Abstract
Urban sprawl is recognized to homogenize biota, with several species that fail to adapt to these new human scenarios. However, some species can live and breed successfully in urbanized habitats. We compared the breeding performance of the relatively common raptor and poorly studied, chimango caracara (Milvago chimango) in an urban gradient of central Argentina. Breeding data of 359 nests were collected during breeding seasons from 2010 to 2012. Birds nested in colonies of 3 – 75 pairs. Overall breeding success was 49.9% with productivity at 1 ± 1.14 chicks per nest. Models revealed that reproductive success and productivity were higher in nests with earlier laying dates and sited in larger colonies and that urbanization gradient did not affect either reproductive output or laying day. Urban habitats in central Argentina appear to provide similar reproductive success of chimango caracara than rural or natural habitats. Thus, chimango caracara shows behavioral plasticity for their successful persistence to human changes as reflected in successfully breeding in a wide variety of habitats such as natural, rural, and urbanized environments that have been impacted by humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural habitats have been strongly altered by urbanization. Although urban development has been responsible for local extinctions of native species (McKinney 2006), some birds have been able to exploit these urbanized environments (Blair 1996). These successful species have a generalist diet or flexible behaviors that permit them to found supplementary food and novel nesting sites to adapt and persist in urbanized habitats (Møller 2009; Sol et al. 2013; Marzluff 2017; Boal 2019).
Changes in reproductive output indicate the responses of populations to environmental conditions such as urbanization. For many bird species that have been studied, urbanization has had a negative impact on reproductive parameters such as laying dates, clutch sizes, nestling weights and fledging success (Chamberlain et al. 2009). However, intermediate levels of urbanization can represent high quality breeding habitats for some native species, with females in better condition, laying larger eggs and having higher overall reproductive success than their agricultural counterparts in suburban rather than agricultural environments (Cardilini et al. 2013).
However, there are cases in which urbanized habitats can act as an ecological trap (Bonnington et al. 2015). This situation can occur if birds choose poor-quality habitats for breeding based on environmental cues that incorrectly indicate habitat quality, affecting negatively the reproductive output of these individuals (Schlaepfer et al. 2002; Battin 2004). In this case, birds breeding in urbanized habitats have failed to respond to predation risk (i.e., avoiding breeding in areas with high concentration of nest predators or adjusting their behavior to reduce the nest predation risk) thereby decreasing reproductive output with higher nest predation rates.
Raptor responses to urbanization varies from having enhanced reproductive success (Botelho and Arrowood 1996; Gehlbach 1996; Parker 1996; Stout et al. 2006; Rebolo-Ifrán et al. 2017; Welch-Acosta et al. 2019), to showing a neutral response (Stout et al. 1998; Millsap et al. 2004; Kübler et al. 2005; Dykstra et al. 2009; Rose et al. 2017) or experiencing negative effects during breeding (Tella et al. 1996; Bond et al. 2005; Sumasgutner 2013; Sumasgutner et al. 2014a). Raptor species that respond well to urban mosaic landscapes usually expand their range into urban and suburban areas (Carrete et al. 2009). If these habitats or their surrounding areas have an adequate food supply, they can allow raptors to breed in these areas that might otherwise be considered unsuitable.
Breeding density, generalist diet, flexibility in the use of habitat and in use of native or exotic trees to nest and extension of breeding season have been cited as important strategies permitting successful reproduction of raptors in urban mosaic landscapes (Kübler et al. 2005; Sumasgutner 2013; Boggie and Mannan 2014; Kreiderits et al. 2016; Sumasgutner et al. 2016; Rose et al. 2017). However, there are cases in which these habitats are selected for nesting but reproductive output can be lower than in non-urbanized or less urbanized habitats. In these cases, it is recognized that urbanized populations have entered an ecological trap (Boal and Mannan 1999; Sumasgutner et al. 2014b).
In colonial species, distance to nearest neighbor nest is a measure of spatial distribution of nests and although this aspect of breeding ecology has not been sufficiently studied in raptors, distance between nests of Eurasian kestrel (Falco tinnunculus) decreases with the percentage of urbanized areas covered by buildings or areas used by traffic (Sumasgutner et al. 2014b). This measure also has been examined in breeding chimango caracaras (Milvago chimango) and no effects of nearest neighbor distance on the reproductive success were found, but this variable explained variation in clutch and brood size (Solaro and Sarasola 2015).
Here, we worked with a relatively common raptor species in southern South America, the chimango caracara, in which it is unclear whether this species is an urban exploiter or adapter. It is unclear whether there is a variation in features such as reproductive output and the use of tree species to nest in function of human disturbance gradients. In this study, we investigated the breeding biology of chimango caracara to evaluate the variation in reproductive parameters in a gradient of human disturbance and see if these parameters are affecting the phenology and behavioral traits related to coloniality. In this sense, we expect to found an adjustment of reproductive parameters of chimango caracara in function of human disturbance, phenology and coloniality.
Methods
Study species
We used the chimango caracara as a study model. The chimango caracara is a relatively common raptor in southern South America that inhabits a wide diversity of habitats included those highly modified by humans (White et al. 1994; Leveau et al. 2022). These characteristics together with a wide trophic ecology of this species (Biondi et al. 2005), allows chimango caracaras to better meet their ecological requirements in heterogeneous landscapes (i.e. complex matrix of disturbed and undisturbed plots), thus benefiting from certain levels of human disturbances (Pedrana et al. 2008). For its part, it has been found that abundances of chimango caracara can be increased by agricultural intensification (Travaini et al. 1995; Carrete et al. 2009). However, chimango caracara abundance has shown to be significantly lower in commercial or residential areas of urbanized habitats than in rural areas (Bellocq et al. 2008). The chimango caracara has the capacity to breed in varied habitats from natural or cultivated to urbanized habitats, in both native and exotic trees and can nest solitarily or by forming colonies of different sizes (Fraga and Salvador 1986; Morrison and Phillips 2000; Solaro and Sarasola 2015).
Study area
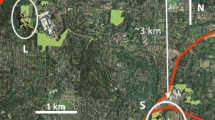
This study was conducted in La Pampa province, central Argentina, over a gradient of human disturbance defined by human presence in different areas. Greatest human disturbance occurred in suburban habitats included peripheral residential areas of Santa Rosa and Toay cities in which there were a strong human disturbance due to presence of inhabitants in their houses and to vehicular and pedestrian transit in roads. Intermediate disturbance occurred in rural habitats that included agricultural lands with crops and pastures for livestock with scattered tree stands of exotic tree species, mainly Eucalyptus spp., for cattle shelter. Human presence was limited to a single ranch house with three or four persons. An area with the least human disturbance occurred in natural habitat represented by a 7600 ha area protecting calden (Prosopis caldenia) forest in which human presence is limited to the tourist sector of the reserve. Thus, considering our human disturbance gradient, we consider suburban areas as the habitats with greater human disturbance, rural as intermediate disturbance, and natural areas as less human disturbance.
The six colonies of chimango caracara that we studied included three in suburban areas, two in the rural habitat and one in the natural habitat (Fig. 1). The three suburban colonies were Club de Caza Mapú Vey Puudú (36.65° S, 64.34° W, CC), La Cuesta del Sur (36.72° S, 64.27° W; CS) and the Golf Club (36.61° S, 64.23° W; GC). The two rural colonies were La Armonía ranch (36.56° S, 64.13° W; LA) and the campus of the Universidad Nacional de La Pampa (36.56° S, 64.30° W; UC). The natural colony was in Parque Luro (36.90° S, 64.25° W; PL).
Nest localization and monitoring
We monitored colonies during three reproductive seasons from 2010 to 2012. We began searching for occupied nests in September of each year. A nest was considered occupied if it contained eggs, chicks or if an adult was incubating. We recorded the species of tree or the structure that chimangos used for the nest. We visited nests weekly to verify: (i) phenological state; (ii) clutch-size (number of eggs per nest); (iii) breeding success (a nest was considered as successful if at least one young achieved 80% of mean age of first flights; Steenhof and Newton 2007), and (iv) productivity (number of young that achieved the minimum age considered to be successful). We estimated the population breeding success for each colony and year (number of successful breeding pairs divided by the total number of pairs attempting to breed in a colony in a year).
We estimated the duration (in days) of incubation and nestling periods considering only those nests visited on days of egg laying, hatching and when young took their first flights. As not all nests were visited on the day that the eggs were laid, in several nests laying day was indirectly estimated by backdating from later stage in the cycle using the previously known duration of incubation and nestling periods.
Spatial analysis of nests
We mapped nests and calculated the nearest neighbor nest distances to the external limit of its colony to evaluate the relation between nest distributions inside a colony and the reproductive output of each nest. The perimeter of each colony was defined through a minimum convex polygon enlarged in 10 m using all nests presents each year in each colony. These geographic analyses were performed using software QGis 2.18.13.
An aggregation index was calculated for each nest using the distances between the nests of each colony. This index describe the relative position of each nest within the spatial distribution of pairs occupying the same breeding colony in each year (Cardador et al. 2012). Aggregation index for each nest was calculated as \(\sum epx({-d}_{ij})\) where \({d}_{ij}\) was the linear distance between breeding pair i and j, and j represented all other nests within a colony (Carrete et al. 2006). Values of aggregation index ranged from 0 to 1, with lower values indicating greater isolation.
Statistical analyses
We used Chi-square tests to analyze independence between the origin of nest trees (native vs exotic) and among degrees of human disturbance (suburban, rural, and natural colonies).
Linear Mixed Models (LMMs) were used to evaluate the variation in laying dates due to year (2010, 2011 and 2012), degree of human disturbance (suburban, rural, and natural) and colony size (number of nests). Laying date (expressed as Julian date) was considered only for a subset of nests for which this data was known or indirectly estimated. Julian laying dates were included in the models as a response variable, with reproductive season and degree of human disturbance as explanatory variables and colony identity included as a random intercept. Due to lack of normality of laying dates (Shapiro–Wilk normality test: W = 0.98, p < 0.01), this variable was transformed as square root for use in models. Before transformation, maximum absolute value of Julian laying date (2.9) was added to each value to obtain positive numbers for taking the square root; this transformation provided normal data (W = 0.99, p = 0.4585).
Generalized Linear Mixed Models (GLMMs) were used to evaluate the variation in nearest neighbor distance (NND) in function of three explanatory variables: distance to the external limit of a colony, colony size and degree of human disturbance. Using the function fitdist of “fitdistrplus” R package (Delignette-Muller and Dutang 2015), we tested to best fit to NND response variable and then used a Gamma distribution in GLMMs. Statistical plots were used to check the residuals of global model. A post hoc comparisons were performed with Tukey contrasts performed on the final model. All tests were two tailed.
GLMMs were performed to evaluate the variation in reproductive parameters in function of temporal, coloniality and human disturbance variables. Response variables were clutch size, reproductive success, and productivity. We built three separate sets of models to evaluate the effects of laying date, coloniality, annual and human disturbance variables. In a first set of models we evaluated the effect of laying date (LAY) on clutch size, reproductive success, and productivity. In the second set of models, we evaluated the effects of coloniality variables on the same response variables. As covariate we used nearest neighbor distance (NND), distance to the external limit of the colony (DISTEDGE), colony size (COLSIZE) and the aggregation index (INDEX). Before we ran the models, covariates were discarded if significant Pearson correlation coefficients existed. These variables were scaled to be used in the models. In the last set of models, we evaluated the effect of year (2010, 2011 and 2012 [YEAR]) and degree of human disturbance (suburban, rural, and natural [HUMAN]) on the same response variables. Due to the under dispersion detected using a Poisson distribution in the global model, clutch size was modeled using a Conway–Maxwell–Poisson distribution (Sellers and Shmueli 2010). Reproductive success was modeled using a binomial family and a link function Logit. Productivity was modeled using a Poisson family and a link function Log adding an ID as correction factor in random component to improve the over dispersion detected in a global model. In all models, colony identity was included as a random intercept.
All prospective models were built within each set of models stated above. Akaike’s information criterion corrected for small sample sizes (AICc) and AICc weight (wi) were used to select the best models (Burnham and Anderson 2002). When there was uncertainty in model choice (lower wi), multimodel inference and model averaging were used. Model-averaged estimates were obtained by computing means and 95% confidence intervals (95% CI) using the weighted average of the corresponding coefficient to the top models, with ΔAIC < 4. Where the 95% CI for an effect size did not span zero, this effect could be considered statistically significant at the 5% level (Burnham and Anderson 2002). All statistical analysis were performed using R 3.6.1 and RStudio 1.2.1335.
Results
Nesting and phenology
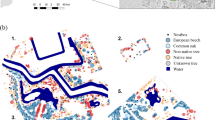
We monitored 359 nests during years 2010, 2011 and 2012 in rural (n = 104), suburban (n = 234) and natural (n = 21) habitats. Nests were located in both native (53.83%) and exotic (43.17%) species trees. The birds did not use the native and exotic trees to the same proportion across the urban gradient (χ2 = 176.5, df = 2, P < 0.01, Fig. 2).
Chimango caracara egg laying started in the second half of September and continued until the final week of December. Chicks left their nests from the second half of November until first half of February. Incubation period lasted 26.5 ± 2.4 days (n = 6) and the nestling period was 30.4 ± 4.6 days (n = 9). Laying date was influenced by neither colony size nor degree of human disturbance degree nor year (AICc weight null model = 0.977).
Coloniality
Colony size varied from 3 to 75 nests. Aggregation index was 0.04 ± 0.07 and NND was 35. ± 28.50 m (2 – 171 m) (Table 1). This last variable was negatively affected by distance of nest to external limit of colony and positively by colony size (Tables 2 and 3, Fig. 3A, B). Human disturbance was retained in the best model to explain the variation in NND. Tukey tests revealed that rural habitats had lower NND than nests in suburban and natural habitats (Tukey tests, p < 0.05), but there were no differences in NDD between nests in suburban and natural habitats (Tukey test, p = 0.662, Tables 2 and 3, Fig. 3C).
Results of GLMMs that evaluated the variation in nearest neighbor distance (NND) in function of (a) distance of nest to external limit of colony (DISTEDGE), (b) colony size (COLSIZE) and (c) human disturbance degree (HUMAN). Different capital letters indicate significant pairwise differences (Tukey’s test) between urbanization categories
Reproductive output
Clutch size was 2.6 ± 0.59 eggs per nest (n = 316). Overall breeding success was 49.9% (Table 1). Productivity was 1 ± 1.14 chicks per nest (range = 0 – 3 chicks). Models revealed that laying date had a negative effect on clutch size (coefficient estimate ± SE: -0.02 ± 0.01, Fig. 4a), reproductive success (-0.29 ± 0.15, Fig. 4b) and productivity (-0.16 ± 0.05, Fig. 4c), and colony size had a positive effect only on reproductive success and productivity (Fig. 5). All other tested variables did not affect any of these parameters (Online resource, Table S4 and S5).
Discussion
We examined the variation in breeding parameters of chimango caracara with regard to variables related to phenology, coloniality and degree of human disturbance in a data pool of 359 nests in three reproductive seasons. Overall, we were able to find support for two of our predictions, our analysis did not detect any effect of urban gradient on reproductive output; however, these parameters were affected by the phenology and coloniality variables finding earlier nests and sited in larger colonies with better reproductive output.
Chimango caracaras in suburban and natural habitats used native tree species more often than exotic species, but caracaras in rural habitats used a higher proportion of exotic tree species than native trees. As we did not measure availability of trees for nesting in each habitat, we cannot confirm that chimango caracaras choose tree type (native or exotic) based on availability. There is a clear preponderance of exotic trees in rural habitats which would explain the use exotic tree species that exist in this habitat (mainly groves with Eucalyptus sp.). In natural habitats (a protected area assigned to protection of natural forest) the main nest tree is the caldén (Prosopis caldenia) a native species. In suburban habitats, there are many exotic ornamental trees but there are abundant patches of native forest which chimango caracaras prefer to nest. The use of both native and exotic trees for nesting has already been reported for other studies both in central Argentina (Fraga and Salvador 1986) and southern Chile (Morrison and Phillips 2000) and this plasticity allows this species to breed even in non-natural habitats.
The breeding phenology values we observed match reports for this species for central Argentina (Fraga and Salvador 1986) which record egg laying from the second half of September until the final week of December. Morrison and Phillips (2000) noted that the start of laying occurred in the first half of September for southern Chile. Our values for the incubation (26.5 days) and nestling (30.4 days) periods match with the values reported by Fraga and Salvador (1986), but disagree with Morrison and Phillips (2000) who reported 32 and 41 days for incubation and nestling periods, respectively. This difference could be due to sampling error associated to each of these measures and to low number of nests in which the exact date of laying and hatching eggs could be known (two in Chile and 6–9 in this study).
The timing of egg laying has a great impact on fitness and will influence reproductive parameters as clutch size, nestling quality, reproductive success, and productivity. Several studies have shown that the earlier laying dates in urban landscapes is due, presumably, to females being in better condition (Chamberlain et al. 2009). Although clutch size, reproductive success and productivity were affected by laying date, there were no differences in laying date among different years, colony size or among habitats with different levels of human disturbance likely because chimangos have found sufficient food in the appropriate time to breed both in natural, rural, or suburban habitats which would allow to females reach a necessary body condition to breed in the correct time. This similar pattern was found by Rose et al. (2017) for the Black Sparrowhawk (Accipiter melanoleucus) across an urban gradient in Africa.
Following the classification proposed by Newton (1979), and considering mean NND values of this study, chimangos nest in dense colonies in all habitats studied. This behavior has been mentioned for this species in a suburban colony, however without strong evidence the authors considered NND as an important characteristic that would affect reproductive output of chimango caracaras in this habitat (Solaro and Sarasola 2015). We found that nests in smaller colonies and placed in the center of the colony were closer to other nests than those of larger colonies or placed at a colony’s periphery. Moreover, rural colonies showed smaller NND than in suburban and natural colonies. This pattern of separation of nests within a colony may be influenced by the distribution of available trees. In suburban and natural habitats chimangos used native tree species for which the distances between trees can be greater and more varied without a regular pattern. For colonies in rural habitats tree distribution is clumped and equidistant and is determined by requirements, needs and utility of people that planted them. Although Morrison and Phillips (2000) did not work in urban habitats, they analyzed chimango colonies in a diverse mosaic of forest patches, linear strips, fields with secondary growth of trees and shrubs, open pastures, and agricultural fields. They found that the nearest neighbor distances between nests are almost five times greater than the values we found. The Eurasian kestrel (Falco tinnunculus), for example, is commonly associated with urbanized landscapes because they often nest in cavities of old buildings in the center of cities; therefore, the distribution and NND of their nests are conditioned by the distribution of these structures with the smallest NND found in the city center (Sumasgutner et al. 2014b). This pattern could be compared to what we noted for rural colonies in which chimangos nest in trees that that had been planted and have smaller NND at these sites.
We found mean clutch size to be 2.65 ± 0.59 eggs per nest. This value is similar to that found by Fraga and Salvador (1986) for a rural colony of Buenos Aires province, Argentina (2.77 eggs per nest) and was slightly greater than the report of Morrison and Phillips (2000) for Chiloe island, Chile (2.26 chicks for nest). Overall reproductive success reported in this study (49.9%) was higher than values found by both Fraga and Salvador (1986) (30%) and Solaro and Sarasola (2015) for a suburban colony in La Pampa province, Argentina (32%). However our reproductive success was lower than the report of Morrison and Phillips (2000) (57%). The values of productivity found in this study (1 ± 1.14 chicks) agree with Morrison and Phillips (2000), who found a productivity of 1.06 and 2.14 chicks for nest for beach and inland colonies respectively.
Timing of breeding has an important influence on reproductive output in birds (Dunn and Møller 2014). Food availability varies seasonality and birds adjust laying dates to synchronize hatching so that chicks can be reared during the peak of food abundance. Earlier egg laying is often associated with larger clutch sizes, greater production of young and higher reproductive success (Verhulst and Nilsson 2008). In our study, laying date had a negative effect on clutch size, reproductive success and productivity, with late breeders laying fewer eggs, failing more and rearing fewer chicks. If adjusting breeding phenology to optimal conditions is profitable in reproductive terms, our results support the idea that earlier breeders were able to better adjust their phenology to achieve a better reproductive performance in producing a larger number of nestlings.
Colony size had a positive effect on reproductive success and productivity with nests placed in larger colonies having a greater probability of being more successful and producing more chicks in each season. Large colonies reflect a favorable environment for breeding (Serrano et al. 2004). Although it was not measured in this study, it is likely that larger colonies were located in places with higher food availability and less predation pressure which allowed these breeders to be more successful than birds nesting in smaller colonies. In our study larger colonies occurred both in rural and suburban habitats which indicates that both habitats are beneficial for breeding (see later). There have been proposals that those species that nest in both solitary and in colonies would have adaptive benefits compared with those species that nest only as either a solitary or colonial breeder (Sasvari and Hegyi 1994). The chimango caracara breeds both solitarily and colonially (Fraga and Salvador 1986; Morrison and Phillips 2000; Solaro and Sarasola 2015). Here we reported colonies of different sizes and the variability in the pattern of nest distribution which could be a behavior that enhances the flexibility and adaptability of this species to a wide variety of habitats as studied here (Solaro and Sarasola 2019).
The ways that raptors respond to urbanization during the reproductive season are highly variable (Solaro 2018), and these responses depend on nesting and feeding requirements and vulnerability to human disturbance (Kettel et al. 2017). Some species are negatively impacted by urbanization (Tella et al. 1996; Charter et al. 2007; Sumasgutner 2013; Sumasgutner et al. 2014a, b), others may respond positively (Gehlbach 1996; Parker 1996; Stout et al. 2006; Lin et al. 2015; Welch-Acosta et al. 2019), and some may not be influenced by urbanization (Rosenfield et al. 1995; Gahbauer et al. 2015; Rose et al. 2017). We found that clutch size, reproductive success, productivity and laying date were not affected by the human disturbance gradient we studied. The lack of urbanization effect on chimango caracara breeding could be a response to three factors. First, the cities of our study were moderately small (around 115,000 and 12,000 inhabitants to Santa Rosa and Toay, respectively) in which the strong effects of urbanization might not be so marked in suburban areas where colonies were placed. This is important from a conservation standpoint since suburban areas could be important sites in which raptor birds can live, feed and breed (Hogg and Nilon 2015), and it would be useful to increase our understanding of breeding biology of urban-adapted species in smaller town and cities (Reynolds et al. 2019). Second, chimango caracaras that breed in suburban habitats can find sufficient food for themselves and their young inside of urban areas, or their daily home range would allow them to find this resource outside of it (Sumasgutner 2013), so they could respond to nutritional needs of their progeny in the same way as non urbanized breeders, getting then similar reproductive parameters. Finally, urban habitats have a variety of threats to raptors (Solaro 2018), and losses during reproduction can reduce reproductive output (Rebolo-Ifrán et al. 2017). Although depredation was not quantified in this study, losses may not have been strong enough to affect the reproductive output of urbanized breeders. Since clutch size, reproductive success and productivity did not vary with degree of urbanization, we do not consider breeding in suburban habitats to be an ecological trap for the chimango caracara.
Conclusions
One interesting finding from this study is that urbanization has no effect on either laying date or reproductive output. However, earlier nests and larger colonies had higher reproductive output. To summarize, the chimango caracara shows behavioral plasticity for their successful persistence to human modified habitats as reflected in being a successful breeder in a wide variety of habitats such as natural, rural, and urbanized environments studied here.
Data availability
Data are available on request.
References
Battin J (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18:1482–1491
Bellocq M, Filloy J, Garaffa P (2008) Influence of agricultural intensity and urbanization on the abundance of the raptor chimango caracara (Milvago chimango) in the Pampean region of Argentina. Ann Zool Fennici 45:128–134
Biondi L, Bó M, Favero M (2005) Dieta del chimango (Milvago chimango) durante el periodo reproductivo en el sudeste de la provincia de Buenos Aires, Argentina. Ornitol Neotrop 16:31–42
Blair RB (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–519
Boal C (2019) Urban raptor communities: why some raptors and not others occupy urban environments. In: Boal C (ed) Urban Raptors. Island Press, Washington, D.C.
Boal CW, Mannan RW (1999) Comparative breeding ecology of Cooper’s hawks in urban and exurban areas of southeastern Arizona. J Wildl Manage 63:77. https://doi.org/10.2307/3802488
Boggie MA, Mannan RW (2014) Examining seasonal patterns of space use to gauge how an accipiter responds to urbanization. Landsc Urban Plan 124:34–42. https://doi.org/10.1016/j.landurbplan.2014.01.001
Bond G, Burnside NG, Metcalfe DJ et al (2005) The effects of land-use and landscape structure on barn owl (Tyto alba) breeding success in southern England, U.K. Landsc Ecol 20:555–566. https://doi.org/10.1007/s10980-004-5037-7
Bonnington C, Gaston KJ, Evans KL (2015) Ecological traps and behavioural adjustments of urban songbirds to fine-scale spatial variation in predator activity. Anim Conserv 18:529–538. https://doi.org/10.1111/acv.12206
Botelho E, Arrowood P (1996) Nesting success of western burrowing owls in natural and human-altered environments. In: Bird CD, Verlan DE, Negro JJ (eds) Raptors in Human Landscapes: Adaptations to Built and Cultivated Environments. Academic Press Limited, New York, pp 61–68
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach (2nd ed)
Cardador L, Carrete M, Mañosa S (2012) Inter-individual variability and conspecific densities: Consequences for population regulation and range expansion. PLoS ONE 7:1–8. https://doi.org/10.1371/journal.pone.0033375
Cardilini A, Weston M, Nimmo D et al (2013) Surviving in sprawling suburbs: suburban environments represent high quality breeding habitat for a widespread shorebird. Landsc Urban Plan 115:72–80. https://doi.org/10.1016/j.landurbplan.2013.04.001
Carrete M, Sánchez-Zapata J, Tella J et al (2006) Components of breeding performance in two competing species: habitat heterogeneity, individual quality and density-dependence. Oikos 112:680–690
Carrete M, Tella J, Blanco G, Bertellotti M (2009) Effects of habitat degradation on the abundance, richness and diversity of raptors across Neotropical biomes. Biol Conserv 142:2002–2011. https://doi.org/10.1016/j.biocon.2009.02.012
Chamberlain D, Cannon A, Toms M, et al (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis (Lond 1859) 151:1–18. https://doi.org/10.1111/j.1474-919X.2008.00899.x
Charter M, Izhaki I, Bouskila A, Leshem Y (2007) Breeding success of the Eurasian Kestrel (Falco tinnunculus) nesting on buildings in Israel. J Raptor Res 41:139–143
Delignette-Muller M-L, Dutang C (2015) fitdistrplus: An R Package for Fitting Distributions. J Stat Softw 64:1–34
Dunn PO, Møller AP (2014) Changes in breeding phenology and population size of birds. J Anim Ecol 83:729–739. https://doi.org/10.1111/1365-2656.12162
Dykstra CR, Hays JL, Simon MM (2009) Spatial and temporal variation in reproductive rates of the Red-shouldered Hawk in suburban and rural Ohio. Condor 111:177–182. https://doi.org/10.1525/cond.2009.080002
Fraga R, Salvador S (1986) Biología reproductiva del Chimango (Polyborus chimango). El Hornero 12:223–229
Gahbauer MA, Bird DM, Clark KE et al (2015) Productivity, mortality, and management of urban peregrine falcons in northeastern North America. J Wildl Manage 79:10–19. https://doi.org/10.1002/jwmg.803
Gehlbach F (1996) Eastern Screech Owls in suburbia: a model of raptor urbanization. In: Raptors in Human Landscapes Adaptations to built and cultivated environments. pp 69–74
Hogg JR, Nilon CH (2015) Habitat associations of birds of prey in urban business parks. Urban Ecosyst 18:267–284. https://doi.org/10.1007/s11252-014-0394-8
Kettel EF, Gentle LK, Quinn JL, Yarnell RW (2017) The breeding performance of raptors in urban landscapes: a review and meta-analysis. J Ornithol 159:1–18. https://doi.org/10.1007/s10336-017-1497-9
Kreiderits A, Gamauf A, Krenn HW, Sumasgutner P (2016) Investigating the influence of local weather conditions and alternative prey composition on the breeding performance of urban Eurasian Kestrels Falco tinnunculus. Bird Study 3657:1–11. https://doi.org/10.1080/00063657.2016.1213791
Kübler S, Kupko S, Zeller U (2005) The kestrel (Falco tinnunculus L.) in Berlin: investigation of breeding biology and feeding ecology. J Ornithol 146:271–278. https://doi.org/10.1007/s10336-005-0089-2
Leveau LM, Gorleri FC, Roesler I, González‐Táboas F (2022) What makes an urban raptor? Ibis (Lond 1859) 1–14. https://doi.org/10.1111/ibi.13062
Lin WL, Lin SM, Lin JW et al (2015) Breeding performance of Crested Goshawk Accipiter trivirgatus in urban and rural environments of Taiwan. Bird Study 62:177–184. https://doi.org/10.1080/00063657.2015.1005570
Marzluff JM (2017) A decadal review of urban ornithology and a prospectus for the future. Ibis (Lond 1859) 159:1–13. https://doi.org/10.1111/ibi.12430
McKinney M (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Millsap B, Breen T, McConnell E et al (2004) Comparative fecundity and survival of Bald Eagles fledged from suburban and rural natal areas in Florida. J Wildl Manage 68:1018–1031. https://doi.org/10.2193/0022-541X(2004)068[1018:CFASOB]2.0.CO;2
Møller A (2009) Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159:849–858
Morrison J, Phillips L (2000) Nesting habitat and success of the Chimango Caracara in southern Chile. Wilson Bull 112:225–232
Newton I (1979) Population ecology of raptors. T & AD Poyser, London
Parker JW (1996) Urban ecology of the Mississippi Kite. In: Bird CD, Verlan DE, Negro JJ (eds) Raptors in human landscapes. Academic Press Limited, New York, Adaptation to built and cultivate environments, pp 45–52
Pedrana J, Isacch J, Bó M (2008) Habitat relationships of diurnal raptors at local and landscape scales in southern temperate grasslands of Argentina. Emu 108:301–310
Rebolo-Ifrán N, Tella JL, Carrete M (2017) Urban conservation hotspots: Predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-03853-z
Reynolds SJ, Ibañez-Álamo JD, Sumasgutner P, Mainwaring MC (2019) Urbanisation and nest building in birds: a review of threats and opportunities. J Ornithol 160:841–860. https://doi.org/10.1007/s10336-019-01657-8
Rose S, Sumasgutner P, Koeslag A, Amar A (2017) Does seasonal decline in breeding performance differ for an African raptor across an urbanization gradient? Front Ecol Evol 5:1–9. https://doi.org/10.3389/fevo.2017.00047
Rosenfield RN, Bielefeldt J, Affeldt JL, Beckmann DJ (1995) Nesting density, nest area reoccupancy, and monitoring implications for Cooper’s hawks in Wisconsin. J Raptor Res 29:1–4
Sasvari L, Hegyi Z (1994) Colonial and solitary nesting choice as alternative breeding tactics in Tree Sparrow Passer-Montanus. J Anim Ecol 63:265–274. https://doi.org/10.2307/5545
Schlaepfer M, Runge M, Sherman P (2002) Ecological and evolutionary traps. Trends Ecol Evol 17:474–480. https://doi.org/10.1016/S0169-5347(02)02580-6
Sellers KF, Shmueli G (2010) A flexible regression model for count data. Ann Appl Stat 4:943–961. https://doi.org/10.1214/09-AOAS306
Serrano D, Forero M, Donázar J, Tella J (2004) Dispersal and social attraction affect colony selection and dynamics of Lesser Kestrels. Ecology 85:3438–3447
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. https://doi.org/10.1016/j.anbehav.2013.01.023
Solaro C (2018) Costs and benefits of urban living in raptors. In: Sarasola J, Grande J, Negro J (eds) Birds of Prey Biology and conservation in the XXI century. Springer International Publishing AG part, Cham, Switzerland, pp 177–196
Solaro C, Sarasola JH (2019) Urban living predicts behavioural response in a neotropical raptor. Behav Processes 169:103995. https://doi.org/10.1016/j.beproc.2019.103995
Solaro C, Sarasola JH (2015) Nest-spacing, not human presence, influences the breeding of Chimango Caracaras (Milvago chimango) in a peri-urban reserve. Emu 115:72–75. https://doi.org/10.1071/MU14038
Steenhof K, Newton I (2007) Assessing nesting success and productivity. In: Bird DM, Bildstein KL (eds) Raptor Research and Management Techniques. Hancock House Publisher, pp 181–192
Stout W, Anderson RK, Papp JM (1998) Urban, suburban and rural Red-tailed Hawk nesting habitat and populations in southeast Wisconsin. J Raptor Res 32:221–228. https://doi.org/10.1080/00958970600794008
Stout W, Temple S, Papp J (2006) Landscape correlates of reproductive success for an urban-suburban Red-tailed Hawk population. J Wildl Manage 70:989–997
Sumasgutner P (2013) Diet specialisation and breeding success along an urban gradient: the kestrel (Falco tinnunculus) in Vienna, Austria. Beiträge Zur Jagd Und Wildforsch 38:385–397
Sumasgutner P, Millán J, Curtis O et al (2016) Is multiple nest building an adequate strategy to cope with inter-species nest usurpation? BMC Evol Biol 16:97. https://doi.org/10.1186/s12862-016-0671-7
Sumasgutner P, Schulze CH, Krenn HW, Gamauf A (2014a) Conservation related conflicts in nest-site selection of the Eurasian Kestrel (Falco tinnunculus) and the distribution of its avian prey. Landsc Urban Plan 127:94–103. https://doi.org/10.1016/j.landurbplan.2014.03.009
Sumasgutner P, Nemeth E, Tebb G et al (2014b) Hard times in the city – attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front Zool 11:48. https://doi.org/10.1186/1742-9994-11-48
Tella J, Hiraldo F, Donázar-Sancho J, Negro J (1996) Costs and benefits of urban nesting in the lesser kestrel. In: Bird CD, Verlan DE, Negro JJ (eds) Raptors in Human Landscapes: Adaptations to Built and Cultivated Environments. Academic Press Limited, New York, pp 53–60
Travaini A, Rodríguez A, Ceballos O et al (1995) Roadside raptor surveys in Central Argentina. Hornero 014:64–66
Verhulst S, Nilsson J-A (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc B Biol Sci 363:399–410. https://doi.org/10.1098/rstb.2007.2146
Welch-Acosta BC, Skipper BR, Boal CW (2019) Comparative breeding ecology of Mississippi Kites in urban and exurban areas of West Texas. J F Ornithol 90:248–257. https://doi.org/10.1111/jofo.12303
White C, Olsen P, Kiff L (1994) Order Falconiformes – Family Falconidae (Falcons and Caracaras). In: del Hoyo J, Elliot A, Sargatal J (eds) Handbook of the Birds of the World, vol 2. New World Vultures to Guineafowl. Lynx, Barcelona, pp 216–277
Acknowledgements
We express our thanks to Andrea Costán and countless collaborators for their field assistance, to Juana, Juan and Maximiliano of La Armonía ranch for their help and permission to work in this site, to community of neighbors of La Cuesta del Sur for their permission to enter their land and to the commissionors of Golf Club and Club de Caza Mappú Vey Puudú for authorizing our work at these sites. We appreciate the improvements in English usage made by Peter Lowther and Pam Denmon through the Association of Field Ornithologists' program of editorial assistance. This work was conducted under permits for the use of wild animals in research awarded by Dirección de Recursos Naturales, Ministerio de la Producción, Gobierno de La Pampa.
Author information
Authors and Affiliations
Contributions
Conceptualization: Claudina Solaro and José Hernán Sarasola; Methodology: Claudina Solaro; Formal analysis and investigation: Claudina Solaro; Writing—original draft preparation: Claudina Solaro; Review: Claudina Solaro.
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solaro, C., Sarasola, J.H. Breeding performance is explained for coloniality and phenology but not for urbanization in a generalist raptor bird. Urban Ecosyst 26, 743–753 (2023). https://doi.org/10.1007/s11252-022-01319-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01319-3