Abstract
Urban forest ecosystems, the structure, and functions therein are subjected to anthropogenic disturbances. Native and sensitive species from those forests might be lost due to such disturbances. At the same time, supplemented anthropogenic resources might create opportunities for exotic and invasive species. Although invasive species are considered one of the major threats to the urban biodiversity and ecosystems, the research on invasion dynamics in the Himalayas has primarily focused on the impacts of invasion on forest structure and productivity. This study aims to understand the influence of forest structure and anthropogenic factors in invasion success that are poorly covered in the existing literature. We selected 11 urban forest patches for the study considering the presence-absence of selected invasive species and structural attributes. We used Principal Component Analysis (PCA) to reduce co-linearity in the covariates and generalized linear mixed effects model (GLMM) to identify the factors affecting invasion success. We found that forest structural attributes, namely, tree diameter, height and canopy cover, and anthropogenic disturbances regulate invasion success in urban forests. This implies that maintaining urban forest structural attributes, especially the stands with large-sized trees, is essential to control invasion in the context of urbanization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban green spaces represented by diverse habitat types ranging from the highly managed green infrastructures like green roofs to the remnant patches of urban forest are crucial for habitat connectivity and ecosystem service perspectives (Milanovich et al. 2012; Aronson et al. 2017). They play a vital role to make towns and cities habitable areas through the abatement of pollutants such as ozone, acting against the impacts of urban heat islands, moderating local climate, and enhancing thermal comfort (Klemm et al. 2015; Ballinas and Barradas 2016; Calfapietra et al. 2016; Jaganmohan et al. 2016; Livesley et al. 2016). Globally, as the rate of urbanization and biodiversity loss are analogous to each other, understanding the ecology of urban forests individually and within the network is essential for biodiversity conservation (Brook et al. 2008; Aronson et al. 2017). Due to the high density of people and the greater prevalence of the human-mediated mobility of the commodities, human-ecosystem interactions differ significantly in the city areas compared to other natural sites. This difference in interaction results in different patterns and processes of invasion in the urban context compared to natural sites (Gaertner et al. 2017).
Habitat patches within the cities are highly fragmented and heterogeneous, and abiotic and biotic environments are significantly altered, affecting local (alpha) or within-patch biodiversity (Faeth et al. 2012). Urban areas are often considered inhospitable for native species, especially sensitive ones, but the case is different for invasive species (Cadotte et al. 2017). As invasive species are opportunistic, the niches created due to the disappearance of the native species are occupied by invasive ones. As urbanization proceeds, the non-native invasive species gradually replace the native species from the ecosystem of city areas (Cadotte et al. 2017). Urban areas are important in invasion research as they act both as entry points and dispersal locations for the invasive species (Gaertner et al. 2017; McLean et al. 2017). As a result, the diversity and abundance of non-native invasive species are greater in city areas than their rural counterparts and other natural sites (Cadotte et al. 2017). Urban green space, including the urban forests, is the hotspots of biological invasion (Gaertner et al. 2017). As the urban green spaces are subject to high human pressure, they often consist of high human trails and other interactions, which enhance edge and serve as propelling factors for opportunistic invasive species (McDonald and Urban 2006). However, the level of invasion and ecological success of invasive species are often regulated by context-specific factors (Dyderski and Jagodziński 2019).
Urban ecosystems may be one of the final frontiers of ecological exploration (Dolan et al. 2013). Mainstays of urban biodiversity are the urban forest patches embedded in the matrix of urban infrastructure, remnants, or naturalized plantations. Though urban ecological research is swirling in recent times (Lepczyk et al. 2017), limited information exists about the dynamics of urban forests in developing countries. Increased anthropogenic activities and increasing demands of ecosystem services have intensified such areas' stress and appeals. However, the invasion of such important areas as one of the most important threats has not been dealt with in detail. Invasive plant species profoundly impact the trophic structure of the forest ecosystem (McCary et al. 2016). As developing countries have limited capacity to apply remedy measures against the problem of ecological invasion (Early et al. 2016), it is crucial to understand the factors that offer resistance to the ecosystem against invasion.
Rapidly urbanizing landscapes in developing nations with several urban forest patches provide an opportunity to deal with the ecological role of forest structure relating to the invasion and future vulnerability (Steenberg 2015). In the face of growing challenges, understanding the factors which regulate the temporal and spatial dynamics is crucial for conserving urban biodiversity. Despite invasion being the subject of interest for more than six decades following the pioneering work of Elton (1958), we are yet to reach a common understanding of the factors responsible for driving the invasion of the species. In urban areas, the problem is even severe as invasion science has yet to give significant attention to the invasion dynamics, patterns, and processes in the urban ecosystem (Gaertner et al. 2017). There is a considerable debate on the factors associated with the invasion of the urban sites with invasive species. Some studies relate to the characteristics of invaded sites, while others relate to the attributes of invading species or other context-dependent factors (Dyderski and Jagodziński 2019). Native species richness can buffer the impact of invasive species when the sites are undisturbed but not the same when the sites are disturbed (Pinto and Ortega 2016). Understanding the role of forest dynamics and structural control on the spread of invasive species is essential to formulate the management plan (Baret et al. 2008) of the urban forest in the face of growing urbanization. In the context of knowledge gap regarding the implication of tree species richness, abundance, and forest structure on the regulation of invasion of the urban forest, this study aimed at generating information on two fronts: i) to assess the role site-level disturbances play on the invasion probability within the urban forests and ii) comprehend the importance of forest structural attributes to contend invasion. Our study focuses on Kathmandu valley, Nepal, to fill the knowledge gap on the factors regulating the presence of invasive species in urban forest patches.
Materials and methods
Study area
Kathmandu, a bowl-shaped valley, encompassing an area of around 899 km2 of three districts, Kathmandu, Lalitpur, and Bhaktapur, lies at 1,300 m above sea level (m asl) and is located between latitudes 27°32′13″ and 27°49′10″ N and longitudes 85°11′31″ and 85°31′38″ E (Khanal et al. 2019). The valley is surrounded by the Mahabharat mountain range, with four hills, namely Chandragiri, Nagarjun, Phulchowki, and Shivapuri, with the highest altitude being 2,831 m asl (Phulchowki). The climate is subtropical, temperate, and cool-temperate, with four distinct seasons: spring (March–May), summer (June–August), autumn (September–November), and winter (December-February) (Pandey et al. 2010). The mountains are forested in the upper reaches, most of these in the regenerating stage and mature hardwood forests confined to parks and sacred areas.
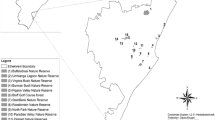
Nepal is one of the fastest urbanizing nations in the world, with the urban population growth exceeding 6% since the 1970s, and Kathmandu has been the center of urbanization where the built-up area almost doubled, resulting in the expansion of settlements outwards in all directions in the period between 2000 and 2018 (Khanal et al. 2019). In the valley slope, agricultural land has been converted to forest land while, on the valley floor, nearly 1,000 ha of forest have been converted to agricultural land and built-up area (Ishtiaque et al. 2017). Agriculture in the valley is characterized by very intensive farming use of fertilizers, irrigation, human labor, and terracing of farmland. The Bagmati is the principal river originating from Shivapuri ridge at an elevation of about 2,650 m asl. It drops to 1,340 m asl over a distance of about 8 km with a network of 20 tributaries (ICIMOD/MoEST/UNEP 2007). Several forest patches are embedded in the urban matrix of Kathmandu valley, creating a mosaic of green areas. We studied 11 patches of forests within three districts of Kathmandu valley (Fig. 1; Table 1). Selected 11 forest patches represent significant urban forests in the valley in terms of either religious values viz. Pashupatinath, Swyambhunath, Neel-Barahi, Bhandarkhal, Bajrabarahi, Gokarna, Mahepi, Hattiban, planted forests - UN Park, and Tribhuvan Univeristy Forest. Ranibari is the only community forest in core area of urban Kathmandu. Nevertheless, these areas represent significant green spaces and biodiversity hotspots in rapidly urbanizing landscape.
Vegetation surveys
A total of 200 random points were generated within the selected urban forest patches using a geographic information systems (GIS). However, during the survey, plot selection criteria - at least 10 m away from forest boundary, at least 50 m away from the adjacent plot (s), and not in a forest path wider than 2 m, lead to the selection of only 146 sample plots. The sample points were tracked using a global positioning system (GPS, Garmin Etrex 10). We sampled an area of 3.65 ha, which is around 1% of the total forest patch area (364.51 ha) considered in the study (Table 1).
Vegetation surveys were conducted in October and November 2015. These surveys included vegetation sampling using quadrates of size 250 m2 (circular). All trees in the plots were measured for diameter at breast height (DBH) (Diameter tape) and height (Clinometer- Suunto PM-5/360 PC Clinometer) and were identified up to species level in the field. For unidentified species, specimens like leaves and flowers were collected and identified in Tribhuvan University Herbarium (TUH), Kathmandu. If the identity was not clear, the tree species were placed in the unidentified category (abbreviated as UN1, UN2, etc.). We considered three main invasive species for the study: Ageratina adenophora, Lantana camera, and Parthenium hysterophorus, as they are considered obnoxious weeds in the valley (Taylor et al. 2012; Maharjan et al. 2015; Shrestha et al. 2015; Thapa et al. 2017). We recorded the presence of invasive species in a plot if it had at least a species established in a plot. Although we did not measure the height or biomass of the individuals or clumps, we ensured the established species using expert judgments in the field.
Canopy cover estimates were made visually, as the portion of sky covered, from four points in the circumference and one at the center of the plot. The five values obtained were averaged to obtain the plot level canopy cover (%) value. Trail distance was measured as the shortest distance (m) of the sample plot from the nearest trail using a measuring tape. Ground cover and Leaf litter conditions were classified based on visual estimation of the coverage in the sample plots (Table 2).
Data management and analysis
We used data exploration techniques such as descriptive statistics and visualization for the general idea about the data and variables. Since our predictor variables (related to tree structure) were highly correlated, we used principal component analysis (PCA) using package FactoMineR (Lê et al. 2008) to obtain orthogonal principal components. We selected the first five components that explained > 90% cumulative variance for further analysis. We checked the major contribution of individual covariates to these components. We used the selected PCs, litter conditions, and trail distance as predictor variables for regression model building. We used the generalized linear mixed effects model (GLMM) using package lme4 (Bates et al. 2015) to model the probability of the presence of invasive alien plants (IAPs). Since we modeled the presence/absence of the IAPs in the plots, we build the regression model using the binomial error structure of the response.
As the design involved plots nested in forests, we modeled two-level grouping factors. However, the variance due to plots was too low; thus, we removed the ‘plot’ as a grouping variable and used only ‘forest’ as random intercept in the model. We started with the most complex model following Zuur et al. (2009), where we used two grouping variables (plots nested in forests) and checked the model outputs to decide what variable to retain in the model. For the fixed effects variables, the model selection used the information criteria approach (Burnham and Anderson 2004) using the backward elimination method, removing an insignificant variable one at a time until all the variables and design variables were significant, checking AIC values and chi-square test significance. We used Bobyqa optimizer with 105 iterations while modeling. We did not scale the covariates in continuous scale as the model did not show any convergence issues without scaling. We built models with Laplace Approximation, Gauss Hermite Quadratures with 10 and 25 Quadratures. Although the models did not vary among three methods (Fig. SI–A), we preferred adaptive Gauss-Hermite quadrature approach with AGQ = 25, since the samples between clusters (groups) were unequal and some groups had smaller sample sizes.
The trail distance had marginal effects on invasion probability. We checked the model by removing ‘Trail’ and AIC value increased (though non-significant, AIC of the model without trail was 148.56, and while included AIC was 147.74 with 1 degree of freedom lost). Moreover, the residual behavior showed significant problems in excluding the trail, and therefore it was retained in the final model. Overall model diagnostics was carried out using simulated residuals following library DHARMa (Hartig 2021) which also provides a test for overall uniformity using the One-sample Kolmogorov–Smirnov test. Model diagnostics also involved visual plots of Pearson Residuals for linearity using each numeric covariate and sensitivity using Pearson residuals and leverage (Figs. SI–B1 and SI–B2). The final model showed no significant issues. We used 2000 simulations of model coefficients to obtain credible intervals using posterior distribution in the package arm (Gelman and Su 2020) to overcome the issues of confidence intervals in GLMM. Pseudo R2 values were calculated using package MuMIn (Barton 2020) following (Nakagawa et al. 2017). We calculated the intraclass correlation coefficient (ICC) for the final model to see the portion of variance captured by grouping variables. All the analyses were carried out using R (R Core Team 2020), and visualization used ggplot2 (Wickham 2016).
Results
All the measured variables within studied forest patches showed variations in values (Table 3).
The Eigenvalues for the first three principal components were more than one, with the only first component Eigenvalue more than two. It took five principal components to capture more than 90% of the cumulative variance in data (Table 4).
Among the variables used in PCA, plot-level average DBH and Height, and largest tree DBH and Height viz. tree size showed a high correlation to the first principal component. Plot level tree number and richness had the highest correlation with the second principal component. The third principal component was found positively correlated to canopy cover, and the fourth component showed a high correlation to the number of trees in the plot. The results show the main contribution of tree size, richness and number, and canopy cover in the first, second, and third principal components, respectively, as shown in the correlation circle (Fig. 2). We selected the first five components (Fig. SI–F) that explained around 93% of the variance.
The correlation circles showing the correlation of variables and principal components. The first correlation circle shows variable relations to PC1 and PC2, while the second circle shows variable relations to PC2 and PC3. It is seen that PC1 is represented by tree structure parameters viz. stand-level average DBH and height, as well as largest tree DBH and height, whereas PC2 is represented by tree species richness and number. PC3 is represented mainly by canopy cover
This is clear that the probability of invasion decreases with an increase in PC1 values and PC3 values. In terms of forest trails, the anthropogenic disturbance has a negative relationship with invasion, whereas; higher leaf litter condition is seen to lower the invasion probability (Fig. 3).
The model dispersion test showed no significant over dispersion (ratio = 0.93, χ2 = 128.63, rdf = 139, p = 0.72). The R2 value (marginal = 0.34 and conditional = 0.44) showed that about 10% more variance was contributed by forests as grouping variable which is further confirmed by intra-class correlation (ICC = 0.35).
Probability of invasive species presence was found significantly negatively related with PC1 (β = -0.318, z = -2.19, p = 0.027) and PC3 (β = -0.719, z = -2.62, p = 0.008). The unit increase in PC1 value is found to reduce the probability of invasive species presence by about 7%. The unit increase in PC3 reduces the probability of invasive species presence by about 17%, considering other covariates constant. The mean invasive species presence probability for abundant leaf litter condition was found 0.57, and that increased to 0.89 and 0.91 for sparse and rare litter conditions (Table 5). The distance of forest trails to sampling plots was found to have a negative relationship (Fig. SI–G) with the presence of IAPS but was not significant (β = -0.03, z = -1.62, p = 0.105).
It is seen that IAPs invasion decreases under abundant (Abu) and sparse (Spr) leaf litter conditions whereas increases under rare (Rar) leaf litter conditions with values of PC1 (Fig. 4; Fig. SI–H). The probability of invasive species presence is found to decrease singificantly with values of PC3 for all the categories of leaf litter condition namely, abundant (Abu), sparse (Spr), and rare (Rar) (Fig. 5; Fig. SI–I).
Discussion
Communities around the world traditionally protect natural sites that are dedicated to ancestral spirits or deities.These sacred grooves and other urban forests are crucial for habitat protection and biodiversity conservation in the face of the constant anthropogenic pressure subjected towards a natural ecosystem in urban areas (Bhagwat and Rutte 2006; Tordoni et al. 2017). Similarly, most of the urban green space in Kathmandu valley, prominently the larger patches, are represented by urban sacred grooves (Mansberger 1991). However, in Nepal’s urban areas like Kathmandu, we have not managed the information to show the biodiversity status of the urban regions and how the urban biodiversity should be maintained. In this study focusing on Kathmandu valley, we found that structural components of the forest exert resistance against the invasion by selected exotic plants, namely, Lantana camara, Ageratina adenophora, and Parthenium hysterophorus. These three species are reported from the degraded forest areas with Lantana camera reported having tolerance for mild canopy coverage (Ghisalberti 2000; Adkins and Shabbir 2014; Yang et al. 2017; Negi et al. 2019). Additionally, the response of invasive species also varies significantly among the forest patches. This finding implies that the structural complexity of the forest should be integrated into the management decision of urban forests.
Forest structure and invasion
The dominance of the tree was found to be the significant factor in resisting the invasion. In our case, the probability of the occurrence of invasive species decreased along the first principal axis represented by both stand level and individual tree size (DBH and height). The trees’ horizontal and vertical measures,especially those of the large-sized trees are important contributors to the structural complexity of the forest ecosystem (Seidel et al. 2019). The inverse relation of the probability of invasion with the first principal axis can be interpreted as the structural control of trees on the invasion. This entails maintaining dominance is crucial to resist the invasion. Different functional aspects of the forest ecosystem such as carbon sequestration, ecosystem regulation, biomass production, etc., are regulated mainly by large-sized trees and are considered essential components of the ecosystem (Lutz et al. 2018; Ali et al. 2019). Large trees enhance the heterogeneity of the forest and regulate the micro-and meso-climatic variables of the environment (Lutz et al. 2013; Lindenmayer and Laurance 2017). Availability of the resources such as light, moisture, and soil fertility is crucial for the successful establishment of invasive species (Dyderski and Jagodziński 2019). The filtering the invasive species in the area with more giant trees can be related to the regulation of the resources such as light, moisture, soil fertility, though not quantified in our study, to the invasive species in the forests.
The negative relation of occurrence of the invasive species to the third principal axis, contributed by canopy coverage, implies that canopy cover regulates the amount and intensity of the light received by the understory invasive species. The amount and intensity of the light, in turn, regulate the nutrient uptake and germination success of invasive species (Dyderski and Jagodziński 2019). This ultimately results in a low occurrence of invasive species in high canopy areas.
Disturbance and invasion
High population density proximity to urban forests poses intense recreational pressures that contribute to biodiversity loss (Vakhlamova et al. 2016). When the forests are exposed to a high level of disturbances, native species are removed, creating space for invasion by the alien species (Bonanomi et al. 2018). Areas with high human interference usually contain a high variety of invasive and alien species (Mavimbela et al. 2018). Canopy cover represents the structural attribute of the forest, and canopy opening is considered an indicator of the disturbance. With increased disturbance, gaps are created on the canopy. Furthermore, though marginally significant in our case, the occurrence of the invasive species is negatively associated with the distance from the trail. Trails and the gaps in the canopy will create an open niche for species (Baret et al. 2008). Trails have been reported to be the facilitator of invasive species dispersal in urban forest ecosystems (Kang et al. 2019). Furthermore, trails influence understory vegetation (Lapaix et al. 2012), resulting in soil compaction and litter removal from the surface, thus exposing the soil to opportunistic species.
Leaf litter cover was another significant predictor of the invasive species occurrence in the urban forests. The probability of the occurrence of the invasive species found significantly higher in the areas with rare or sparse leaf litter conditions compared to the abundant leaf litter conditions. This can be related to the disturbance-invasion relationship. In the area with the abundance of leaf litter, the amount of light that gets transmitted is low compared to the areas with rare to sparse leaf litter conditions, impacting the germination success of the invasive species seeds (Setterfield et al. 2005). Researches have shown that the alien species richness, not considered in our study, is found to increase significantly with disturbance in the forest habitat (Alston and Richardson 2006). Furthermore, the probability of the occurrence of the invasive species increased even along with the first principal component when the leaf litter condition was rare. In contrast, the opposite relation was observed for sparse and abundant litter coverage. However, the probability of the occurrence of invasive species decreased along with the third principal component in all conditions of leaf litter coverage. This indicates that the leaf litter cover regulates structural controls of the invasion while the canopy itself can contend the invasion.
According to the theory proposed by Elton (1958), increased species richness should confer a higher degree of invasion resistance. In our case, neither species richness nor tree abundance was retained in the final model indicating the influence of the structural complexity and disturbance to be more important than the species richness. It is inferred from the results that leaf litter management and maintaining structural complexity are paramount in urban forests to control the invasion. Furthermore, trail management can somehow help to contain invasive species distribution in urban forests. Currently, a large number of informal trails criss-cross the urban forest patches within Kathmandu valley. Approximately a population of about 2.5 million is expected to interact with forests for pilgrimage or recreation. The informal trails are then turned into formal trails with increased width and soil erosion. The unmanaged trail can initiates the widespread network of trails by trampling impacts that ultimately facilitate invasion. Unmanaged trail systems promote invasion by decreasing the canopy cover of urban forest patches. Therefore, reducing the amount of light received by the invasive species. Low light intensity will reduce the germination success of the invasive species (Setterfield et al. 2005) and is vital for controlling invasive species in urban forests.
Conclusion
Several species of invasive plants invade the urban forests. The features of urban forest patch such as tree size and stand height has a role in promoting or filtering the success of the invasive plant species. As urbanization-induced mobility creates a suitable niche for invasive species through disturbance of the forest floor, structural control can be applied through the increased stock of large trees. As larger trees with wider canopy are seen to control plant invasion in urban green spaces, conserving the remnant trees in such areas is essential. Leaf litter conditions when left high with minimal disturbance also contribute to checking the invasion. The large trees, an ecologically critical habitat for urban birds and other animal taxa act as a natural control of invasive species burden. Thus, urban plantation and trail management can be leveled to reduce the burden of plant invasions in urban green spaces of subtropical regions. Rather than maintaining a dense canopy of only small trees, we recommend retaining larger trees with a wider canopy in mixed stands to maintain the structural complexity. The presence of large trees favors forming the multilayered canopy cover, which might be more effective in contending against invasion. However, the quantification of invasive species burden in terms of biomass or density will clarify the notion of safe areas in urban forests in urban centers in the subtropical Himalayas.
References
Adkins S, Shabbir A (2014) Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci 70(7):1023–1029. https://doi.org/10.1002/ps.3708
Ali A, Lin SL, He JK, Kong FM, Yu JH, Jiang HS (2019) Big-sized trees overrule remaining trees’ attributes and species richness as determinants of aboveground biomass in tropical forests. Glob Change Biol 25(8):2810–2824. https://doi.org/10.1111/gcb.14707
Alston KP, Richardson DM (2006) The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol Cons 132(2):183–198. https://doi.org/10.1016/j.biocon.2006.03.023
Aronson MFJ, Lepczyk CA, Evans KL, Goddard MA, Lerman SB, MacIvor JS, Nilon CH, Vargo T (2017) Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ 15(4):189–196. https://doi.org/10.1002/fee.1480
Ballinas M, Barradas VL (2016) The urban tree as a tool to mitigate the urban heat island in Mexico City: A simple phenomenological model. J Environ Qual 45(1):157–166. https://doi.org/10.2134/jeq2015.01.0056
Baret S, Cournac L, Thébaud C, Edwards P, Strasberg D (2008) Effects of canopy gap size on recruitment and invasion of the non-indigenous Rubus alceifolius in lowland tropical rain forest on Réunion. J Trop Ecol 24(3):337–345. https://doi.org/10.1017/S0266467408004987
Barton K (2020) MuMIn: Multi-Model Inference. https://cran.r-project.org/package=MuMIn
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bhagwat SA, Rutte C (2006) Sacred groves: Potential for biodiversity management. Front Ecol Environ 4(10):519–524. https://doi.org/10.1890/1540-9295(2006)4[519:SGPFBM]2.0.CO;2
Bonanomi G, Incerti G, Abd El-Gawad AM, Sarker TC, Stinca A, Motti R, Cesarano G, Teobaldelli M, Saulino L, Cona F, Chirico GB, Mazzoleni S, Saracino A (2018) Windstorm disturbance triggers multiple species invasion in an urban mediterranean forest. Iforest 11(1):64–71. https://doi.org/10.3832/ifor2374-010
Brook BW, Sodhi NS, Bradshaw CJA (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23(8):453–260. https://doi.org/10.1016/j.tree.2008.03.011
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33(2):261–304. https://doi.org/10.1177/0049124104268644
Cadotte MW, Yasui SLE, Livingstone S, MacIvor JS (2017) Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol Invasions 19(12):3489–3503. https://doi.org/10.1007/s10530-017-1586-y
Calfapietra C, Morani A, Sgrigna G, Di Giovanni S, Muzzini V, Pallozzi E, Guidolotti G, Nowak D, Fares S (2016) Removal of ozone by urban and peri-urban forests: Evidence from laboratory, field, and modeling approaches. J Environ Qual 45(1):224–233. https://doi.org/10.2134/jeq2015.01.0061
Dolan RW, Carter T, Ryan T, Salsbury C, Dolan TE, Hennessy M (2013) Trials of the urban ecologist. Front Ecol Environ 11(3):163–164. https://doi.org/10.1890/1540-9295-11.3.163
Dyderski MK, Jagodziński AM (2019) Context-dependence of urban forest vegetation invasion level and alien species’ ecological success. Forests 10(1):1–20. https://doi.org/10.3390/f10010026
Early R, Bradley BA, DukesJS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibañez I, Miller LP, Sorte CJB, Tatem AJ (2016) Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun 7. https://doi.org/10.1038/ncomms12485
Elton CS (1958) The Ecology of Invasions by Animals and Plants
Faeth SH, Saari S, Bang C (2012) Urban Biodiversity: Patterns, Processes and Implications for Conservation. ELS. https://doi.org/10.1002/9780470015902.a0023572
Gaertner M, Wilson JRU, Cadotte MW, MacIvor JS, Zenni RD, Richardson DM (2017) Non-native species in urban environments: patterns, processes, impacts and challenges. Biol Invasions 19(12):3461–3469. https://doi.org/10.1007/s10530-017-1598-7
Gelman A, Su Y-S (2020) Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. https://cran.r-project.org/package=arm
Ghisalberti EL (2000) Lantana camara L. (Verbenaceae). Fitoterapia 71(5):467–486. https://doi.org/10.1016/S0367-326X(00)00202-1
Hartig F (2021). DHARMa: Residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.4.3. http://florianhartig.github.io/DHARMa/
ICIMOD/MoEST/UNEP (2007) Kathmandu Vvlley environmental outlook, International centre for integrated mountain development (ICIMOD)
Ishtiaque A, Shrestha M, Chhetri N (2017) Rapid urban growth in the Kathmandu Valley, Nepal: monitoring land use land cover dynamics of a himalayan city with landsat imageries. Environments 4(4):72. https://doi.org/10.3390/environments4040072
Jaganmohan M, Knapp S, Buchmann CM, Schwarz N (2016) The bigger, the better? The influence of urban green space design on cooling effects for residential areas. J Environ Qual 45(1):134–145. https://doi.org/10.2134/jeq2015.01.0062
Kang W, Song Y, Lee D, Kim G, Chae H (2019) Identifying habitats and corridors of an invasive plant, Ageratina altissima, in an urban forest. Landscape Ecol Eng 15(3):277–287. https://doi.org/10.1007/s11355-019-00381-y
Khanal N, Uddin K, Matin MA, Tenneson K (2019) Automatic detection of spatiotemporal urban expansion patterns by fusing OSM and Landsat data in Kathmandu. Remote Sens 11(19). https://doi.org/10.3390/rs11192296
Klemm W, Heusinkveld BG, Lenzholzer S, Jacobs MH, Van Hove B (2015) Psychological and physical impact of urban green spaces on outdoor thermal comfort during summertime in The Netherlands. Build Environ 83:120–128. https://doi.org/10.1016/j.buildenv.2014.05.013
Lapaix R, Harper K, Freedman B (2012) Patterns of exotic plants in relation to anthropogenic edges within urban forest remnants. Appl Veg Sci 15(4):525–535. https://doi.org/10.1111/j.1654-109X.2012.01195.x
Lê S, Josse J, Husson F (2008) FactoMineR: An R package for multivariate analysis. J Stat Softw 25(1):1–18. https://doi.org/10.18637/jss.v025.i01
Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, Macivor JS (2017) Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. Bioscience 67(9):799–807. https://doi.org/10.1093/biosci/bix079
Lindenmayer DB, Laurance WF (2017) The ecology, distribution, conservation and management of large old trees. Biol Rev 92(3):1434–1458. https://doi.org/10.1111/brv.12290
Livesley SJ, McPherson GM, Calfapietra C (2016) The urban forest and ecosystem services: Impacts on urban water, heat, and pollution cycles at the tree, street, and city scale. J Environ Qual 45(1):119–124. https://doi.org/10.2134/jeq2015.11.0567
Lutz JA, Furniss TJ, Johnson DJ, Davies SJ, Allen D, Alonso A, Anderson-teixeira KJ, Becker KML, Andrade A, Baltzer J, Blomdahl EM, Bourg NA, Bunyavejchewin S, Burslem DFRP, Cansler CA, Fischer GA, Fletcher C, Freund JA, Giardina C, Germain SJ (2018) Global Importance of Large-Diameter Trees. https://doi.org/10.1111/geb.12747
Lutz JA, Larson AJ, Freund JA, Swanson ME, Bible KJ (2013) The importance of large-diameter trees to forest structural heterogeneity. PLoS One 8(12). https://doi.org/10.1371/journal.pone.0082784
Maharjan S, Joshi S, Shrestha BB, Devkota A, Jha PK (2015) Life History Traits and Invasion Success of Parthenium hysterophorus L. in Kathmandu Valley, Nepal. Nepal J Sci Technol15(1):31–38. https://doi.org/10.3126/njst.v15i1.12007
Mansberger JR (1991) Ban yatra: a biocultural survey of sacred forests in Kathmandu Valley. University of Hawaii
Mavimbela LZ, Sieben EJJ, Procheş Ş (2018) Invasive alien plant species, fragmentation and scale effects on urban forest community composition in Durban, South Africa. N Z J For Sci 48(1). https://doi.org/10.1186/s40490-018-0124-8
McCary MA, Mores R, Farfan MA, Wise DH (2016) Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: A meta-analysis. Ecol Lett 19(3):328–335. https://doi.org/10.1111/ele.12562
McDonald RI, Urban DL (2006) Edge effects on species composition and exotic species abundance in the North Carolina Piedmont. Biol Invasions 8(5):1049–1060. https://doi.org/10.1007/s10530-005-5227-5
McLean P, Gallien L, Wilson JRU, Gaertner M, Richardson DM (2017) Small urban centres as launching sites for plant invasions in natural areas: insights from South Africa. Biol Invasions 19(12):3541–3555. https://doi.org/10.1007/s10530-017-1600-4
Milanovich JR, Peterman WE, Barrett K, Hopton ME (2012) Do species distribution models predict species richness in urban and natural green spaces? A case study using amphibians. Landsc Urban Plan 107(4):409–418. https://doi.org/10.1016/j.landurbplan.2012.07.010
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14(134). https://doi.org/10.1098/rsif.2017.0213
Negi GCS, Sharma S, Vishvakarma SCR, Samant SS, Maikhuri RK, Prasad RC, Palni LMS (2019) Ecology and Use of Lantana camara in India. Bot Rev 85(2):109–130. https://doi.org/10.1007/s12229-019-09209-8
Pandey S, Khanal SN, Giri D, Singh A (2010) The influence of meterological parameters and PM10 concentrations on visibility of Kathmandu Valley. HARMO 2010 - Proceedings of the 13th International Conference on Harmonisation within Atmospheric Dispersion Modelling for Regulatory Purposes 1:872–876
Pinto SM, Ortega YK (2016) Native species richness buffers invader impact in undisturbed but not disturbed grassland assemblages. Biol Invasions 18(11):3193–3204. https://doi.org/10.1007/s10530-016-1208-0
Seidel D, Ehbrecht M, Dorji Y, Jambay J, Ammer C, Annighöfer P (2019) Identifying architectural characteristics that determine tree structural complexity. Trees. https://doi.org/10.1007/s00468-019-01827-4
Setterfield SA, Douglas MM, Hutley LB, Welch MA (2005) Effects of canopy cover and ground disturbance on establishment of an invasive grass in an Australia Savanna. Biotropica 37(1):25–31. https://doi.org/10.1111/j.1744-7429.2005.03034.x
Shrestha BB, Shabbir A, Adkins SW (2015) Parthenium hysterophorus in Nepal: A review of its weed status and possibilities for management. Weed Res 55(2):132–144. https://doi.org/10.1111/wre.12133
Steenberg JWN (2015) Urban forest vulnerability and its implications for ecosystem service supply at multiple scales [Doctoral dissertation, Environmental Applied Science and Management, Ryerson~…]. Growth. https://digital.library.ryerson.ca/islandora/object/RULA:5097/datastream/OBJ/download/Urban_forest_vulnerability_and_its_implications_for_ecosystem_service_supply_at_multiple_scales.pdf
Taylor S, Kumar L, Reid N, Kriticos DJ (2012) Climate change and the potential distribution of an invasive shrub. Lantana Camara l Plos One 7(4):1–14. https://doi.org/10.1371/journal.pone.0035565
Team RC (2020) R: A Language and Environment for Statistical Computing. R Fou Stat Comput 1(4.0.2):409. https://doi.org/10.1007/978-3-540-74686-7
Thapa LB, Kaewchumnong K, Sinkkonen A, Sridith K (2017) Plant invasiveness and target plant density: high densities of native Schima wallichii seedlings reduce negative effects of invasive Ageratina adenophora. Weed Res 57(2):72–80. https://doi.org/10.1111/wre.12238
Tordoni E, Napolitano R, Nimis P, Castello M, Altobelli A, Da Re D, Zago S, Chines A, Martellos S, Maccherini S, Bacaro G (2017) Diversity patterns of alien and native plant species in Trieste port area: exploring the role of urban habitats in biodiversity conservation. Urban Ecosystems 20(5):1151–1160. https://doi.org/10.1007/s11252-017-0667-0
Vakhlamova T, Rusterholz HP, Kamkin V, Baur B (2016) Recreational use of urban and suburban forests affects plant diversity in a Western Siberian city. Urban For Urban Green 17:92–103. https://doi.org/10.1016/j.ufug.2016.03.009
Wickham H (2016) Elegant Graphics for Data Analysis. Springer-Verlag New York. http://ggplot2.org
Yang G, Gui F, Liu W, Wan F (2017) Crofton Weed Ageratina adenophora (Sprengel). Biol Invasions Manag China 111–129. Springer Singapore. https://doi.org/10.1007/978-981-10-3427-5_8
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. In Mixed effects models and extensions in ecology with R. Springer New York. https://doi.org/10.1007/978-0-387-87458-6
Acknowledgement
We would like to acknowledge funding support from University Grants Commission, Nepal. We acknowledge the cooperation from the management committees of study forests in Kathmandu Valley. Uttam Aryal, Sagar Dhakal of GoldenGate International College and Laxman Phuyal, supported the field survey. We are indebted to Nepal Army for providing us permission to survey Gokarna Forest and GoldenGate International College for logistic support.
Funding
This study was funded by University Grants Commission Nepal.
Author information
Authors and Affiliations
Contributions
PCA conceptualized the study, PCA, KB, DC, SRM, MKD conducted the field. PCA & CA analyzed the data, PCA & CA wrote the draft paper, and all the authors reviewed and edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aryal, P.C., Aryal, C., Bhusal, K. et al. Forest structure and anthropogenic disturbances regulate plant invasion in urban forests. Urban Ecosyst 25, 367–377 (2022). https://doi.org/10.1007/s11252-021-01159-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-021-01159-7