Abstract
Urbanization generally reduces wildlife populations. Individual species responses, however, are often highly variable, and such variability can be explained by differences in species ecological traits. To examine this hypothesis, we focused on two co-occurring land snails, Ezohelix gainesi and Euhadra brandtii sapporo; the former is ground-dwelling and the latter is arboreal. We first estimated their population densities at nine sites distributed along an urbanization gradient: three were located in continuous natural forests, three at the edge of natural forests, and the rest in small isolated forests in urban areas. As a result, the ground-dwelling E. gainesi occurred at highest density in urban forests, followed by forest edges and continuous forests. By contrast, the arboreal E. b. sapporo occurred at highest density in continuous forests, but declined in forest edges and urban forests. We then conducted manipulative field experiments to quantify changes in predation pressure on these species. Ground-tethered E. gainesi and E. b. sapporo were repeatedly predated upon by forest-living mammals in continuous forests, but their survival rates increased in forest edges and urban forests. By contrast, canopy-tethered E. b. sapporo maintained high survival rates in all three forest types. The results indicate that a lack of mammalian predators enables ground-dwelling species to occur at high densities in urban forests, whereas the arboreal species was not affected by this predator relaxation effect. The combination of species-specific behavioural traits and changes in predator communities across an urbanization gradient has important effects on the biodiversity of urban ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural ecosystems are increasingly being affected by anthropogenic disturbances, and urbanization is among the most impactful of these disturbances. Habitat loss and fragmentation in urbanized areas promote local extinction (McKinney 2002; Marzluff 2005), declining species richness (Miyashita et al. 1998; Martinson and Raupp 2013), increasing populations of synanthropic and non-native species (Riley et al. 2005; Shochat et al. 2010), and reduction of beta diversity or homogenization (Horsák et al. 2013; Hodges and McKinney 2018). Anthropogenic disturbances create drier and warmer environments (Gallo and Owen 1999; Kalnay and Cai 2003; Yang et al. 2017), and species sensitive to those changes (e.g., lichens and mosses) are known to develop markedly different communities in urban versus non-urban areas (Lättman et al. 2014; Oishi 2018). Recent studies have demonstrated that land-use changes have a large influence on functional diversity, which is closely related to ecosystem services (Kissick et al. 2018).
Numerous studies have demonstrated that urbanization has tremendous effects on biodiversity. Yet the actual mechanisms regulating these effects are poorly understood (Faeth et al. 2005; Shochat et al. 2006, 2010; Fischer et al. 2012; El-Sabaawi 2018). In general, species are highly variable in their response to anthropogenic disturbances (e.g., Patten and Bolger 2003; Gagné and Fahrig 2011; Saito and Koike 2013; Ehlers Smith et al. 2018). Many species populations decline with increasing urbanization, but some species populations actually increase (Hansen et al. 2005; McDonnell and Hahs 2008). We predicted that such variability could be explained by differences in species’ ecological traits. Species’ responses to urbanization are often closely related to changes in predator–prey interactions (Gering and Blair 1999; Shochat et al. 2006; Ritchie and Johnson 2009; Rodewald et al. 2011; El-Sabaawi 2018). Loss of top-down control and release of prey populations are a common trophic consequence of land-use change (Crooks and Soulé 1999; Faeth et al. 2005; Fischer et al. 2012). However, this mechanism may not be applicable to certain functional groups; for example, two closely-related species may respond differently to land-use change because of differences in their anti-predator behaviours.
To examine this hypothesis, we investigated the effects of urbanization on two co-occurring land snails, Ezohelix gainesi and Euhadra brandtii sapporo; the former is ground dwelling and the latter is arboreal (Saeki et al. 2017a). We selected these species because they have differing behavioural traits that may trigger different ecological processes following anthropogenic disturbances. One of these processes, predator–prey interaction, is often difficult to quantify in the field, with the exception of a few bird studies that use artificial nests (Gering and Blair 1999). However, land snails’ slow movement and low sensitivity to close human observation allow us to record predation rates in detail. The two snail species in our study are found predominantly in the same regional landscape of southern Hokkaido, Japan, which is severely affected by forest fragmentation and the introduction of non-native species. We took advantage of these regional characteristics to quantify changes in predator–prey interactions along a gradient of urbanization.
Although there has been increased interest in biodiversity–urbanization relationships in recent years, the available literature is biased towards vertebrate studies, especially birds (Ritchie and Johnson 2009; Gagné and Fahrig 2011). Yet invertebrates represent a majority of animal diversity (Groombridge and Jenkins 2002) and have distinctive ecological functions (Mason 1970; Kissick et al. 2018). Moreover, molluscs are important conservation targets because of their high diversity, large number of extinction records (Lydeard et al. 2004), and ability to be used as ecosystem indicators due to their limited dispersal ability (Hodges and McKinney 2018).
Our general objective was to identify the effects of urbanization on two ecologically-distinct land snails with a special focus on predator–prey interactions. Our specific objectives were to (i) compare the spatial distribution of arboreal and ground-dwelling land snails in continuous forests and forests that have been highly fragmented by urbanization, (ii) use manipulative field experiments to examine the effects of predation, or top-down control, on snail abundance, and (iii) analyse the relationship between species occurrence and predator communities along an urbanization gradient.
Methods
Study area
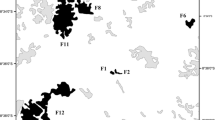
Field studies were conducted in Tomakomai city, Hokkaido, Japan. Our study region covers latitudes of 42.64–42.71°N and longitudes of 141.55–141.70°E with an elevation range of 7–128 m (Table 1, Fig. 1). We used nine study sites in this region: three were located in large, continuous natural forests, three at forest edges, and three in small isolated forests in urbanized areas (hereafter ‘continuous forests’, ‘edge forests’, and ‘urban forests’, respectively). The forest area of continuous, edge, and urban forests ranged from over 50 km2 (continuous forests) to less than 0.1 km2 (urban forests) (Table 1). Data obtained from aerial photographs (provided by the Geospatial Information Authority of Japan) show that there was relatively little fragmentation of edge and urban forests until the 1950s. These forests were gradually fragmented and or converted to residential and industrial areas in the 1970s to 2000s. Of our nine sites, Itoi (ITO), Takuyuu (TKU), and Numanohata Centre (NUC) are managed by local governments, Uenae (UEN), Futou (FUT), and Numanohata South (NUS) are owned by private companies, and Horonai River (HOR), TOEF-North (TOE), and Takaoka (TKA) are located in the Tomakomai Experimental Forest, which is owned by Hokkaido University (TOEF).
Map of nine study sites in Tomakomai city, Hokkaido Island, Japan. See Table 1 for abbreviation of site names. Sites 1–3, continuous forest; 4–6, edge forest; 7–9, urban forest. The base map is created from Google Earth
The climate of our study area is characterized by cool summers and cold winters. Maximum winter snow depth ranges from 20 to 50 cm. There was little pre-existing microclimatic data for our study sites; instead, we conducted our own monitoring of temperature at the forest floor (just below the litter layer, height = 0 m; TidbiT, Onset, Bourne, MA, USA) and in the canopy (height = ca. 6–8 m; HOBO U23 Pro v2, Onset), and measured canopy relative humidity at 4-h intervals from November 2017 to October 2018. Mean annual ground and canopy temperatures were 8.1–8.8 °C and 6.7–8.0 °C, respectively, and mean annual canopy relative humidity was 78.9–85.2% (Supporting Information 1). Mean annual temperature differed between canopy positions (canopy vs. ground) and also among the three forest types (Supporting Information 2). These differences were small in magnitude, however (<0.5 °C). Relative humidity differed among the three forest types in summer (F = 20.7, P = 0.002), but did not differ in winter (F = 1.54, P = 0.29). Again, these differences were small in magnitude (< 4%).
Our study area is situated within the cool-temperate deciduous forest zone. Dominant tree species are Quercus crispula, Acer pictum, A. amoenum, and Magnolia obovata. The topography is generally flat, with no steep slopes. The soil originates from volcanic ash, which was deposited when Mt. Tarumae, located approximately 20 km away from the area, erupted about 330 years ago.
Density of land snails
To estimate the population density of E. gainesi and E. b. sapporo, we counted the number of individuals per unit land area in ground litter from 24 to 30 October 2017 at our nine study sites. We conducted our investigation during this period because we found in previous research that arboreal E. b. sapporo move to the ground in late fall and stay there until the following spring (Saeki et al. 2017a). We established three 5.4 × 5.4 m plots at each study site and recorded the number of living individuals and dead shells in the ground litter. Surveys were conducted by two to four persons, and sampling effort was maintained at approximately 30 min/person/plot. Ezohelix gainesi and E. b. sapporo were the two most dominant land snails in our sample, and all the other snail species we found at our study sites were < 5 mm in shell diameter. Relationships between forest type and E. b. sapporo and E. gainesi abundance were examined using a generalized linear model (GLM) constructed using the statistical software JMP 9.0 (SAS Institute, Cary, NC, USA).
Predation pressure
To quantify predation pressure at continuous, edge, and urban forests, we performed two manipulative field experiments. The first experiment was conducted from 24 August to 7 September 2017 (hereafter referred to as ‘2017-fall’), and the second was from 29 June to 12 July 2018 (hereafter referred to as ‘2018-summer’). In the first experiment, we collected 96 E. b. sapporo and 48 E. gainesi in a natural forest in the TOEF. We selected adult-sized individuals of shell diameter 20–27 mm for E. b. sapporo and 25–40 mm for E. gainesi. Sample collection was conducted over 2 days, and we brought the collected individuals alive to a laboratory at the TOEF. Then, an approximately 50-cm long string (Kevlar, diameter 0.25 mm) was tied around the shell of each snail, and a numbered label was also glued to the shell. A previous study (Saeki et al. 2017a) indicated that this treatment has little effect on snail survival and allows reasonably free movement within a radius equal to the length of the string. Attachment of strings and number labels was completed within 24 h after collection, and the snails were stored at room temperature for another 12–36 h until the first day of the manipulative field experiment.
The first experiment in 2017-fall was performed at six study sites: three continuous forest sites, two edge forest sites, and one urban forest site (Table 1, Fig. 1). We conducted this experiment at a relatively limited scale (i.e., six sites) to grasp the general trend of species occurrences in Tomakomai city along a gradient of forest fragmentation and urbanization. At each site, we selected one Q. crispula tree to serve as the location for the manipulative field experiment. For all selected trees, diameter at breast height was ca. 20–50 cm and tree height was ca. 15–20 m. Individual snails that had been collected and prepared as described above were brought to each site. For each selected tree, eight E. b. sapporo were tethered to the trunk at a height of 5–10 m, and eight were tethered on the ground. Eight E. gainesi were also tethered on the ground. We did not tether E. gainesi to tree trunks because mature individuals of this species are almost completely ground dwelling. Canopy-tethered E. b. sapporo were placed at least 0.5 m apart, and ground-tethered E. b. sapporo and E. gainesi were placed ca. 3–5 m apart.
Infrared camera trap (HGC SG-007, Shenzhen Siyuan Digital Technology, Dongguan City, China) were set up at each study tree to record predation events. One was placed in the crown of the study tree to monitor one of the eight individuals in the canopy treatment. The others were positioned to monitor individuals on the ground. The number of cameras on the ground was one to three per study tree depending on the availability of camera devices and batteries. After preparing this set-up, we visited the study trees every 1–3 days and noted whether each of the total 144 land snails (i.e., 96 E. b. sapporo and 48 E. gainesi) was dead or alive. Because it took 2 days to place these snails in the field, we started the experiment 1 day earlier for two-thirds of the samples (i.e., four study sites), followed by the remaining one-third (i.e., two study sites) the next day. Canopy access was achieved by using single-rope techniques (Ishii 2000; Lowman et al. 2012).
In the first experiment, a clearly different survival trend was perceived across an urbanization gradient (see Results). Thus, we decided to perform the second experiment with a larger sample size by adding one edge and two urban forest sites. The total sample size was therefore 144 for E. b. sapporo and 72 for E. gainesi. The experimental procedure was similar to the one in the first experiment (Table 1). This second experiment (2018-summer) also enabled us to examine species responses in a different season from that of the 2017-fall experiment.
After the experiments, survival curves were constructed using the Kaplan–Meier method and compared by a log-rank test for each pair (Fox 2001) by using the statistical software JMP 9.0 (SAS Institute). Because of the small sample size (eight individuals) for each tree and the similarity of survival curves among the study trees within each treatment, data from the trees in the same treatment (i.e., land snail species × forest type) were pooled in the analysis. A few individuals escaped from the string during the experiment, and we treated them as “censored” in this model. When a land snail was found dead, the cause of death (predation or otherwise) was inferred from the available data.
Predator community
Four pitfall traps (9 cm in diameter, 12 cm in depth, without bait or preservatives) were placed approximately 20 m from the study tree during manipulative field experiments at each study site to identify the species composition of ground-dwelling beetles, which are putative predators of our two land snail species. The traps were opened for 12 days during both the 2017 and 2018 experiments. Invertebrates that fell into the traps were collected every 3 days, identified and counted in situ, and then released several metres away from the traps. Although a wide range of ground-living invertebrates was captured in the traps, we focused on carabine beetles (Carabidae: Carabinae) because this taxon contains specialists that consume land snails; their predation was also confirmed in the laboratory (S. Niwa, unpublished data).
In addition to the pitfall traps, we performed camera-trap surveys to identify putative mammal and bird predators. Animal sensor cameras were set up during the manipulative field experiments at each site as described above, as well as before and after the experiments. Camera-trap monitoring was conducted from 24 August to 26 October in 2017 and from 29 May to 27 September in 2018. These data were used to estimate mammalian and avian community differences among study sites. One camera, which was used in the canopy before and after the experiment, was excluded from the analysis because the frequency of animal detection was quite low (only 18 detections during the entire study period).
Species composition data obtained by the pitfall and camera traps were collated into a matrix of site × species information, respectively. Then, we ran a non-metric multidimensional scaling (NMDS) analysis to visualize predator–community changes with urbanization. We used Bray–Curtis dissimilarity as a distance measure. The analysis was run by using the package vegan (Oksanen et al. 2019) in R 3.5.2 (R Core Team 2018). All experiments and sample collection procedures were performed in accordance with the Institutional Policy on Animal Experimentation of the University of Tsukuba.
Results
Density of land snails
The population density of arboreal E. b. sapporo and ground-dwelling E. gainesi differed among our study sites (Fig. 2). The density of E. b. sapporo was highest at site HOR followed by TOE, both of which are located in continuous forests, and decreased markedly with forest loss and fragmentation; it was low or zero in edge sites, and zero at all three urban sites. This pattern was reversed with E. gainesi. Its density was lowest in continuous forests but increased in edge forests, and was highest at site NUS, which is located in an urban forest. Our GLM analysis shows that E. b. sapporo and E. gainesi abundance differed significantly among forest types (χ2 = 117.78, df = 2, p < 0.0001 for E. b. sapporo and χ2 = 280.55, df = 2, p < 0.0001 for E. gainesi). We obtained similar patterns based on our count of dead shells (Supporting Information 3).
Euhadra brandtii sapporo (a) and Ezohelix gainesi (b) abundance at nine study sites located across un urbanization gradient in Tomakomai city, Hokkaido, Japan. Basis: three 5.4 × 5.4 m plots were surveyed by two to four persons (approximately 30 min/person/plot). Error bars indicate standard deviation. Please refer to Table 1 and Fig. 1 for site numbers, abbreviations, and locations
Predation pressure
For both species, the survival rate of ground-tethered individuals differed significantly among forest types in the 2018-summer experiment; it was highest in urban forests and decreased in edge and continuous forests (Fig. 3, Table 2a). A similar pattern was found in the 2017-fall experiment, although the differences were not statistically significant due to a relatively small sample size. By contrast, the survival rate of canopy-tethered E. b. sapporo did not differ significantly among forest types in either experiment. Survival rates were consistently high in all forest types in both years. When comparing between canopy and ground-tethered treatments, the survival rate of canopy-tethered E. b. sapporo was significantly higher than that of ground-tethered individuals in continuous forests in both years (Fig. 3, Table 2b). Yet these differences became less conspicuous in edge and urban forests. In edge forests, canopy-tethered individuals survived more than ground-tethered individuals in the 2018-summer experiment but not in the 2017-fall experiment, and no statistical difference was detected in urban forests in both years. In terms of comparisons between species, the survival rates of ground-tethered E. gainesi did not significantly differ from those of E. b. sapporo across all three forest types in both years (Table 2c).
Comparison of survival curves of Euhadra brandtii sapporo and Ezohelix gainesi for canopy and ground treatments in the 2017-fall (left) and 2018-summer (right) experiments. Error bars indicate 1 SE based on results of 8 samples × 3 sites (i.e., n = 24) for one forest type. See Table 2 for results of pairwise comparisons by log-rank tests
Mortality events among ground-tethered E. b. sapporo and E. gainesi were mainly due to predation by ground-dwelling mammals (Fig. 4). We attributed a predation event to ground- dwelling mammals in instances when the shell was broken or the string was cut and the sample disappeared. This inference is consistent with our camera-trap data, in which we observed multiple instances of mammalian predation leading to this outcome. At least four ground-tethered land snails were predated upon by Nyctereutes procyonoides, and two were predated upon by Vulpes vulpes, across the two experiments (Supporting Information 4). The average incidence rate of broken shells in the 2017-fall experiment was 23% for ground-tethered E. b. sapporo and 46% for E. gainesi in continuous forests, followed by 0% and 31% in edge forests and 0% and 13% in urban forests (Fig. 4), respectively. We obtained similar results in the 2018-summer experiment: the incidence of broken shells was highest in continuous forests for both species. Some ground-tethered E. b. sapporo, however, were found dead without any discernible shell damage. We observed a few cases of carabine beetles (both larvae and adults) eating the soft body of snails (Supporting Information 4). Thus, we assumed that some portion of the mortality events without discernible shell damage were due to predation by beetles. The survival rate of canopy-tethered E. b. sapporo was consistently high across all forest types, and our camera traps did not record any canopy predation events in the 2017-fall or 2018-summer experiments, although birds were occasionally observed in the canopy.
Comparisons of mortality rate and its estimated causes for Euhadra brandtii sapporo and Ezohelix gainesi obtained through the 2017-fall (left) and 2018-summer (right) manipulative field experiments. See Table 1 for site name abbreviations
Predator communities
The species composition of carabine beetles, which are known to prey on land snails, changed along an urbanization gradient. Carabus arboreus and Cychrus morawitzi were the dominant carabine beetles in all forest types in the 2017-fall experiment (Fig. 5a). In the 2018-summer experiment, Carabus albrechti was dominant in continuous and edge forests whereas Carabus granulatus and exotic Carabus insulicola were dominant in urban forests. Cychrus morawitzi and Carabus blaptoides, both snail specialists, were found in all three forest types.
Species composition of carabine beetles (a) and mammals and birds (b) in nine study sites along an urbanization gradient. See Table 1 for site name abbreviations. Carabine beetles were observed with pitfall traps, and mammals and birds were observed with camera traps. Specialist predators of land snails (carabine beetles) and species that preyed on land snails in our manipulative field experiments (mammals and birds) are indicated by red and yellow bars on the figure, and by an asterisk after the species name in the legend
Camera-trap records show that the species composition of ground-dwelling mammals also differed along the urbanization gradient (Fig. 5b). In total, we recorded four omnivores (Nyctereutes procyonoides, Vulpes vulpes, Procyon lotor, Sciurus vulgaris), one carnivore (Felis catus), and one herbivore (Cervus nippon). Nyctereutes procyonoides and C. nippon were frequently detected in continuous forests, but V. vulpes appeared in edge forests, and exotic F. catus and P. lotor were observed in urban forests. We also recorded three bird species on the ground, but their detection was limited to the urban forest sites.
Ordination analysis (NMDS) of carabine beetle communities revealed marked differences in species composition among forest types and seasons (Fig. 6a). Urban sites were assigned lower scores on NMDS Axis 2, whereas edge and continuous sites were assigned higher scores. Seasonal gradients were also detected: community composition data from the 2017-fall experiment were assigned low Axis 1 scores and high Axis 2 scores. The autumn-breeding carabine species (C. morawitzi, Carabus opaculus, C. arboreus) were assigned low and high Axis 1 and Axis 2 scores, respectively, whereas the spring-breeders (C. albrechti, C. insulicola, C. granulatus, Carabus conciliator, C. blaptoides) generally had high and low scores.
Ordination analyses (non-metric multidimensional scaling) of carabine beetle (a) and mammal and bird (b) communities at nine sites along an urbanization gradient. See Table 1 for site name abbreviations. Exotic species are underlined
Changes in mammalian and avian species composition were also clearly detectable along an urban to continuous forest gradient (Fig. 6b). The community composition in continuous forests was assigned a low score along NMDS Axis 1, and the community composition of the 2017-fall data was assigned a higher score along Axis 2. The 2017 data for the urban site TKU were not plotted on these coordinates because we did not record any mammal or bird species during that year at the site. There were distinct differences between the mammal and bird communities observed in continuous forests versus those in urban forests, and those in edge forests were of intermediate composition (Fig. 6a, b). Inter-site differences in species composition were larger for mammals and birds than for carabine beetles.
Discussion
Urbanization commonly alters trophic interactions (Faeth et al. 2005; Fischer et al. 2012), but the direction and magnitude of this alteration has rarely been estimated empirically. In this study, we investigated changes in the abundance, predation rate, and putative predator community of two land snails along a gradient of forest fragmentation. This approach enabled us to characterize their contrasting responses to urbanization.
The density of the ground-dwelling E. gainesi was highest in urban forests, followed by edge forests, and was lowest in continuous forests. By contrast, the density of the arboreal E. b. sapporo was highest in continuous forests and decreased markedly in edge forests, and the species was completely absent in urban forests (Fig. 2). The high density of E. gainesi in urban forests was likely due to a lack of predators. Our manipulative field experiments demonstrate that E. gainesi mortality is extremely low in urban forests, whereas many individuals were predated upon in continuous forests (Figs. 3 and 4). Ground-living mammals, such as N. procyonoides and V. vulpes, were the principal predators (Supporting Information 4). Predation events were repeatedly recorded by camera-trap monitoring, and many dead individuals were observed with damaged shells. We interpret these density and predation patterns as a typical case of prey-population release by reduction of top-down control in urbanized areas (Faeth et al. 2005, Fischer et al. 2012). Similar patterns also were observed for the arboreal E. b. sapporo when individuals were tethered to the ground, indicating that a reduction of predation pressure in urban areas probably applies to a wide range of large ground-dwelling snails.
This predator relaxation effect, however, was not seen in non-ground-tethered E. b. sapporo, which markedly decreased in population density along an urbanization gradient. Instead, the species’ survival rate in the canopy remained high in all three forest types (Figs. 3 and 4), most likely because arboreality allows it to avoid predators in both urban and continuous forests. This suggests that canopy-dwelling land snails may be more sensitive to dispersal limitations caused by forest fragmentation. Fragmentation causes a loss of canopy habitat, and likely interferes with the horizontal movement of arboreal species. In fact, a previous investigation revealed extremely low E. b. sapporo density in clear-cut and conifer-plantation forests (Saeki 2015). Unfortunately, we were unable to determine whether the presence of E. b. sapporo at the urbanized sites (TKU, NUC, and NUS) pre-dated the current state of severe forest fragmentation. One possibility is that the species was originally present at the sites, but was extirpated due to habitat fragmentation or environmental perturbation caused by urbanization. We observed the presence of exotic P. lotor in these urban sites using camera trap surveys (Figs. 5 and 6). Unlike native predators, this species is able to climb trees and prey on arboreal land snails (Saeki et al. 2017b). Although we did not record any predation events during this experiment (Fig. 4), episodic predation by exotic species may have driven local extinction in the past. In our study area, urban sites were warmer and more humid than non-urban sites (Supporting Information 1 and 2). However, this difference in microclimate is not likely to have substantially affected snail survival rates because the differences were slight and likely within the range of inter-annual variation in this region. Similarly, bottom-up processes (i.e., differences in food availability) are unlikely to have accounted for the effect of urbanization, as we did not observe large differences in ground litter, epiphytic lichen, or canopy moss availability.
The species composition of carabine beetles, which prey on land snails, changed from urban to natural forests (Figs. 5 and 6). This shift appears to have had relatively little effect on land snail survival rates in both the 2017-fall and 2018-summer experimental periods. In E. gainesi, almost all of the mortality was associated with shell-breaking predation by ground-living mammals (Fig. 4). This species is known to display a unique anti-predator behaviour, whereby it attacks predatory beetles by swinging its shell (Morii et al. 2016). In addition, juveniles of the species climb woody and tall herbaceous plants (Yuta Morii, personal communication), which is also an effective strategy for avoiding ground beetles. These behavioural traits likely account for the lack of mortality from ground-beetle predation. However, we did occasionally observe dead ground-tethered E. b. sapporo with undamaged shells (Fig. 4), which is an indicator of ground-beetle predation, and found one individual that had been predated upon by C. blaptoides (Supporting Information 4). These results indicate that there is some predation pressure from beetles on the ground, which may be sufficient to affect snails that do not possess anti-predator traits.
Relative to carabine beetles and birds, predation by mammals had the greatest effect on land snail survival rates (Fig. 4). Although ground beetles are closely linked to land snails both in terms of ecological interactions and evolutionary history (e.g., Konuma et al. 2011; Morii et al. 2016), predation by terrestrial mammals may be more important than predation by ground beetles in anthropogenically-disturbed landscapes. Comparative studies of top-down controls from multiple predators are extremely limited. Our results provide more evidence that prey responses to predators are highly variable in complex food webs, thereby highlighting the importance of identifying key predators and their effects. Regarding the trophic dynamics of urban ecosystems, a phenomenon called the predation paradox has been discussed (Fischer et al. 2012). That is, vertebrate predator populations increase with urbanization at the same time that predation rates decline. We suspect that this paradox may not apply to either of the snails we studied here, as we did not find any evidence of an increase in predator populations in edge and urban forests (Fig. 5).
Summary and management implications
We hypothesized that differences in ecological traits influence species’ responses to urbanization. This hypothesis was supported by the results of our study on land snails. Arboreal species, such as E. b. sapporo, are able to avoid predation simply by virtue of their arboreal behaviour. Thus, predation is not a major factor in the urban and non-urban survival rates of this species. Instead, habitat destruction and dispersal limitation caused by forest fragmentation may be more important for population sustainability than trophic interactions. By contrast, ground-dwelling E. gainesi experienced a marked decline in predation pressure from continuous to urban forests. The species has strong anti-predator defences against beetles but not against terrestrial mammals, which had a substantial role in regulating its abundance. These contrasting responses imply that the combination of species’ behavioural traits and changes in predator communities across an urbanization gradient can strongly affect biodiversity in urban ecosystems.
Biodiversity conservation planning is often discussed in terms of the presence and absence of species of concern. Yet changes in species interactions should be included more often in management planning. For example, connecting isolated urban forests to more natural, continuous forests not only helps avoid local extirpation of fragmentation-sensitive species, but also helps to restore more natural food-web networks. Complex trophic systems tend to prevent runaway population growth of a few prey species, thereby helping to maintain total biodiversity and ecosystem services. Nevertheless, it remains difficult to generalize the effects of urbanization on wildlife populations (Gagné and Fahrig 2011). We encourage further study on a wider range of taxonomic groups as well as in different geographic regions.
Change history
03 March 2020
The original version of the article unfortunately contained mistakes in the legends of Fig. 3. The legends (1) Blue square: <Emphasis Type="Italic">Euhadra brandtii sapporo</Emphasis>, ground is changed to Blue square: <Emphasis Type="Italic">Euhadra brandtii sapporo</Emphasis>, canopy (2) Red circle: <Emphasis Type="Italic">Euhadra brandtii sapporo</Emphasis>, canopy is changed to Red circle: <Emphasis Type="Italic">Euhadra brandtii sapporo</Emphasis>, ground.
References
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Ehlers Smith YC, Ehlers Smith DA, Ramesh T, Downs CT (2018) Forest habitats in a mixed urban-agriculture mosaic landscape: patterns of mammal occupancy. Landsc Ecol 33:59–76
El-Sabaawi R (2018) Trophic structure in a rapidly urbanizing planet. Funct Ecol 32:1718–1728
Faeth SH, Warren PS, Shochat E, Marussich WA (2005) Trophic dynamics in urban communities. BioScience 55:399–407
Fischer JD, Cleeton SH, Lyons TP, Miller JR (2012) Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. BioScience 62:809–818
Fox GA (2001) Future-time analysis. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, New York, pp 235–266
Gagné SA, Fahrig L (2011) Do birds and beetles show similar responses to urbanization? Ecol Appl 21:2297–2312
Gallo KP, Owen TW (1999) Satellite-based adjustments for the urban heat island temperature bias. J Appl Meteorol 38:806–813
Gering JC, Blair RB (1999) Predation on artificial bird nests along an urban gradient: predatory risk or relaxation in urban environments? Ecography 22:532–541
Groombridge B, Jenkins MD (2002) World atlas of biodiversity. UNEP World Conservation Monitoring Centre, Berkeley
Hansen AJ, Knight RL, Marzluff JM, Powell S, Brown K, Gude PH, Jones K (2005) Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol Appl 15:1893–1905
Hodges MN, McKinney ML (2018) Urbanization impacts on land snail community composition. Urban Ecosyst 21:721–735
Horsák M, Lososová Z, Čejka T, Juřičková L, Chytrý M (2013) Diversity and biotic homogenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 central European cities. PLoS One 8:e71783
Ishii H (2000) Canopy research using single-rope techniques in the temperate coniferous forests of the Pacific northwest, USA. Japanese Journal of Ecology 50:65–70 (in Japanese)
Kalnay E, Cai M (2003) Impact of urbanization and land-use change on climate. Nature 423:528
Kissick AL, Dunning JB Jr, Fernandez-Juricic E, Holland JD (2018) Different responses of predator and prey functional diversity to fragmentation. Ecol Appl 28:1853–1866
Konuma J, Nagata N, Chiba S (2011) Factors determining the direction of ecological specialization in snail-feeding carabid beetles. Evolution 65(2):408–418
Lättman H, Bergman KO, Rapp M, Tälle M, Westerberg L, Milberg P (2014) Decline in lichen biodiversity on oak trunks due to urbanization. Nord J Bot 32:518–528
Lowman MD, Schowalter TD, Franklin JF (2012) Methods in Forest canopy research. University of California Press, Berkeley
Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark SA, Cummings KS, Frest TJ, Gargominy O, Herbert DG, Hershler R, Perez KE, Roth B, Seddon M, Strong EE, Thompson FG (2004) The global decline of nonmarine mollusks. BioScience 54:321–330
Martinson HM, Raupp MJ (2013) A meta-analysis of the effects of urbanization on ground beetle communities. Ecosphere 4:art60
Marzluff JM (2005) Island biogeography for an urbanizing world: how extinction and colonization may determine biological diversity in human-dominated landscapes. Urban Ecosyst 8:157–177
Mason CF (1970) Snail populations, beech litter production, and the role of snails in litter decomposition. Oecologia 5:215–239
McDonnell MJ, Hahs AK (2008) The use of gradient analysis studies in advancing our understanding of the ecology of urbanizing landscapes: current status and future directions. Landsc Ecol 23:1143–1155
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52:883–890
Miyashita T, Shinkai A, Chida T (1998) The effects of forest fragmentation on web spider communities in urban areas. Biol Conserv 86:357–364
Morii Y, Prozorova L, Chiba S (2016) Parallel evolution of passive and active defence in land snails. Sci Rep 6:35600
Oishi Y (2018) Urban heat island effects on moss gardens in Kyoto. Japan Landscape and Ecological Engineering 15:177–184. https://doi.org/10.1007/s11355-018-0356-z
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. (2019) Package 'vegan'. https://cran.r-project.org/web/packages/vegan/vegan.pdf
Patten MA, Bolger DT (2003) Variation in top-down control of avian reproductive success across a fragmentation gradient. Oikos 101:479–488
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Riley SPD, Busteed GT, Kats LB, Vandergon TL, Lee LFS, Dagit RG, Kerby JL, Fisher RN, Sauvajot RM (2005) Effects of urbanization on the distribution and abundance of amphibians and invasive species in Southern California streams. Conserv Biol 19:1894–1907
Ritchie EG, Johnson DN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Rodewald AD, Kearns LJ, Shustack DP (2011) Anthropogenic resource subsidies decouple predator–prey relationships. Ecol Appl 21:936–943
Saeki I (2015) Conservation biology at field research stations: five potential roles in forest ecosystem management. Biodiversity 16:42–46
Saeki I, Niwa S, Osada N, Hyodo F, Ohta T, Oishi Y, Hiura T (2017a) Adaptive significance of arboreality: field evidence from a tree-climbing land snail. Anim Behav 127:53–66
Saeki I, Niwa S, Osada N (2017b) Predation of a rare arboreal land snail Euhadra brandtii sapporo by introduced common raccoon Procyon lotor. Venus: Japanese Journal of Malacology 75:83–87
Saito M, Koike F (2013) Distribution of wild mammal assemblages along an urban–rural–forest landscape gradient in warm-temperate East Asia. PLoS One 8:e65464
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191
Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH (2010) Invasion, competition, and biodiversity loss in urban ecosystems. BioScience 60:199–208
Yang P, Ren G, Hou W (2017) Temporal–spatial patterns of relative humidity and the urban dryness island effect in Beijing City. J Appl Meteorol Climatol 56:2221–2237
Acknowledgments
We sincerely thank Dr. T. Nakaji, Dr. H. Ishii, Dr. K. Nakai, Dr. A. Agetsuma, Dr. O. Kishida, Dr. Y. Okuzaki, Ms. R. Uchida, Mr. J. Uchida, Mr. A. Okuda, Mr. Y. Sasabe, Mr. W. Mukaimine, and staff members of the Tomakomai city government, the Hokkaido Prefectural government, and the Nihon-seishi Corporation for their support. This research was funded by the Japan Society for Promotion of Sciences (grant no. 17 K07270).
Author information
Authors and Affiliations
Contributions
IS, SN, and NO designed research, collected and analysed data, and wrote the manuscript. WA and TH collected data and revised the manuscript. All authors contributed critically the drafts and gave final approval for publication
Corresponding author
Electronic supplementary material
ESM1
(DOCX 2.16 MB)
Rights and permissions
About this article
Cite this article
Saeki, I., Niwa, S., Osada, N. et al. Contrasting effects of urbanization on arboreal and ground-dwelling land snails: role of trophic interactions and habitat fragmentation. Urban Ecosyst 23, 603–614 (2020). https://doi.org/10.1007/s11252-020-00930-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-00930-6