urbanization on plants: a global meta-analysis. Ecol Evol 13:1–9. https://doi.org/10.1002/ece3.9894
Hughes A (2010) Disturbance and diversity: an ecological chicken and Egg Problem. Nat Sci Educ 3(10):48
Husseini R, Abubakar A, Nasare LI (2019) Effect of anthropogenic disturbances on insect diversity and abundance in the sinsablegbini forest reserve, Ghana. UDSIJD 6(3):24–33. https://doi.org/10.47740/388.UDSIJD6i
Kondratyeva A, Knapp S, Durka W, Kühn I, Vallet J, Machon N, Martin G, Motard E, Grandcolas P, Pavoine S (2020) Urbanization effects on Biodiversity revealed by a two-scale analysis of Species Functional Uniqueness vs. Redundancy Front Ecol Evol 8:1–16. https://doi.org/10.3389/fevo.2020.00073
Korovchinsky NM (1992) Sididae and Holopediidae (Crustacea: Daphniiformes). In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; SPB Academic Publishing: The Hague, The Netherlands, Volume 3, pp. 1–82
Koste W, Shiel RJ (1989) Rotifera from Australian inland waters III: Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta).Trans. R. Soc. S. Aust. 113: 85–114
Koste W, Shiel RJ (1990) Rotifera from Australian inland waters V. Lecanidae (Rotifera: Monogononta). Trans R Soc S Aust 114:1–36
Kuczynska-Kippen N (2020) Response of Zooplankton indices to Anthropogenic pressure in the Catchment of Field ponds. Waters 12:758. https://doi.org/10.3390/w12030758
Kulkarni MR, Padhye SM (2021) Does habitat restoration disturb? A case study of a shallow urban water reservoir in western India using cladoceran zooplankton. bioRxiv. https://doi.org/10.1101/2021.06.18.448979
Kumar KH, Kiran BR (2016) A report on diversity of cladocera in sewage fed tank of Bhadravathi taluk. Karnataka Int J Fauna Biol 3(2):18–20
Leita ̃o RP, Zuanon J, Villeger S, Williams SE, Baraloto C, Fortunel C, Mendonca FP, Mouillot D (2016) Rare species contribute disproportionately to the functionalstructure of species assemblages. Proc Biol Sci 283:1–9. https://doi.org/10.1098/rspb.2016.0084
Lepori F, Hjerdt N (2006) Disturbance and aquatic biodiversity: reconciling contrasting views. Bioscience 56(10):809–818. https://doi.org/10.1641/0006-3568(2006)56[809:DAABRC]2.0.CO;2
Lewis WMJ (1996) Tropical lakes: How latitude makes a difference. In: Schiemer F, Boland KT (eds) Perspectives in Tropical Limnology. 43–64
Lind L, Schuler MS, Hintz WD, Stoler AB, Jones DK, Mattes BM, Relyea RA (2018) Salty fertile lakes: how salinization and eutrophication alter the structure of freshwater communities. Eosphere 9 9(9). https://doi.org/10.1002/ecs2.2383
Liu P, Xu S, Lin J, Li H, Lin Q, Han BP (2020) Urbanizatio nincreases biotic homogenization of zooplankton communities in tropical reservoirs. Ecol Indic 110:1–10. https://doi.org/10.1016/j.ecolind.2019.105899
Lomartire S, Marquesa JC, Gonçalves AMM (2021) The key role of zooplankton in ecosystem services: a perspective of interaction between zooplankton and fish recruitment. Ecol Indic 129:1–8. https://doi.org/10.1016/j.ecolind.2021.107867
Luo D, Ganesh S, Koolaard J (2022) Predictmeans: Predicted Means for Linear and Semi Parametric Models_. R package version 1.0.8, https://CRAN.R-project.org/package=predictmeans. Accessed 26 June 2022
Maiphae S, Pholpunthin P, Dumont HJ (2008) Taxon richness and biogeography of the Cladocera (Crustacea:Ctenopoda, Anomopoda) of Thailand. Ann Limnol - Int J Lim 44(1):33–43. https://doi.org/10.1051/limn:2008021
Martín J, Ortega J, García-Roa R, Rodríguez-Ruiz G, Pérez-Cembranos A, Pérez-Mellado V (2023) Effects of Anthropogenic Disturbance of Natural habitats on the Feeding Ecology of Moorish Geckos. Animals 13:1413. https://doi.org/10.3390/ani13081413
McKinney ML (2002) Urbanization, Biodiversity, and Conservation. Bioscience 52:883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. https://doi.org/10.1007/s11252-007-0045-4
McMichael AJ (2000) The urban environment and health in a world of increasing globalization: issues for developing countries. Bull World Health Organ 78(9):1117–1126. https://doi.org/10.1590/S0042-96862000000900007
Moyle PB, Leidy RA (1992) Loss of Biodiversity in aquatic ecosystems: evidence from Fish Faunas. : the theory and practice of Nature Conservation. Springer New York, New York, pp 127–169
Negreiros NF, Santos-Wisniewski MJ, Santos RM, Rocha O (2010) The influence of environmental factors on the seasonal dynamics and composition of Rotifera in the Sapucaí River arm of Furnas Reservoir, MG, Brazil. Biota Neotrop 10(4):173–182. https://doi.org/10.1590/S1676-06032010000400023
Nichols J, Hubbart JA, Poulton BC (2016) Using macroinvertebrate assemblages and multiple stressors to infer urban stream system condition: a case study in the central US. Urban Ecosyst 19:679–704
Nielsen DL, Brock MA, Crossle K, Harris K, Healey M, Jarosinski I (2003) The effects of salinity on aquatic plant germinationand zooplankton hatching from two wetland sediments. Freshw Biol 48:2214–2223. https://doi.org/10.1046/j.1365-2427.2003.01146.x
Nogrady T, Pourriot R, Segers H (1995) Rotifera: Notommatidae and Scaridiidae. In: Dumont HJF, Nogrady T (eds) Guides to the identification of the microinvertebrates of the Continental Waters of the World. SPB Academic Publishing, The Hague, The Netherlands, pp 1–248
Padhye SM (2020) Seasonal variation in functional composition and diversity of cladoceran zooplankton of a lotic eutrophic habitat from India. Ann Limnol - Int J Lim 56(11):1–11. https://doi.org/10.1051/limn/2020011
Padhye SM, Dahanukar N (2015) Determinants of ‘water fleas’ (Crustacea: Branchiopoda: Cladocera) diversity across seasonal and environmental gradients of a polluted river. Curr Sci 109:1777–1780
Paerl HW, Paul VJ (2012) Climate change: links to global expansion of harmful Cyanobacteria. Water Res 46:1349–1363. https://doi.org/10.1016/j.watres.2011.08.002
Pajk F, Zhang J, Han BP, Dumont HJ (2017) Thermal reaction norms of a subtropical and a tropical species of Diaphanosoma (cladocera) explain their distribution. Limnol Oceanogr 63(3):1204–1220. https://doi.org/10.1002/lno.10766
Pecorari S, José de Paggi S, Paggi JC (2006) Assesment of the urbanization effect on a Lake by Zooplankton. Water Resour 33(6):677–685. https://doi.org/10.1134/S0097807806060091
Phan NT, Duong QH, Tran-Nguyen QA, Dang MT (2021) The species Diversity of Tropical Freshwater rotifers (Rotifera: Monogononta) in relation to environmental factors. Water 13(1156):1–13. https://doi.org/10.3390/w13091156
Plangklang N, Athibai S (2021) Comparisons of Zooplankton Community structure between with- and without- pesticide applications on Rice Fields. Diversity 13(12):1–13. https://doi.org/10.3390/d13120644
Qin H, Cao X, Cui L, Lv Q, Chen T (2020) The influence of human interference on Zooplankton and Fungal Diversity in Poyang LakeWatershed in China. Diversity 12:296. https://doi.org/10.3390/d12080296
Razak SBA, Sharip Z (2019) Spatio-temporal variation of zooplankton community structure in tropical urban waterbodies along trophic and urban gradients. Ecol Process 8(44):1–12. https://doi.org/10.1186/s13717-019-0196-2
Resh VH, Giap DH (2010) Biomonitoring methods for the Lower Mekong Basin. Mekong River Commission, Vientiane
Riley WD, Potter ECE, Biggs J, Collins AL, Jarvie HP, Jones JI, Kelly-Quinn M, Ormerod SJ, Sear DA, Wilby RL, Broadmeadow S, Brown CD, Chanin P, Copp GH, Cowx IG, Grogan A, Hornby DD, Huggett D, Kelly MG, Naura M, Newman JR, Siriwardena GM (2018) Small Water bodies in Great Britain and Ireland: ecosystem function, human-generated degradation, and options for restorative action. Sci Total Environ 645:1598–1616. https://doi.org/10.1016/j.scitotenv.2018.07.243
Ritchie H, Roser H (2018) Urbanization. Published online at OurWorldInData.org. Retrieved from: ‘https://ourworldindata.org/urbanization’ [Online Resource]
Romanowska-Duda Z, Mankiewicz J, Tarczynska M, Walter Z, Zalewski M (2002) The effect of toxic cyanobacteria (blue-green algae) on water plants and animal cells. Pol J Environ 11(5):561–566
Sa-ardrit P, Beamish F (2005) Cladocera diversity, Abundance and Habitat in a Western Thailand Stream. Aquat Ecol 39(3):353–365. https://doi.org/10.1007/s10452-005-0783-4
Sanoamuang L (2002) T. Nogrady, H. Segers (Eds.), Rotifera, vol. 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochospheridae and Filinia, vol. 18, Backhuys Publishers, Leiden, The Netherlands, pp. 224–257
Saulnier-Talbot É (2016) Paleolimnology as a tool to achieve environmental sustainability in the Anthropocene: an overview. Geosci J 6(2):1–11. https://doi.org/10.3390/geosciences6020026
Schobben M, Stebbins A, Ghaderi A, Strauss H, Korn D, Korte C (2016) Eutrophication, microbial- sulfate reduction and mass extinctions. Commun Integr Biol 9(1):1–27. https://doi.org/10.1080/19420889.2015.1115162
Segers H (2007) Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature. Taxonomy and Distribution Zootaxa 1564:1–104. https://doi.org/10.11646/zootaxa.1564.1.1
Segers H, Koste W, Yussuf SM (1996) Contribution to the knowledge of the Monogonont Rotifera of Zanzibar, with a note on Filinia Novaezealandiae Shiel and Sanoamuang, 1993 Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie. 81(4):597–603. https://doi.org/10.1002/iroh.19960810413
Sharaf MB, Khalaf-Allah HMM, Hassan AKM, Zeina AF, Abo-Taleb HA (2023) Species composition, abundance and distribution of Freshwater Rotifera at El-Mahmoudia Canal. Egypt Egypt J Aquat Biol Fish 27(2):591–607. https://doi.org/10.21608/EJABF.2023.295420
Shen J, Qin G, Yu R, Zhao Y, Yang J, An S, Liu R, Leng X, Wan Y (2021) Urbanization has changed the distribution pattern of zooplankton species diversity and the structure of functional groups. Ecol Indic 120:106944. https://doi.org/10.1016/j.ecolind.2020.106944
Shiel RJ, Koste W (1992) Rotifera from Australian inland waters. VIII: Trichocercidae (Monogononta). Trans R Soc S Aust 116:1–27
Shiel R, Sanoamuang L (1993) Trans-Tasman variation in Australasian Filinia populations. Hydrobiologia 255/256:455–462
Sinev AY (2016) Key for identification of Cladocera of the subfamily Aloninae (Anomopoda: Chydoridae) from South-East Asia. Zootaxa 4200(4):451–486. https://doi.org/10.11646/zootaxa.4200.4.1
Smirnov NN (1992) The Macrothricidae of the world. In: Dumont HJF (ed) Guides to the identification of the microinvertebrates of the Continental Waters of the World. SPB Academic Publishing, Amsterdam, The Netherlands, pp 1–143
Smirnov NN (1996) Cladocera: the Chydorinae and Sayciinae (Chydoridae) of the world. In: Dumont HJF (ed) Guides to the identification of the microinvertebrates of the Continental Waters of the World. SPB Academic Publishing, Amsterdam, The Netherlands, pp 1–197
Sripanya J, Rattanawilai K, Vongsombath C, Vannachak V, Hanjavanit C, Sangpradub N (2022) Benthic macroinvertebrates and Trichoptera adults for Bioassessment Approach in streams and Wadeable Rivers in Lao people’s Democratic Republic. Nat Hist 22:12–24
Stansfield JH, Perrow MR, Tench LD, Jowitt AJD, Taylor AAL (1997) Submerged macrophytes as refuges for graz-ing Cladocera against fish predation: observations on seasonal changes in relation to macrophyte cover and predation pressure. Hydrobiologia 342:229–240. https://doi.org/10.1023/A:1017091407556
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J North Am Benthol Soc 29:344–358. https://doi.org/10.1899/08-171.1
Townsend C, Hildrew AG (1994) Species traits in relation to a habitat templet for river systems. Freshw Biol 31(3):265–275. https://doi.org/10.1111/j.1365-2427.1994.tb01740.x
Van Damme K, Sinev AY, Dumont HJ (2011) Separation of Anthalona gen.n. From Alona Baird, 1843 (Branchiopoda: Cladocera: Anomopoda): morphology and evolution of scraping stenothermic alonines. Zootaxa 2875:1–64. https://doi.org/10.11646/zootaxa.2875.1.1
Velthuis M, Teurlincx S, Van Dijk G, Smolders AJ, De Senerpont DLN (2023) Salinisation effects on freshwater macrophyte growth and establishment in coastal eutrophic agricultural ditches. Freshw Biol 68(4):547–560. https://doi.org/10.1111/fwb.14046
Vincent H, Bornand CN, Kempel A, Fischer M (2020) Rare species perform worse than widespread species under changed climate. Biol Conserv 246:1–7. https://doi.org/10.1016/j.biocon.2020.108586
Wenjie L, Binxia L, Cuijuan N (2019) Effects of temperature on Life History Strategy of the Rotifer Euchlanis dilatata. Zool Sci 36(1):52–57. https://doi.org/10.2108/zs170096
Acknowledgements
We would like to thank the editor and the reviewers for taking the time and effort to review the manuscript. We appreciate all the valuable comments and suggestions that helped us improve the quality of the manuscript. We thanks Dr.Rapeepan Jaturapruek and Dr.Thanida Saetang for assistance in field sampling. We also appreciate the facilities provided by the Department of Zoology and the Faculty of Science, Kasetsart University.
Funding
This research was financially supported by the Centre of Excellence on Biodiversity (BDC) Office of Higher Education Commission (BDC-PG2-161004).
Author information
Authors and Affiliations
Contributions
Conceptualization, SM, NJ; sample collection and investigation, NJ, SM; methodology, SM, NJ, SMP; data analysis, SMP, SM; All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This project was approved by the Institutional Animal Care and Use Committee, Faculty of Science, Kasetsart University, Thailand under Project number ACKU63-SCI-006.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jantawong, N., Padhye, S.M. & Maiphae, S. Biodiversity and species-environment relationships of freshwater zooplankton in tropical urban ponds. Urban Ecosyst 27, 827–840 (2024). https://doi.org/10.1007/s11252-023-01491-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01491-0