Abstract
How urban habitats contribute to biodiversity conservation is a key challenge in a rapidly urbanising world. Urban parks can provide important habitats for native species, but previous studies are geographically biased; fast growing megacities, in particular in South America, are clearly understudied. To assess habitat functions and underlying drivers in parks of Santiago de Chile, we analysed the assemblages of wild growing plant species in two ubiquitous park habitat types (grasslands, wooded areas) in 15 parks (150 plots) along an urban-rural gradient. We first used linear contrasts to compare species richness, beta diversity and the proportion of introduced species. We then tested for the explanatory value of environmental variables operating at different spatial scales (plot, park, urban matrix). Unlike in most previous studies, biodiversity patterns were not related to the position of the parks on the urban-rural gradient. Introduced species, mostly from Europe, generally dominated both habitat types (>90 %). Socio-economic (population growth or density), but not spatial, variables were retained in most models. Maintenance intensity was most influential in predicting species assemblages, complemented by park age in wooded areas. A high proportion of European grassland species indicates a trend of homogenisation in park grassland at a cross-continental scale. We conclude that habitat functions of urban parks for native species that have been mainly demonstrated for Europe cannot be generalised to South American megacities. This highlights the need for innovative and locally appropriate conservation approaches (e.g., re-introduction of native species) to foster biodiversity functions in urban parks of South American megacities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban growth clearly conflicts with biodiversity conservation outside cities (Millennium Ecosystem Assessment 2005, Ellis 2015). Since urbanisation increases in rapidly growing megacities (Güneralp and Seto 2013) as well as in regions with limited economic development (Haase et al. 2013), enhancing urban habitat functions for native species becomes a key issue in biodiversity conservation at the global scale (McKinney 2002; Lundholm and Richardson 2010; Kowarik 2011; Shwartz et al. 2014). Mechanistic insights into biodiversity responses to urban land-use types and their dynamics in time and space would clearly help adjust conservation approaches in urban settings (Ramalho and Hobbs 2012).
Many analyses along urban-rural gradients (McDonnell and Hahs 2008) and comparisons between urban and rural areas (Knapp et al. 2008a) have revealed some general responses to urbanisation. Cities can be rich in both native and introduced species (Kühn et al. 2004), with species richness peaking often in transition zones (McKinney 2008). Native species usually decrease with increasing urbanisation, with the exception of some human-adapted native species, and opposed to introduced species (Hansen et al. 2005; McKinney 2008; Schmidt et al. 2014), yet with differences among groups of plants and animals (McKinney 2008) and among species with different traits (Knapp et al. 2008b). Gradient analyses have been criticised because they do neither reflect the spatial complexity, the non-linearity, nor the temporal dynamics in modern urban land-use (Ramalho and Hobbs 2012). In their response to this critique, McDonnell et al. (2012) argue that it is not simply spatially linear gradients but related environmental gradients that drive biodiversity patterns in cities. Spatial gradients may be relevant, but do not account for the multiple cores of newer urban areas (McIntyre et al. 2000), in particular in fast growing urban regions (McDonald et al. 2013). The question thus arises which parameters relate to urban biodiversity patterns beyond the spatial position of a plot on an urban-rural gradient (McDonnell and Hahs 2008).
Previous studies have related biodiversity patterns to different indices of urban growth, including the year of the city’s establishment (Wang et al. 2012), the distance to the nearest house, the number of houses (Gavier-Pizarro et al. 2010) as well as the style of urban development (Sushinsky et al. 2013). Others have related socio-economic variables such as human population density to biodiversity (Luck 2007). However, most studies use just a single or a few of such indices to reveal biodiversity responses to urbanisation. Likewise, many urban studies consider a single spatial scale in combination with site-specific parameters (e.g., Sushinsky et al. 2013, but see Wang et al. 2012). Yet the complexity of urbanisation, especially in rapidly changing megacities, might implicate a variety of spatial scales as illustrated by Cook et al. (2012) for residential landscapes. Beyond the spatial gradient, socio-economic and site-specific variables and their dynamics in time might affect urban biodiversity patterns from the plot to the city scale.
While most urban biodiversity studies have been conducted in temperate regions (Faeth et al. 2011), Latin America is generally understudied (Pauchard et al. 2006; Shwartz et al. 2014). This holds in particular for studies about the relationship between population density and biodiversity (Luck 2007). Such geographical bias critically hampers our understanding of biodiversity responses to urbanisation because urban land-use dynamics clearly differs among continents (Sala et al. 2000), and most megacities are located outside the temperate zones (UN-Habitat 2009; Singh 2015). South American cities are predicted to have the fastest growing urban sprawl in biodiversity hotspot areas (Güneralp and Seto 2013), with decreasing distances between protected areas and urban conglomerations (McDonald et al. 2008). Chile’s urban areas, for example, currently face rapid growth in already highly urbanised metropolitan areas (Rojas et al. 2013) and within mid-cities (Aguayo et al. 2007). Research on urbanisation impacts on biodiversity is vastly limited there (but see de la Maza et al. 2002; Pauchard et al. 2006; Pauchard and Barbosa 2013), and, just recently, Fuentes et al. (2014) revealed that alien plant richness was related to human population density at the province scale in Chile.
As part of the urban green infrastructure, parks may function as biodiversity hotspots—depending on their history and characteristics (Nielsen et al. 2014). As drivers of biodiversity patterns may differ among continents due to differences in the history or pace of urbanisation, we tested the traditional gradient-analysis approach in a rapidly growing South American megacity by using urban parks as model ecosystems. We explored the extent to which the position of the plots within parks on an urban-rural gradient explain the local biodiversity patterns, and particularly, the performance of native versus introduced species. Additionally, we investigated if other variables that relate to different spatial scales (urban matrix, parks, plots within parks) correlate with our biodiversity measures.
Urban parks, with grasslands (lawns, meadows) and woods as major components, are ubiquitous land-use types today. Habitat functions of parks for native species have been largely demonstrated for Europe (e.g., Cornelis and Hermy 2004; Celesti-Grapow et al. 2013; Fischer et al. 2013a; Nielsen et al. 2014). Since park design, plant use and maintenance have converged internationally due to similar trends in European countries and their former colonies (Ignatieva 2011), it remains an open question whether urban parks outside Europe function as habitats for native species as well. Alternatively, the propagule pressure that results from a large proportion of cultivated exotic species (e.g., Loeb 2010; Nagendra and Gopal 2011; Abendroth et al. 2012) might contribute to a prevalence of introduced species in the wild park flora. Specifically for urban grassland, trends of a global biotic homogenization have been hypothesized due to the use of similar seed sources and unifying management approaches that are expected to enhance the similarity among grassland communities in parks (Ignatieva 2011; Ignatieva et al. 2015). However, comparisons between species assemblages of park grassland from different regions are largely lacking thus far.
Santiago de Chile (Santiago) represents a rapidly growing city in South America that is located in a global biodiversity hotspot (Arroyo et al. 2006) and also features a high density of introduced species (Fuentes et al. 2013). Whether the large regional pool of native plant species contributes to a high richness in urban parks is unknown thus far. We assessed biodiversity measures for grasslands and the herb layer in wooded areas in 15 parks (150 plots) in Santiago and related these to environmental and socio-economic variables at different spatial scales. We hypothesised that (1) the composition of urban park vegetation differs along the urban-rural gradient, in that (a) species richness decreases, (b) the proportion of introduced species increases, and (c) species composition becomes more homogenous towards downtown areas; (2) variables at the plot and park level and variables related to the surrounding urban matrix have more influence on species composition than the urban-rural gradient at the city scale, and (3) biodiversity patterns are similar in two common park habitat types, grasslands and wooded areas. Moreover, we compared our results on introduced species versus native species to urban grassland studies from other continents.
Material and methods
Study area and study design
Surrounded by the Andes, Santiago is situated in a global biodiversity hotspot (Arroyo et al. 2006). The approximately 7 million inhabitants of the metropolitan region account for 40 % of Chile’s population, and have increased by about 10 % within the last 10 years (INE 2014).
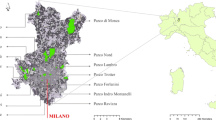
We distinguished three urbanisation zones (downtown, transition zone, suburb) for the metropolitan area of Santiago based on spatial data and population density (for a detailed definition see urban-rural gradient, Table 1). In each zone, we chose five parks which were evenly distributed across the city (Table 2). The minimum distance between parks was 2.5 km. We analysed biodiversity patterns in two different habitat types (grasslands, wooded areas) per park. Grassland areas were at least 5 m from woody plants, and wooded areas had a tree canopy of >50 %. In each habitat type, plots were set up in a randomised design. We investigated four wooded plots (each 15 × 15 m2) and six grassland plots (each 4 × 4 m2) per park, resulting in a total of 150 plots.
In addition to the plot positions on the urban-rural gradient, we assessed five environmental and socio-economic variables that covered four spatial scales: the metropolitan area (urban-rural gradient), the urban matrix surrounding each park (human population density, human population growth), each park (park age, park area) and each plot (maintenance intensity) (see Table 1 for details on the variables). The relevance of these variables for biodiversity patterns has been demonstrated previously, although mainly in other geographic contexts (Luck 2007; Nielsen et al. 2014).
The choice of the parks for our study based primarily on their spatial location and their position on the urban-rural gradient, and relied on the occurrence of both habitat types (grassland and wooded areas) within each park. The studied parks have different origins and may comprise designed or informal areas. For example, one park was initially used for military purposes, including parades and training, and was later on transformed to an area that served the public as a recreational greenspace (Parque O’Higgins). Another park was established as a site for learning and experimenting with agriculture and botany, while at the same time a great aesthetic and recreational value was created (Parque Quinta Normal). Other parks were primarily designed to directly increase the quality of life of the inhabitants, including sports and recreational uses (Parque Padre Hurtado), or to green the neighbourhood and to create a green corridor (Parque Inés de Suarez). Within some parks, both native and exotic species were used deliberately during the park establishment (e.g., Parque O’Higgins, Parque Araucano) or for agricultural/botanical issues (e.g., orchards, vineyards in Parque Quinta Normal) (see Laborde 2007 for details).
Data collection
From October to December 2009, all spontaneous plant species in the herb layer of a plot were recorded, including both the grassland and the wooded areas. Nomenclature and species origin follows Hoffmann (1998a, 1998b) for most species; additional information for a few species was taken from Schmeil and Fitschen (2009) and Al-Shehbaz (1993). We henceforth distinguish between native and introduced species, with native species originating from the area of central Chile. Habitat demands and characteristics of exemplary species are given in the discussion, with information on the mentioned species compiled from Jäger (2011).
Statistical analyses
As measures of biodiversity we used species richness, beta diversity within the 15 parks, and the proportion of introduced species. Beta diversity was calculated as mean Bray-Curtis dissimilarity between the plots of a habitat type within each of the 15 parks. All environmental variables shown in Table 2 were tested for intercorrelation; we used Pearson’s product moment correlation coefficient for metric variables, and Spearman’s rank correlation for ordinal variables. Human population density and population growth were significantly correlated (rS = −0.557; p = 0.03) but were both kept in the models as the correlation coefficient was below 0.7.
We first used linear contrasts (Crawley 2007) to assess if (1) species richness, (2) proportion of introduced species, and (3) beta diversity differed along the urban-rural gradient for the two habitat types (grasslands, wooded areas). Proportion of introduced species was logit-transformed prior to analysis (Warton and Hui 2011). In a second step, we used analyses of covariance (ANCOVA) to assess significant effects of the environmental and socio-economic variables on (1) species richness, (2) proportion of introduced species, and (3) beta diversity for both habitat types (Crawley 2007). We calculated minimal adequate models using the step function of the Mass package in R that selects a model on the basis of AIC (Venables and Ripley 2002). We related the same variables to a combined data set of the two habitat types and included a variable “habitat type” to determine similarities between habitat types. For the metric variables retained in the models, we conducted single-variable linear regression models to determine their sole explanation value for each response variable. We used linear contrasts to assess significant interactions among factors for the ordinal variable (maintenance intensity).
To assess which of the five environmental and socio-economic variables significantly influence species composition we conducted nonmetric multidimensional scaling (Oksanen et al. 2011; NMDS, vegan package in R) based on Bray-Curtis distances. We included all species that occurred in more than one park in the data set to level variations due to park design and resulting differences in vegetation and environmental data within a park. As a result NMDS incorporated the frequency of 38 species in grassland habitats and of 50 in wooded habitats. We pooled the data sets of the plots belonging to the same habitat type within a park and used the frequency of a species across all plots of a park to generate the distance matrices. We then calculated a 999-permutation model with a two-dimensional solution for both habitat types. All analyses were conducted with R version 2.15.3 (R Development Core Team 2013). Bray-Curtis dissimilarity index was calculated with BiodiversityR (1.6) for TinnR (version 1.0.3).
Results
Overall, mean species richness was significantly higher in wooded areas (19.7 species) than in grasslands (14.4 species; Table 2). In grassland habitats and wooded areas, introduced species clearly prevailed with a mean proportion of 95 % and 94 %, respectively, without significant differences between habitat types (Table 2).
Different to our hypothesis, the urban-rural gradient did not significantly relate to the tested biodiversity patterns. We did neither detect significant differences in species richness or in the proportion of introduced species in grassland or wooded habitats when we used linear contrasts to compare all 15 parks along the urban-rural gradient, nor did we find significant differences in beta diversity in the corresponding analyses (Table 3).
In contrast, environmental and socio-economic variables at the matrix, park and plot level had better explanation values, with clear differences between habitat types, and a combination of the most important variables explained biodiversity patterns when analysing both habitat types together (Table 4). For the combined data set of both habitat types, ANCOVA showed that species richness was negatively influenced by human population growth and positively related to the wooded habitat type (Table 4). Beta diversity was significantly positively related to the variable park area and negatively to the urban-rural gradient and maintenance intensity.
For grassland habitats, ANCOVA revealed that species richness was related to population density and maintenance intensity; both variables were retained in the final model but were not significant (Table 4). For the proportion of introduced species, the variables population density and urban-rural gradient were retained in the model, but had no significant effect. Beta diversity related significantly to low maintenance with a negative relation, and park age and human population density remained in the model non-significantly. Maintenance intensity was the only variable in the models for grassland habitats that showed a significant relationship to the corresponding biodiversity measure (beta diversity) when using linear contrasts (Table 5b; Fig. 1a–c).
Biodiversity patterns in urban parks in Santiago de Chile and underlying drivers. Relation of a species richness and b proportion of introduced species to population growth in the park surroundings for grassland habitats. c Beta diversity (Bray-Curtis dissimilarity) of grassland plots compared for parks with high and low maintenance regimes. Relation of d species richness and e proportion of introduced species to population growth in the park surroundings for wooded habitats. f Beta diversity (Bray-Curtis dissimilarity) of wooded plots compared for parks with high and low maintenance regimes
For wooded habitats, species richness was explained by park area, a non-significant variable in the model, and human population growth, which showed a significant negative relation (Table 4). The proportion of introduced species was explained by human population growth as a single significant explanatory variable with a positive relation. ANCOVA determined park area and the urban-rural gradient as non-significant explanatory variables for beta diversity. The variable human population growth was significantly related to species richness and proportion of introduced species in single-variable linear regression analyses (Table 5a, Fig. 1d–f).
NMDS revealed that species composition in grassland habitats was strongly influenced by the variable maintenance intensity (Fig. 2a; final stress value: 10.94, p = 0.001 for maintenance intensity). For wooded habitats, NMDS showed that maintenance intensity and park area significantly determined the composition of the understorey vegetation (Fig. 2b; final stress value 11.62, p = 0.002 for maintenance intensity, p = 0.032 for park area).
Ordination plots according to NMDS, which combined species composition of 15 urban parks in Santiago de Chile with the influential environmental variables. The variables are shown either by their name (ordinal variable: maintenance intensity) or by the ordination line (metric variable: park area) for a grassland and b wooded habitats
A comparison with urban grassland studies in other geographic contexts disclosed a much higher proportion of introduced species in park grasslands in Santiago (Online Resource A.1). Grassland species found in our study originated mostly from Europe and were largely shared with European urban grasslands (79 %), but less with urban grasslands on other continents (Fig. 3, Online Resource A.2).
Discussion
Predictive power of variables at different scales
Analyses of urban-rural gradients are an established method to assess the influence of urban structures on biota (McIntyre et al. 2000). However, this method has disadvantages as the urban matrix and associated ecological and socio-economic parameters can induce diversity patterns independently from concentrically arranged zones (McDonnell and Hahs 2008). For urban parks, the review of Nielsen et al. (2014) showed mainly for European and North American cities that native species diversity decreases towards city centres. In contrast, our results clearly demonstrate that the urban-rural gradient did not explain species richness, the proportion of introduced species or beta diversity in neither grasslands nor wooded habitats in our study area (linear contrasts, Table 3). Also, the gradient did not influence species composition (NMDS, Fig. 2) nor did it significantly improve the model fit of any model in combination with other variables to explain species richness, the proportion of introduced species or beta diversity in grassland or wooded habitats (ANCOVA, Table 4). As the variable was however retained in the models but no significant effect was revealed, it may contribute to the model fit indirectly, as through the interplay with other variables. Also, the effect may have been small since — beside the spatial location — population density was used to define the gradient beforehand. This parameter was retained in some models as well, but also without significant effect. Only in the integrated model for the two habitat types the urban-rural gradient was positively related to beta diversity. Although the parks in downtown areas had higher mean dissimilarity values, the differences between gradient zones were not significant (Table 3).
A major finding of our study was therefore that differences in biodiversity patterns within urban parks of Santiago were best explained by individual environmental and socio-economic variables at different spatial scales (plot, park or urban matrix level) or a combination of these. Whether these contrasting results arose due to different geographic contexts, urbanisation patterns or socio-economic structures, remains an open question.
Population growth and density
Both human population growth and population density were retained in the models for the studied habitat types; population growth related to some of the response variables significantly (Table 4, Fig. 1). This variable was negatively related to species richness, but positively to the proportion of introduced species in wooded areas. Our results thus indicate that it is the human population growth in the park surroundings that relates to the biodiversity patterns within parks more than population density. We assume that both variables are linked to visitor pressure, and related disturbances are generally known to enhance introduced species (Hobbs and Huenneke 1992). Capsella bursa-pastoris in the grassland areas and Cerastium holosteoides in the wooded areas are examples for introduced species that we found only in those parks that were characterised by a population growth in the neighbourhood. Both species are known especially from ruderal sites in their native ranges (Jäger 2011), and C. bursa-pastoris also in Chile (Castro et al. 2014), and thus are good representatives for colonising sites with regular disturbances. Correspondingly to our results, human population density is positively related to the proportion of introduced species in parks in the northeastern USA (Loeb 2006). Yet population growth, a major characteristic of rapidly developing megacities, is also associated with highly dynamic urban environments, where construction activities generate plenty of open habitats. These usually harbour abundant populations of introduced species, resulting in a propagule pressure of disturbance-adapted alien species that is likely higher than in quarters with a similar human population density but a less dynamic development. By human-mediated dispersal (Wichmann et al. 2009; von der Lippe and Kowarik 2012; Auffret and Cousins 2013) propagules of such species may be tranferred into the parks, finally resulting in elevated proportions of introduced species (Fig. 1). Similar to the species that are found solely in areas of urban growth, our species set shows that areas with high population density include introduced species that are known from frequently disturbed sites, e.g., Cirsium arvense in the grassland areas and Chenopodium album in the wooded areas.
Fuentes et al. (2014) disclosed human population density as one predictor of alien species richness among others at the province scale in Chile. At a smaller scale—zoomed in for an urban area—we have shown that urban growth variables together with others (maintenance intensity, park age and area) best explained biodiversity patterns in Santiago. That is, influences on the species assemblages did not mainly arise from single environmental and socio-economic variables, but from a combination of these (Table 4).
Management intensity
Human activities can affect the proportion of native and introduced species by changes in environmental gradients (Davis et al. 2000; Tomasetto et al. 2013). Accordingly, intensity and continuity of maintenance were revealed as important predictors of species richness in urban grasslands in Europe (Wilhelm and Andres 1998; Politi Bertoncini et al. 2012). Surprisingly, maintenance intensity did not relate to the proportion of introduced species in our multivariate analyses, and there were no differences in the proportion of introduced species between plots experiencing low and high maintenance intensity (Table 5). However, maintenance intensity had a major influence on the species composition of both habitat types (Fig. 2) and on the beta diversity of grassland plots (Table 4). This indicates that different levels of maintenance (together with visitor pressure) induced a general heterogeneity in disturbance patterns. In contrast to theories in (grassland) invasion science (Hobbs and Huenneke 1992; Burke and Grime 1996) and other studies on park vegetation (LaPaix and Freedman 2010), higher levels of maintenance thus did not enhance introduced species per se, but led, in combination with human population growth and park age, to a more heterogeneous species composition. Similar to a study from Paris (Politi Bertoncini et al. 2012), where different levels of management intensity and practices (e.g., mowing frequency) were found to affect species composition, our study plots likely had different levels of heterogeneity due to different levels and types of human disturbance (access, maintenance). This relation is more distinct in grassland plots (maintenance intensity was the sole influence in NMDS and was a significant variable in ANCOVA), probably due to faster responses of the herbs and grasses to different management intensities, compared to species in shady and likely less accessed wooded areas. For example, species that are known from arable, cultivated land (e.g., Setaria viridis) were found exclusively in the grassland areas with high maintenance. Also, species such as Urtica dioica that are characteristic for increased nutrient levels were part of the species set only in the high maintenance sites of the wooded habitats.
Park area and age
Theories about the species-area relationship in urban parks are mostly based on studies of fauna (Nielsen et al. 2014). Only a few studies have confirmed that park area is an important predictor for plant diversity (Cornelis and Hermy 2004; Li et al. 2006). In our study, park area, together with other variables, determined the species composition (Fig. 2) and species richness (Table 4) in wooded habitats, but not in grasslands. The latter can be explained by the greater homogeneity in lawns compared to wooded areas. The given species composition in wooded habitats likely only occurs when park area and maintenance practices play in concert (Fig. 2). This may be backed up by some species (e.g., Hypochaeris radicata and Rumex acetosa in wooded areas) that are typical of sites with an extensive management. Such a management may be given especially in larger parks, where visitor pressure and connected management activities may concentrate in some places whereas major areas off the beaten track provide terrain for low-maintenance species.
In contrast to our hypothesis, we did not find a lower proportion of introduced species in older parks (Table 2), and we thus assume that the species assemblages of the herb layer were more influenced by direct parameters such as disturbance and dispersal patterns at either the plot or matrix level. In line with the missing influence of the urban-rural gradient on the proportion of introduced species (Table 3), there is thus no evidence that the proximity to the hinterlands of Santiago, a global biodiversity hotspot, has led to an accumulation of native species in older urban parks due to natural colonization processes. Possible reasons for this include dispersal limitation, competition with introduced species or failure of native species from the regional species pool to adapt to urban park environments. Legacies of former land-use transformations may also account for such low rates of natives (Hahs et al. 2009). This clearly contrasts with European and North American studies, which report a range of native species in parks, including species of conservation concern, either survivors of the original habitat or colonising forest or grassland species (e.g., Cornelis and Hermy 2004; DeCandido et al. 2007; Kümmerling and Müller 2012; Celesti-Grapow et al. 2013; Fischer et al. 2013a).
Dominance of alien species
The global history of urban greening may explain why neither maintenance intensity and park age nor the position of parks on the urban-rural gradient (Table 4) related to the proportion of wild growing introduced species in either park habitat. All over the world, design schemes and associated species assemblages for wooded areas and grasslands were exported from Europe to colonised areas and further propagated throughout the western world (Ignatieva 2011), likely resulting in a considerable propagule pressure of introduced species. In contrast, native (tree) species were rarely incorporated into colonial design schemes (but see Abendroth et al. 2012).
For the wild grassland flora of parks in Santiago, we found an overwhelming dominance of introduced species while urban grasslands in Europe revealed proportions of introduced species <20 % (Online Resource A.1, post-1492 introduced species, e.g., Thompson et al. 2004); the proportion of introduced species in our study was more than fourfold higher (95 %). Studies from other continents reported values between 35 % and 97 % (Online Resource A.1, e.g., Cilliers et al. 2004 for Africa; Kirkpatrick 2004; Stewart et al. 2009 for Oceania). Also, proportions of introduced species in studies of North America were lower (c. 50 %, DeCandido 2004) than in Santiago, but clearly higher than in European studies. Within this species pool, European grassland species play an important role. Almost all of the grassland species from the studied parks in Santiago are non-native to South America, and nearly all of these are native to Europe and have been frequently reported from urban grasslands in different parts of Europe (Fig. 3, Online Resource A.2). This illustrates that in the studied region, only few native species were able to colonize grasslands, whereas a large portion of previously introduced grassland species still prevail in the current park grassland, these likely descending from former seedings. Our study thus supports with quantitative data the idea that urban grasslands, particularly lawns, represent a globally distributed type of ecosystem with a common species pool (Ignatieva 2011).
Conclusions
A main message from our study is that the considerable habitat functions of urban parks for native plant species—as previously mainly demonstrated for Europe and North America—cannot be generalised to Santiago as an example of a South American megacity. Correspondingly, other biodiversity drivers than in most previous studies were revealed for park vegetation in Santiago. The overwhelming prevalence of introduced species clearly contrasts with urban parks in Europe and likely reflects the initial implementation of European design schemes and associated plantings and seed mixtures in the wake of post-colonial urban greening. Our results thus point to global homogenisation in urban grassland, despite a rich native species pool in the rural surroundings of Santiago. To enhance the conservation functions of parks in cities where urban sprawl conflicts with biodiversity hotspots, a better understanding of the drivers that shape the local species pool is needed. To disentangle the interplay of dispersal limitation, competition of introduced species and high human pressure on park habitats is thus an intriguing research question. Adjusting management (e.g., mowing) is a key approach for enhancing the biodiversity functions of urban green spaces in Europe. Our study suggests that different conservation strategies are required for urban parks in South America since the native species pool is strictly limited there. Fostering native species in design schemes as tested in New Zealand (Ignatieva et al. 2008), adding native grassland species to the existing vegetation (Fischer et al. 2013b) and, generally, developing management strategies that are sensitive to biodiversity issues (Niemelä 2014) may be promising—in particular where unplanned urban sprawl threatens highly diverse urban hinterlands.
References
Abendroth S, Kowarik I, Müller N, von der Lippe M (2012) The green colonial heritage: Woody plants in parks of Bandung, Indonesia. Landsc Urban Plan 106:12–22
Aguayo MI, Wiegand T, Azocar GD, Wiegand K, Vega CE (2007) Revealing the driving forces of mid-cities urban growth patterns using spatial modeling: a case study of Los Angeles, Chile. Ecol Soc 12:13
Al-Shehbaz IA (1993) Lepidium tayloriae (Brassicaceae), a new species from Chile. Novon 3:93–95
Arroyo MTK, Marquet P, Marticorena C, Simonetti JA, Cavieres L, Squeo FA, Rozzi R, Massardo F (2006) El Hotspot chileno, prioridad mundial para la conservación. In: Saball P, Arroyo MTK, Castilla JC, Estades C, Guevara JMLD, Larraín S, Moreno C, Rivas F, Rovira J, Sánchez A, Sierralta L (eds) Biodiversidad de Chile. Patrimonio y Desafíos. Comisión Nacional del Medio Ambiente, Santiago de Chile, pp. 94–99
Auffret AG, Cousins SAO (2013) Humans as long-distance dispersers of rural plant communities. PLoS One 8:e62763
Burke MJW, Grime JP (1996) An experimental study of plant community invasibility. Ecology 77:776–790
Castro SA, Espinosa C, Figueroa JA (2014) Two haplotypes of Capsella bursa-pastoris (Brassicaceae) in Continental Chile support multiple introduction. Gayana Bot 71:216–221
Celesti-Grapow L, Capotorti G, Del Vico E, Lattanzi E, Tilia A, Blasi C (2013) The vascular flora of Rome. Plant Biol 147:1059–1087
Cilliers SS, Müller N, Drewes E (2004) Overview on urban nature conservation: situation in the western-grassland biome of South Africa. Urban For Urban Green 3:49–62
Cook EM, Hall SJ, Larson KL (2012) Residential landscapes as social-ecological systems: a synthesis of multi-scalar interactions between people and their home environment. Urban Ecosyst 15:19–52
Cornelis J, Hermy M (2004) Biodiversity relationships in urban and suburban parks in Flanders. Landsc Urban Plan 69:385–401
Crawley MJ (2007) The R Book. John Wiley & Sons, Ltd, Chichester
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
DeCandido R (2004) Recent changes in plant species diversity in urban Pelham Bay Park, 1947-1998. Biol Conserv 120:129–136
DeCandido R, Calvanese N, Alvarez RV, Brown MI, Nelson TM (2007) The naturally occurring historical and extant flora of Central Park, New York City, New York 1857-2007. J Torrey Bot Soc 134:552–569
de la Maza CL, Hernández J, Bown H, Rodríguez M, Escobedo F (2002) Vegetation diversity in the Santiago de Chile urban ecosystem. Arbitr J 26:347–357
De Mattos CA (2004) Santiago de Chile: Metamorfosis bajo un nuevo impulso de modernización capitalista. In: De Mattos CA, Ducci ME, Rodríguez A, Yánez Warner G (eds) Santiago en la globalización ¿una nueva ciudad? Instituto de Estudios Urbanos y Territoriales, Santiago, pp. 42–43
Ellis EC (2015) Ecology in an anthropogenic biosphere. Ecol Monogr. doi:10.1890/14-2274.1
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 1223:69–81
Fischer LK, von der Lippe M, Kowarik I (2013a) Urban land use types contribute to grassland conservation: the example of Berlin. Urban For Urban Green 12:263–272
Fischer LK, von der Lippe M, Rillig MC, Kowarik I (2013b) Creating novel urban grasslands by reintroducing native species in wasteland vegetation. Biol Conserv 159:119–126
Fuentes N, Pauchard A, Sanchez P, Esquivel J, Marticorena A (2013) A new comprehensive database of alien plant species in Chile based on herbarium records. Biol Invasions 15:847–858
Fuentes N, Saldaña A, Kühn I, Klotz S (2014) Climatic and socio-economic factors determine the level of invasion by alien plants in Chile. Plant Ecol Divers 30:609–628
Gavier-Pizarro GI, Radeloff VC, Stewart SI, Huebner CD, Keuler NS (2010) Rural housing is related to plant invasions in forests of southern Wisconsin, USA. Landsc Ecol 25:1505–1518
GORE-RM (2009) Políticas Áeras verdes de la ciudad de Santiago. Cartografía actualizada. Gobierno Reginal de Santigo Región Metropolitana, Santiago de Chile
Güneralp B, Seto KC (2013) Futures of global urban expansion: uncertainties and implications for biodiversity conservation. Environ Res Lett 8:014025
Haase D, Kabisch N, Haase A (2013) Endless urban growth? On the mismatch of population, household and urban land area growth and its effects on the urban debate. PLoS One 8:e66531
Hahs AK, McDonnell MJ, McCarthy MA, Vesk PA, Corlett RT, Norton BA, Clemants SE, Duncan RP, Thompson K, Schwartz MW, Williams NSG (2009) A global synthesis of plant extinction rates in urban areas. Ecol Lett 12:1165–1173
Hansen AJ, Knight RL, Marzluff JM, Powell S, Brown K, Gude PH, Jones A (2005) Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol Appl 15:1893–1905
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion - implications for conservation. Conserv Biol 6:324–337
Hoffmann A (1998a) El Árbol urbano en Chile, 3rd edn. Ediciones Fundación Claudio Gay, Santiago, Chile
Hoffmann A (1998b) Flora silvestre de Chile, zona central, 4th edn. Ediciones Fundación Claudio Gay, Santiago, Chile
Ignatieva M (2011) Plant material for urban landscapes in the era of globalization: roots, challenges and innovative solutions. In: Richter M, Weiland U (eds) Applied urban ecology: a global framework. John Wiley & Sons, Ltd, Chichester, UK, pp. 139–151
Ignatieva M, Ahrné K, Wissman J, Eriksson T, Tidåker P, Hedblom M, Kätterer T, Marstorp H, Berg P, Eriksson T, Bengtsson J (2015) Lawn as a cultural and ecological phenomenon: a conceptual framework for transdisciplinary research. Urban For Urban Green 14(2):383–387
Ignatieva, ME, Stewart, GH, Meurk, CD (2008) Low Impact Urban Design and Development (LIUDD): matching urban design and urban ecology. Landscape Review 12: 61-73INE (2014) Censo 2012. Población, País y Regiones: Actualización Población 2002–2012 y Proyecciones 2013–2020. Instituto Nacional de Estadisticas, Santiago, Chile
INE (2002) Censo 2002. Chile, proyecciones de poblacion al 30 de junio (1990-2020). Instituto Nacional de Estadisticas, Santiago, Chile
INE (2014) Censo 2012. Población, País y Regiones: Actualización Población 2002-2012 y Proyecciones 2013-2020. Instituto Nacional de Estadisticas, Santiago, Chile
Jäger EJ (2011) Rothmaler - Exkursionsflora von Deutschland. Gefäßpflanzen: Grundband. Spektrum Akademischer Verlag, München
Kirkpatrick JB (2004) Vegetation change in an urban grassy woodland 1974–2000. Aust J Bot 52:597–608
Knapp S, Kühn I, Mosbrugger V, Klotz S (2008a) Do protected areas in urban and rural landscapes differ in species diversity? Biodivers Conserv 17:1595–1612
Knapp S, Kühn I, Wittig R, Ozinga WA, Poschlod P, Klotz S (2008b) Urbanization causes shifts in species' trait state frequencies. Preslia 80:375–388
Kowarik I (2011) Novel urban ecosystems, biodiversity, and conservation. Environ Pollut 159:1974–1983
Kühn I, Brandl R, Klotz S (2004) The flora of German cities is naturally species rich. Evol Ecol Res 6:749–764
Kümmerling M, Müller N (2012) The relationship between landscape design style and the conservation value of parks: a case study of a historical park in Weimar, Germany. Landsc Urban Plan 107:111–117
Laborde M (2007) Parques de Santiago. Editorial Catalonia, Santiago de Chile
LaPaix R, Freedman B (2010) Vegetation structure and composition within urban parks of Halifax Regional Municipality, Nova Scotia, Canada. Landsc Urban Plan 98:124–135
Li W, Ouyang Z, Meng X, Wang X (2006) Plant species composition in relation to green cover configuration and function of urban parks in Beijing, China. Ecol Res 21:221–237
Loeb RE (2006) A comparative flora of large urban parks: intraurban and interurban similarity in the megalopolis of the northeastern United States. J Torrey Bot Soc 133:601–625
Loeb RE (2010) Diversity gained, diversity lost: long-term changes in woody plants in Central Park, New York City and Fairmount Park, Philadelphia. Stud Hist Gard Des L 30:124–151
Luck GW (2007) A review of the relationships between human population density and biodiversity. Biol Rev 82:607–645
Lundholm JT, Richardson PJ (2010) Habitat analogues for reconciliation ecology in urban and industrial environments. J Appl Ecol 47:966–975
McDonald RI, Kareiva P, Forman RT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141:1695–1703
McDonald RI, Marcotullio PJ, Güneralp B (2013) Urbanization and global trends in biodiversity and ecosystem services. In: Elmqvist T, Fragkias M, Goodness J, Güneralp B, Marcotullio PJ, McDonald RI, Parnell S, Schewenius M, Sendstad M, Seto KC, Wilkinson C (eds) Urbanization, biodiversity and ecosystem services: challenges and opportunities. Springer, Dordrecht, pp. 31–52
McDonnell M, Hahs A (2008) The use of gradient analysis studies in advancing our understanding of the ecology of urbanizing landscapes: current status and future directions. Landsc Ecol 23:1143–1155
McDonnell MJ, Hahs AK, Pickett STA (2012) Exposing an urban ecology straw man: critique of Ramalho and Hobbs. Trends Ecol Evol 27:255–256
McIntyre NE, Knowles-Yanez K, Hope D (2000) Urban ecology as an interdisciplinary field: differences in the use of "urban" between the social and natural sciences. Urban Ecosyst 4:5–24
McKinney M (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
McKinney M (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: current state and trends. Island Press, Washington
Nagendra H, Gopal D (2011) Tree diversity, distribution, history and change in urban parks: studies in Bangalore, India. Urban Ecosyst 14:211–223
Nielsen AB, van den Bosch M, Maruthaveeran S, Konijnendijk van den Bosch C (2014) Species richness in urban parks and its drivers: a review of empirical evidence. Urban Ecosyst 17:305–327
Niemelä J (2014) Ecology of urban green spaces: the way forward in answering major research questions. Landsc Urban Plan 125:298–303
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2011) vegan: Community Ecology Package. R package version 2.0-0. http://CRAN.R-project.org/package=vegan
Pauchard A, Aguayo M, Pena E, Urrutia R (2006) Multiple effects of urbanization on the biodiversity of developing countries: the case of a fast-growing metropolitan area (Concepcion, Chile). Biol Conserv 127:272–281
Pauchard, A, Barbosa, O (2013) Regional assessment of Latin America: rapid urban development and social economic inequity threaten biodiversity hotspots In: Elmqvist, T, Fragkias, M, Goodness, J, Güneralp, B, Marcotullio, PJ, McDonald, RI, Parnell, S, Schewenius, M, Sendstad, M, Seto, KC, Wilkinson C (eds) Urbanization, biodiversity and ecosystem services: Challenges and opportunities. Springer, Dordrecht, pp 589–608
Politi Bertoncini A, Machon N, Pavoine S, Muratet A (2012) Local gardening practices shape urban lawn floristic communities. Landsc Urban Plan 105:53–61
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramalho CE, Hobbs RJ (2012) Time for a change: dynamic urban ecology. Trends Ecol Evol 27:179–188
Rojas C, Pino J, Basnou C, Vivanco M (2013) Assessing land-use and -cover changes in relation to geographic factors and urban planning in the metropolitan area of Concepción (Chile). Implications for biodiversity conservation. Appl Geogr 39:93–103
SAF (2006) Orthophotos of the Metropolitan Region of Santiago de Chile, scale 1:20.000. Servicio Aerofotogramétrico, Santiago
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schmeil O, Fitschen J (2009) Flora von Deutschland und angrenzender Länder. Quelle & Meyer Verlag, Wiebelsheim
Schmidt KJ, Poppendieck HH, Jensen K (2014) Effects of urban structure on plant species richness in a large European city. Urban Ecosyst 17:427–444
Shwartz A, Turbé A, Julliard R, Simon L, Prévot A-C (2014) Outstanding challenges for urban conservation research and action. Sci Technol 28:39–49
Singh RB (2015) Urban development challenges, risks and resilience in asian mega cities. In: Singh RB (ed) Advances in geographical and environmental sciences. Springer, Japan
Stewart GH, Ignatieva ME, Meurk CD, Buckley H, Horne B, Braddick T (2009) URban Biotopes of Aotearoa New Zealand (URBANZ) (I): composition and diversity of temperate urban lawns in Christchurch. Urban Ecosyst 12:233–248
Sushinsky JR, Rhodes JR, Possingham HP, Gill TK, Fuller RA (2013) How should we grow cities to minimize their biodiversity impacts? Glob Chang Biol 19:401–410
Thompson K, Hodgson JG, Smith RM, Warren PH, Gaston KJ (2004) Urban domestic gardens (III): composition and diversity of lawn floras. J Veg Sci 15:373–378
Tomasetto F, Duncan RP, Hulme PE (2013) Environmental gradients shift the direction of the relationship between native and alien plant species richness. Divers Distrib 19:49–59
UN-Habitat (2009) Planning Sustainable Cities — Global Report on Human Settlements 2009. Earthscan
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
von der Lippe M, Kowarik I (2012) Interactions between propagule pressure and seed traits shape human-mediated seed dispersal along roads. Perspect Plant Ecol 14:123–130
Wang HF, MacGregor-Fors I, Lopez-Pujol J (2012) Warm-temperate, immense, and sprawling: plant diversity drivers in urban Beijing, China. Plant Ecol 213:967–992
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Wichmann MC, Alexander MJ, Soons MB, Galsworthy S, Dunne L, Gould R, Fairfax C, Niggemann M, Hails RS, Bullock JM (2009) Human-mediated dispersal of seeds over long distances. P Roy Soc B-Biol Sci 276:523–532
Wilhelm M, Andres F (1998) Parkrasen und Parkwiesen in Zürich. In: Schmidt E, Sigel B (eds) Kowarik, I. Naturschutz und Denkmalpflege. vdf Hochschulverlag, Zürich, pp. 221–227
Acknowledgments
We thank Sonia Reyes Paecke, Luis Meza (Pontificia Universidad Católica de Chile, Instituto de Estudios Urbanos y Territoriales) and Marianne Katunaric (CONAMA Chile) for cooperation, help with species identification and provision of GIS data. The study was funded by the German Academic Exchange Service DAAD, the International Office of the TU Berlin, and the German Research Foundation (DFG) as part of the graduate research training programme ‘Urban Ecology Berlin’ (GRK 780/III). We thank Kelaine Ravdin for improving our English and two anonymous reviewers for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 56.7 kb)
Rights and permissions
About this article
Cite this article
Fischer, L.K., Rodorff, V., von der Lippe, M. et al. Drivers of biodiversity patterns in parks of a growing South American megacity. Urban Ecosyst 19, 1231–1249 (2016). https://doi.org/10.1007/s11252-016-0537-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0537-1