Abstract
The study was carried out to estimate genetic and phenotypic parameters for growth traits in Dorper crossbred sheep. The data set consisted of 5717 growth records from 1347 individuals of Dorper 50% crossbred sheep descended from 43 sires and 344 dams born between the years 2012 and 2022 at Debre Birhan Agricultural Research Center sheep research station, Ethiopia. Studied traits were birth weight (WT0), weaning weight (WT3), six months weight (WT6), yearling weight (WT12), average daily gain from birth to weaning (ADG1), average daily gain from weaning to six months (ADG2), average daily gain from six months to yearling (ADG3). The (co)variance components were estimated by fitting six different univariate animal models using Average Information Restricted Maximum Likelihood (AI-REML) procedure. Contrary to the genetic trend, phenotypic performance for all studied traits showed a declining trend over the years. Direct heritability estimates of 0.10 ± 0.06, 0.29 ± 0.09, 0.37 ± 0.10, 0.10 ± 0.09, 0.43 ± 0.15, 0.04 ± 0.05, and 0.14 ± 0.09 were obtained for WT0, WT3, WT6, WT12, ADG1, ADG2 and ADG3, respectively. Genetic correlations among the studied traits ranged from -0.43 (between ADG2 and ADG3) to 0.99 (between WT3 and ADG1). Selection for weaning, six months and pre-weaning average daily gain would be expected to yield good response as these traits were found moderately heritable. Strong to moderate genetic correlation of WT3 with WT6, WT12, and ADG1 suggested that selection based on WT3 would result in improvement of other growth traits due to correlated response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indigenous sheep breeds are unable to meet the rapidly increasing demand for animal products that are being created by rapid population growth, urbanization, and income growth despite having adapted to the current environmental situation, which is characterized by poor nutrition and a high prevalence of disease (Getachew et al. 2016). To satisfy the growing demand for animal products, crossbreeding using exotic breeds has been used in many countries. To meet the ever-increasing demand for animal products, Ethiopia has initiated a national sheep crossbreeding program using the Dorper breed, which specializes in meat production. Such a genetic improvement strategy is considered as potentially attractive breed improvement method due to its quick benefits as the result of breed complementarity and heterosis effects (Leymaster 2002).
Debre Birhan Agricultural Research Center (DBARC) Dorper-based crossbreeding program has been implemented since 2012 to improve local sheep productivity and carcass quality. The breeding program was started with the crossing of local ewes with pure Dorper rams to produce 50% crossbred lambs at the research center. The crossbred ewe lambs produced in the first cross are being mated with 50% crossbred ram lambs (Inter se mating) to develop synthetic breed through successive crossing. Whereas crossbred rams not selected for inter se mating are distributed to village based crossbreeding programs. As a result, over the past decade, the research center has produced about 1347 crossbred lambs (Male and Female) for synthetic breed development and for village-based crossbreeding programs. Due to fast growth, large body size, better carcass quality and higher market prices of crossbred lambs to their local counterparts, farmers have often showed interest in the Dorper crossbred lambs (Abebe et al. 2016; Mekonnen et al. 2018).
Evaluation of the breeding program is an important aspect to optimize the breeding program if the designed breeding program is progressing towards the set goal or redesign other alternative if it deviates from the preset goals (Mallick et al. 2016). To increase the efficiency of breeding program, knowledge of the genetic parameters, genetic relationship between the traits and a thorough understanding of the effect of different environmental factors are the prerequisites (Behzadi et al. 2007; Tesema et al. 2022). Phenotypic performance evaluation of Dorper crossbred sheep population were documented by several scholars (Abebe et al. 2023, 2016; Belete et al. 2015; Deribe et al. 2017; Lakew et al. 2014) in Ethiopia. However, only a few attempts have been made to estimate genetic parameters in Dorper × Tumele sheep population (Tesema et al. 2022). Moreover, estimates of the genetic parameters of a trait are influenced by population and environment. Therefore, the present study was conducted to evaluate the ongoing Dorper × Menz sheep crossbreeding program and generate information to optimize the breeding program by estimating the variance and covariance components for different growth traits.
Materials and methods
The breeding flock and management
Data were collected from breeding flock of Dorper × Menz sheep maintained at Debre Birhan Agricultural Research Center, Amhara Regional State, Ethiopia for a period of 11 years (2012 to 2022). The research center is located 120 km North-east of Addis Ababa at an altitude of 2,765 m above sea level and at a latitude of 09°36′23′′N and longitude of 39°39′10′′E. The area is characterized by a bi-modal rainfall pattern, where the main rainy season is from June to September and unreliable short rainy season is expected from February/March to April. According to metrological data obtained from DBARC, average annual rainfall during the studied period was 865 mm and the mean minimum and maximum temperature was 6.95 °C and 20.25 °C respectively. Frost is common from October to December. All animals were allowed to graze during the day on natural pasture daily for 6 to 7 h and penned at night during dry and short rainy seasons. On the other hand, because of high mortality occurrence due to fasciolosis outbreak in 2014, all animals were kept indoors day and night during the main rainy season (from June to September) and fed dry hay as a basal diet without supplementation of vitamins and minerals premix. However, since 2019, the animals were fed green forage during the main rainy season with a cut and carry feeding system. The animals were supplemented with 200 to 400 g/head/day mixed concentrate depending up on status, age and availability of grazing pasture. As a routine flock health management practice of the research center the experimental animals were treated against internal parasites and were vaccinated against common viral diseases of the area. Pure Dorper rams were mated with Menz ewes which is indigenous to the central highland of North Shewa zone to produce 50% crossbred lambs at the research center. The crossbred ewe lambs produced in the first generation were mated with the best 50% crossbred rams (Inter se mating) to develop synthetic breed through successive crossing. For the last 11 years a total of 43 rams (23 pure Dorper to produce F1 crossbred lambs and 20 crossbred Dorper (50%) for successive crossing) were used. Generally, controlled mating was practiced, and one selected breeding rams was allowed to mate with 25 to 30 ewes and mating lasted an average of 60 days. Breeding rams were selected based on estimated breeding values (EBVs) for six months weight using WOMBAT software. Rams with high EBVs and desired physical conformation and coat color were selected with the aim of improving growth and carcass yield. Since 2019, MateSel software Kinghorn (2010) has been applied to make mating group in order to control inbreeding and to maximize genetic gain across generation. At birth each lambs was identified with plastic ear tag and date of birth, sex, birth litter size, weight and color were recorded. Lambs were normally weaned at three months of age.
Data collection, management and analyses
Data were collected over the years 2012 to 2022, with records on a total of 1347 lambs descended from 43 sires and 344 dams. Traits considered for analysis were weight at birth (WT0), weight at three months (WT3), weight at six months (WT6), weight at yearling (WT12), average daily gain from birth to weaning (ADG1), average daily gain from weaning to six months (ADG2) and average daily gain from six months to yearling (ADG3). Birth weight was taken within 24 h of the birth of lamb. Weaning, six months and yearling weight measurements were taken at approximately 90, 180 and 365 days respectively after birth, with a permissible range of plus or minus five days, and subsequently linearly adjusted to precisely 90, 180 and 365 days to ensure precise comparison and reliable analysis. Average daily gain was calculated as ADG1 = ((WT3-WT0)/90) X 1000, ADG2 = ((WT6-WT3)/90) X 1000 and ADG3 = ((WT12-WT6)/180) X 1000.
Fixed effects for body weight and average daily gain were estimated using the GLM procedure of SAS 9.4 software (SAS 2004). The considered fixed effects were generation of lambs in three classes (F1, F2 and F3), sex of lambs in two classes (male and female), birth litter size in two classes (single and twin), parity of dam in five classes (one to five), year of lambing in eleven classes (2012–2022) and season of lambing in three classes (rainy, dry and short rainy season). Means were compared using Tukey-kramers test.
The model used for the analysis of body weight and average daily gain was
where Yijklmnop is an observation for body weight and average daily gain; μ is overall mean; Gi is fixed effect of lamb generation; Yj is fixed effect of year of birth; Btk is fixed effect of birth type; Bsl is fixed effect of birth season; Pm is fixed effect of parity; Sn is fixed effect of sex of lamb and eijklmno is residual error.
The (co)variance components, genetic parameters and inbreeding coefficient were estimated by the Average Information Restricted Maximum Likelihood (AI-REML) and fitting six univariate animal models using WOMBAT software (Meyer 2007). For animal breeding applications, the inclusion of fixed effects is used to protect against downward bias in heritability estimates (Ghafouri-Kesbi & Gholizadeh 2017). Fixed effects with significant effect (P < 0.05) in the linear model analysis were included in the genetic model. When the change in log likelihood between the last two iterations is less than 10–4; convergence was assumed to have been achieved (Meyer 2006). Multivariate analysis was applied for genetic and phenotypic correlation estimates.
Many random factors such as direct genetic effects, maternal genetic effects and environmental factors affects the growth of lambs and its dam (Behzadi et al. 2007). The animal models which ignoring maternal effects lead to upward estimates of direct heritability (Sharif et al. 2022). Thus, to achieve optimum genetic progress maternal effects should be taken into consideration, especially when the direct-maternal genetic correlation is negative (Behzadi et al. 2007; Tesema et al. 2022). By excluding or including permanent environmental or maternal genetics effects, the following six univariate animal models were fitted for each trait.
Model (1) y = Xβ + Zaα + e.
Model (2) y = Xβ + Zaα + Zpepe + e.
Model (3) y = Xβ + Zaα + Zmm + e Cov(α, m) = 0.
Model (4) y = Xβ + Zaα + Zmm + e Cov(α, m) = Aσam.
Model (5) y = Xβ + Zaα + Zmm + Zpepe + e Cov(α, m) = 0.
Model (6) y = Xβ + Zaα + Zmm + Zpepe + e Cov(α, m) = Aσam.
Where y is a vector of observations on the considered traits; β, α, m, pe and e are vectors of significant fixed effects, direct additive genetic effects, maternal genetic effects, permanent environment effects and the residual effects, respectively. Whereas X, Za, Zm and Zpe are corresponding incidence matrices relating the fixed effect, direct additive genetic effects, maternal additive genetic effects and permanent environmental effects of the dam. A is Numerator relationship matrix between animals and σam is covariance between direct and maternal genetic effects. Direct heritability (h2a), maternal heritability (h2m) and relative permanent maternal environmental effects (c2) were calculated as ratios of estimates of σ2a, σ2m and σ2c respectively, to the phenotypic variance σ2p. Total heritability (h2t) was estimated using the following formula according to (Willham 1972).
where σ2a is additive variance, σ2m is maternal variance, σam is covariance between direct and maternal additive genetic effects and σ2p is total phenotypic variance.
A log likelihood ratio tests were conducted to choose the best model for each trait. The log likelihood ratio test were computed as the twice the difference between the log-likelihoods of the full and reduced models, which assumed to be distributed as chi-square distribution with degree of freedom equal to the difference in the number of random covariance components fitted for the two models. A random effect was considered to have significant influence, when its inclusion caused significant (P < 0.05) increase in log-likelihood compared to the model in which it was ignored. However, when log likelihoods did not differ significantly P > 0.05), the model that had the fewer number of parameters was selected as the most suitable model (Wilson et al. 2010).
Phenotypic and genetic trends were estimated by regressing yearly mean phenotype performance and estimating breeding value (EBV) on year of birth. Moreover, inbreeding trend was estimated by regressing yearly mean inbreeding rate on year of birth.
Results and discussion
Phenotypic growth performance
Phenotypic means, standard deviations (S.D.), coefficient of variation (C.V.) and pedigree structure of studied traits are presented in Table 1. Number of observations decreased with increasing age of birth (n = 1347) to yearling (n = 517) because of culling related to death, sale of animals and distribution of rams to villages-based crossbreeding program. The overall least-squares mean of birth weight recorded under the current study was comparable to values reported by Lakew et al. (2014); Tesema et al. (2022) for Dorper × Local sheep at Sirinka agricultural research center. However, the current birth weight was higher than values reported by Abebe et al. (2016) for Dorper × Menz 50% at DBARC (2.77 ± 0.04) and Belete et al. (2015) for Dorper 50% lambs (2.25 ± 1.75 kg) in Wolaita and Silte Zone at farmer’s management conditions. The overall least-squares means of weaning and six months weight recorded in the present study were comparable with the findings of Abebe et al. (2016) for Dorper × Menz 50% at DBARC (12.34 ± 0.25 and 17.25 ± 0.30 respectively). However, values obtained in the present study for weaning, six months and yearling weight were significantly lower than values reported by Lakew et al. (2014); Tesema et al. (2022) for Dorper × Local 50% at Sirinka agricultural research center. The overall least-squares means for pre-weaning average daily gain obtained in the present study was in close agreement with the report of Abebe et al. (2016), but lower than values reported by Lakew et al. (2014); Tesema et al. (2022) for Dorper × local 50% at Sirinka agricultural research center. Furthermore, average daily gain recorded in post-weaning growth periods was lower than values reported by Abebe et al. (2016); Lakew et al. (2014). The difference in phenotypic performance can be attributed to difference in performance of the dam breed, availability of grazing pasture, animal management and other unknown environmental conditions. Coefficient of variation for the studied traits ranged from 16.76% (WT12) to 88.95% (ADG2). The highest coefficient of variation for the studied traits can be explained by higher variability of the traits among the animals, higher changes of the traits by environmental conditions and other unknown factors.
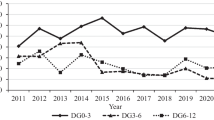
The effect of some non-genetic factors on the phenotypic body weight and average daily gain traits of Dorper crossbred sheep are presented in Tables 2 and 3. Year of birth had a significant effect (P < 0.001) in all studied traits. The impact of birth year on the studied traits can be explained by differences in animal management, feed availability, disease incidence, climatic condition (such as rate of rainfall, humidity and temperature, which affected the quality and quantity of pasture forage) in different years (Bakhshalizadeh et al. 2016). The phenotypic performance for WT3, WT6, WT12 and ADG1, ADG2, ADG3 showed a decline trends a rate of -0.17, -0.69, -0.91 kg and -1.94, -5.88, -2.30 g over years respectively (Figs. 1 and 2). The phenotypic trends obtained by fitting a linear regression on birth year for WT6 and ADG2 were significantly different from zero (P < 0.05), but not significant for WT3, WT12, ADG1, and ADG3. Opposing to phenotypic trends, the genetic performance showed improvement trends of 0.06 kg, 0.03 kg, 0.78 g, 0.07 g for WT6, WT12, ADG1 and ADG3 over year, respectively. Contrary to the genetic trend, the declined trend of phenotypic performance on the studied traits may had occurred due to management and unfavorable environmental effects that hindered expression of genetic potential of crossbred lambs. A significant reduction in phenotypic performance was observed between the year 2015–2018 followed by an improvement trends and then declining trends between 2021–2022. Difference in feed availability, climatic conditions and management practice of the research center are the possible reason for the declined trends during these years. Since 2015, the animals were not allowed to graze on natural pasture during winter season, kept indoors and fed dry hay as a basal diet supplemented with 200 g head/day mixed concentrate but without any supplementation of vitamins and minerals premix. This practice could have exposed the animals to vitamin E deficiency, also, as the center is located in high rainfall (865 mm) area, it is expected to increase chances of selenium deficiency. Deficiency of either or both selenium and vitamin E can reduce growth, reproductive performance and immune response of the animal (Ramírez-Bribiesca et al. 2005; Ziaei 2015).
The finding also indicated that male lambs were superior (P < 0.001) to female lambs in WT0, WT6 and WT12. The difference may be ascribed to difference in endocrine hormone in the two sexes (Behzadi et al. 2007; Rashidi et al. 2008). Similar result was also reported by Abebe et al. (2016); Goshme et al. (2014) who reported that the male Dorper crossbred lambs were heavier than the female counter parts under similar management condition. The current result also showed that lambs with singleton were heavier (P < 0.001) than lambs with twin birth except post-weaning average daily gain. This difference may be attributed to the fact that lesser availability of uterine space, limited capacity of ewes to provide more nutrients during pregnancy among multiple births and also the competition for dam’s milk during pre-weaning period (Ehsaninia 2021; Mokhtari et al. 2012). As indicated in Tables 2 and 3 parity has a significant effects (P < 0.05) on WT0, WT3, ADG1 and ADG3. Accordingly, lambs born from dams in their 1st parity had lighter weight at WT0, WT3 and ADG1 as compared to lambs born from the successive parities. The significant effects of parity can be ascribed to difference in maternal effects and maternal behavior of ewes at different ages (Ehsaninia 2021). The same effects of parity for Dorper crossbred lambs were reported by Abebe et al. (2016); Belete et al. (2015) under different management condition. The present finding also revealed that birth season had a significant effect (P < 0.001) in all studied traits except ADG2. Lambs born during dry season had lower growth performance in most studied traits as compared to lambs born during main and short rainy seasons. This may be attributed to availability of grazing pasture and disease incidence varied across seasons. The non-significant effects of most fixed effects on ADG2 may be due to the presence of a significant weaning shock on lamb performance during separation from their dam. Furthermore, second generation crossbred lambs had significantly higher (P < 0.001) WT0, WT3, WT6 and ADG1 than first generation crossbred lambs. This may be attributed to the difference in uterine effect among Dorper cross and local dams.
Estimates of genetic parameters
Estimates of (co)variance components and genetic parameters for body weight and average daily gain traits are shown in Table 4. Estimates of direct genetic heritability for birth weight depended on the model used, ranging from 0.04 to 0.19. Fitting a permanent environmental effect (Model 2) substantially increased the log-likelihood values over that for Model 1, indicating a significant permanent environmental effect in birth weight. Fitting maternal genetic effect (Model 4) for birth weight also significantly increased log-likelihood when compared with other models. Based on the most appropriate model (Model 4) for birth weight, the estimates of direct and maternal genetic heritability was 0.10 ± 0.06 and 0.26 ± 0.07 respectively. The estimate of direct genetic heritability of birth weight in the present study was comparable to the finding of Mandal et al. (2006) in Muzaffarnagari sheep and while, lower maternal genetic heritability was reported by same author. However, higher estimates of direct and maternal genetic heritability of birth weight was reported by Gizaw et al. (2007); Habtegiorgis et al. (2020) for indigenous sheep breeds in Ethiopia. On the other hand lower direct and maternal genetic heritability estimate were reported by Rashidi et al. (2008) in Kermani sheep by fitting the same model.
Fitting maternal genetic effects without considering covariance between direct and maternal genetic effects (Model 3), was determined as the best model for WT3. Based on the most appropriate model direct and maternal genetic heritability for WT3 was 0.29 ± 0.09 and 0.14 ± 0.05 respectively. The most appropriate model for WT6 and WT12 were model including only direct genetic effects. This revealed that lesser effect of permanent and maternal genetic effect in six months and yearling weight. Based on the appropriate model (Model 1), the estimates of direct genetic heritability for WT6 and WT12 were 0.37 ± 0.10 and 0.10 ± 0.09 respectively. The direct genetic heritability estimates for WT3 and WT6 were moderate in magnitude, while the direct genetic heritability estimates for WT12 was weak. Estimates of direct genetic heritability for WT3, WT6 and WT12 were lower than values reported by Gizaw et al. (2007) in Menz sheep. However, direct genetic heritability estimate in the current study for WT3 and WT6 were higher than values reported by Matika et al. (2003); Mokhtari et al. (2012); Singh et al. (2006) in other sheep breeds. A reliable reason for the negative correlation between direct and maternal genetic effects (Model 4) can be poor environmental conditions and poor data structure (low number of progeny records per dam) (Bakhshalizadeh et al. 2016).
The most appropriate model for ADG1 was model including maternal genetic effects with considering covariance between direct and maternal genetic effects (Model 4). A model including only the direct genetic effects was the most appropriate model for ADG2 and ADG3. This indicated the lesser effect of permanent and maternal genetic effects in ADG2 and ADG3. Based on the most appropriate model direct genetic heritability estimates for ADG1, ADG2 and ADG3 were 0.43 ± 0.15, 0.04 ± 0.05 and 0.14 ± 0.09 respectively. Direct genetic heritability estimate for ADG1 in the current study was higher than values reported by Habtegiorgis et al. (2020); Matika et al. (2003); Mokhtari et al. (2012); Rashidi et al. (2008) in other sheep breeds. Direct genetic heritability estimate for ADG2 was lower than values reported by Habtegiorgis et al. (2020) in Doyogena sheep. However, direct genetic heritability estimates for ADG2 in the current study was higher than values reported by Tamioso et al. (2013) in Suffolk lambs. The current results revealed that maternal genetic effects was a considerable source of phenotypic variation for pre-weaning growth period. Thus, both direct and maternal genetic effects were found to be important for the genetic parameter estimation of pre-weaning growth period. A similar findings was obtained by Behzadi et al. (2007) in Kermani sheep. When maternal effects contribute significant source of variation in the phenotype of progenies, prediction of selection response should be done by estimating total heritability (h2t) (Willham 1972).
Correlation estimates
Multivariate analyses results are presented in Table 5. The genetic correlation between studied traits ranged from low to high in magnitude -0.43 (ADG2-ADG3) to 0.99 (WT3-ADG1). Estimates of genetic and phenotypic correlations between WT3 and ADG1 was 0.99. These high estimates are expected because WT3 partly contributes to the calculation of ADG1. Strong genetic and phenotypic correlation between WT3 and ADG1 were also reported by Rashidi et al. (2008) for Kermani sheep. Moderate (0.66) direct genetic correlations were obtained for WT3-WT6 the corresponding estimates was comparable with estimates of Singh et al. (2006) in crossbred sheep (0.76). However, lower estimate of direct genetic correlation between WT3 and WT6 was reported by Habtegiorgis et al. (2020); Mohammadi et al. (2012). Positive and moderate direct genetic correlation between WT3 and WT6 weight could be an opportunity to select best animal early in the process of developing synthetic breed through successive crossing. Genetic correlation between ADG1 with ADG2 and ADG3 was negative and weak in magnitude. It revealed that lambs with higher daily gain in pre-weaning period were less efficient during the post-weaning period and vice versa. The negative and weak genetic correlations between those traits imply that different genetic mechanisms are involved in expressing those traits at different stage of growth (Mohammadi et al. 2011, 2015). The genetic and phenotypic correlation estimates of WT0 with other traits were weak in magnitude. The weak genetic and phenotypic association of WT0 with other traits can be explained by WT0 is highly influenced by prenatal maternal environment compared to other traits.
Genetic trends and inbreeding coefficient
The genetic trends for WT0, WT6 and WT12 from pooled data analysis showed a positive improvement trends a rate of 0.002, 0.058, 0.026 kg over years respectively. Moreover, ADG1 and ADG3 showed improvement trends a rate of 0.781 and 0.074 g over years respectively. However, WT3 and ADG2 showed a declining trend at rate of 0.005 kg and 0.009 g over years respectively (Figs. 3 and 4). This could be due to existence of poor environmental condition, it could result in lamb not expressing their genetic potential. Therefore, improving management practices of the center during this period could improve the genetic progress. The annual genetic trends across generation for all studied trait showed improvement trends except for WT3 in first generation lambs (Tables 6 and 7). Reasons for low R2 values of most studied traits could be these traits are highly influenced by environmental factors. The average inbreeding coefficient of Dorper crossbred sheep was 0.158% with annual inbreeding rate of 0.009% (P < 0.5875). Total number of inbred animal in the current study were nine and its average inbreeding coefficient was 23.61% (Table 8). The coefficient of inbreeding for first generation lambs was zero due to crossing of distinct breed of sheep. Average inbreeding coefficient recorded in the current study was lower than values reported by Habtegiorgis et al. (2020) in Doyogena sheep (0.3%) and Areb et al. (2021) in Bonga sheep (0.36%). Generally, inbreeding coefficient recorded under the present study was under acceptable ranges. According to Food and Agricultural Organization of the United Nations (FAO) inbreeding rate should be maintained below the range of 0.5–1% per year to avoid risk of genetic disorders and inbreeding depression (Food and Agriculture Organization 2010).
Conclusion
The current study contributes to model comparison and estimates of genetic parameters in Dorper crossbred sheep. Selection for weaning, six months and pre-weaning average daily gain would be expected to yield good response as these traits was found moderately heritable. Strong to moderate genetic correlation of weaning weight with six months weight, yearling and pre-weaning average daily gain suggested that selection based on weaning weight would result in improvement of other growth traits due to correlated response.
Data availability
Data will be made available on request.
References
Abebe A, Berhane G, Gizaw S, Getachew T (2023) Effect of genotype and environment on the productive and survivability traits of lambs under a community-based management system. J Agric Food Res 13(March):100644. https://doi.org/10.1016/j.jafr.2023.100644
Abebe A, Gizaw S, Getachew T, Lemma S, Bisrat A, Goshme S, Besufekad S, Abebe A, Mekonen T, Zewudie T, Chaniyalew Y (2016) Growth Performance of Dorper and its F1 Crossbreds at Debre-Birhan Agricultural Research Center, Ethiopia 1. Proceedings of the 8th Annual Regional Conference on Completed Research Activities of Livestock, January, 1–21
Areb E, Getachew T, Kirmani MA, Silase TG, Haile A (2021) Estimation of co-variance components, genetic parameters, and genetic trends of reproductive traits in community-based breeding program of Bonga sheep in Ethiopia. Animal Bioscience 34(9):1451–1459. https://doi.org/10.5713/AJAS.20.0413
Bakhshalizadeh S, Hashemi A, Gaffari M, Jafari S, Farhadian M (2016) Estimation of genetic parameters and genetic trends for biometric traits in Moghani sheep breed. Small Rumin Res 134:79–83. https://doi.org/10.1016/j.smallrumres.2015.12.030
Behzadi MRB, Shahroudi FE, Van Vleck LD (2007) Estimates of genetic parameters for growth traits in Kermani sheep. J Anim Breed Genet 124(5):296–301. https://doi.org/10.1111/j.1439-0388.2007.00672
Belete E, Goshu G, Tamir B (2015) Productive Performance Evaluation of Dorper Sheep Crosses (50% Dorper × Pure Adilo Indigenous Sheep Breed) under Farmer Conditions in Different Agro Ecological Zones. Int J Livest Prod 6(5):61–68. https://doi.org/10.5897/IJLP2014.0246
Deribe G, Girma A, Gebeyehu G, Asrat T, Belete SG (2017) Early growth and survival rates of crossbred lambs (Dorper x indigenous) under semi-intensive management at Areka, Southern Ethiopia: Effects of non-genetic factors. Afr J Agric Res 12(23):2008–2016. https://doi.org/10.5897/ajar2017.12280
Ehsaninia J (2021) Estimates of (co) variance components and genetic parameters for pre-weaning body weight traits and Kleiber ratio in Sangsari sheep breed. Ital J Anim Sci 20(1):918–927. https://doi.org/10.1080/1828051X.2021.1908860
Food and Agriculture Organization (2010). Breeding Strategies for Sustainable Management of Animal Genetic Resources. FAO Animal Production and Health Guidelines No. 3, Rome, Italy
Getachew T, Haile A, Wurzinger M, Rischkowsky B, Gizaw S, Abebe A, Sölkner J (2016) Review of sheep crossbreeding based on exotic sires and among indigenous breeds in the tropics: An Ethiopian perspective. Afr J Agric Res 11(11):901–911
Ghafouri-Kesbi F, Gholizadeh M (2017) Genetic and phenotypic aspects of growth rate and efficiency-related traits in sheep. Small Rumin Res 149:181–187. https://doi.org/10.1016/j.smallrumres.2017.02.006
Gizaw S, Lemma S, Komen H, Van Arendonk JAM (2007) Estimates of genetic parameters and genetic trends for live weight and fleece traits in Menz sheep. Small Rumin Res 70(2–3):145–153. https://doi.org/10.1016/j.smallrumres.2006.02.007
Goshme S, Gizaw S, Bisrat B, Besufkad S, Abebe A (2014) Comparative growth performance and survival rate of Dorper x improved Menz, Dorper x Menz type and Dorper x Afar cross bred sheep at Debre Birhan research center. Proceeding of the 6th and 7th annual regional conference on completed research activities, 25–27 January, 2012 and 22–24 January, 2013, Amhara Agricultural Research Institute (ARARI), Bahir Dar, Ethiopia, pp 247–251
Habtegiorgis K, Haile A, Kirmani MA, Getachew T (2020) Estimates of Genetic Parameters and Genetic Trends for Growth Traits of Doyogona Sheep in Southern Ethiopia. J Anim Breed Genet 4(2):33–49
Kinghorn BP (2010) MateSel: a software mating tool to aid in selection for improved fertility. University of New England, NSW, Australia. https://bkinghor.une.edu.au/pedigree.htm. Accessed 20 Apr 2023
Lakew M, Haile-Melekot M, Mekuriaw G (2014) Evaluation of growth performance of local and dorper × Local crossbred sheep in Eastern Amhara Region, Ethiopia. Iran J Anim Sci 4:123–126
Leymaster KA (2002) Fundamental aspects of crossbreeding of sheep: use of breed diversity to improve efficiency of meat production. Sheep Goat Res J 17(3):50–59
Mallick PK, Thirumaran SMK, Pourouchottamane R, Rajapandi S, Venkataramanan R, Nagarajan G, Murali G, Rajendiran AS (2016) Genetic trend for growth and wool performance in a closed flock of Bharat Merino sheep at sub temperate region of Kodai hills. Tamil Nadu Veterinary World 9(3):276
Mandal A, Neser FWC, Rout PK, Roy R, Notter DR (2006) Estimation of direct and maternal (co)variance components for pre-weaning growth traits in Muzaffarnagari sheep. Livest Sci 99(1):79–89. https://doi.org/10.1016/j.livprodsci.2005.06.001
Matika O, Van Wyk JB, Erasmus GJ, Baker RL (2003) Genetic parameter estimates in Sabi sheep. Livest Prod Sci 79(1):17–28. https://doi.org/10.1016/S0301-6226(02)00129-X
Mekonnen T, Alemayehu L, Abebe A, Bisrat A, Yitagesu E (2018) Body Weight and Carcass Characterstics of 25% Dorper x Local Crossbred Sheep Supplemented with Legumes and Mungbean Halum in North Shewa, Ethiopia. Proceeding of the 10th Annual Conference on Completed Livestock Research Activities, March 13–16,2017, Bahirdar, Ethiopia
Meyer K (2006) WOMBAT-A program for mixed model analyses by restricted maximum likelihood. User notes, Animal Genetics and Breeding Unit, Armidale, p 55
Meyer K (2007) WOMBAT—A tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). J Zhejiang Univ Sci 8:815–821
Mohammadi K, Rashidi A, Mokhtari MS, Nassiri MTB (2011) The estimation of (co)variance components for growth traits and Kleiber ratios in Zandi sheep. Small Rumin Res 99(2–3):116–121. https://doi.org/10.1016/j.smallrumres.2011.03.058
Mohammadi H, Shahrebabak MM, Vatankhah M, Shahrebabak HM (2012) Direct and maternal (Co)variance components, genetic parameters, and annual trends for growth traits of Makooei sheep in Iran. Trop Anim Health Prod 45(1):185–191. https://doi.org/10.1007/s11250-012-0190-5
Mohammadi K, Abdollahi-Arpanahi R, Amraei F, Mohamadi EM, Rashidi A (2015) Genetic parameter estimates for growth and reproductive traits in Lori sheep. Small Rumin Res 131:35–42. https://doi.org/10.1016/j.smallrumres.2015.07.029
Mokhtari MS, Shahrebabak MM, Shahrebabk HM, Sadeghi M (2012) Estimation of ( co ) variance components and genetic parameters for growth traits in Arman sheep. 1(1):38–47
Ramírez-Bribiesca JE, Tortora JL, Huerta M, Hernández LM, López R, Crosby MM (2005) Effect of selenium-vitamin E injection in selenium-deficient dairy goats and kids on the Mexican plateau. Arquivo Brasileiro De Medicina Veterinaria e Zootecnia 57(1):77–84. https://doi.org/10.1590/s0102-09352005000100011
Rashidi A, Mokhtari MS, Jahanshahi AS, Abadi MRM (2008) Genetic parameter estimates of pre-weaning growth traits in Kermani sheep. Small Rumin Res 74(1–3):165–171. https://doi.org/10.1016/j.smallrumres.2007.06.004
SAS (2004) SAS user's guide version 9.4: Statistics. Cary, NC: SAS Institute Inc
Sharif N, Ali A, Dawood M, Khan MI, ur R, Do DN (2022) Environmental Effects and Genetic Parameters for Growth Traits of Lohi Sheep. Animals 12(24):1–12. https://doi.org/10.3390/ani12243590
Singh D, Kumar R, Pander BL, Dhaka SS, Singh S (2006) Genetic parameters of growth traits in crossbred sheep. Asian Australas J Anim Sci 19(10):1390–1393. https://doi.org/10.5713/ajas.2006.1390
Tamioso PR, Luiz J, Filho A, Dias LT, Teixeira RDA (2013) Estimates of ( co ) variance components and genetic parameters for growth traits in Suffolk lambs. 2215–2220
Tesema Z, Deribe B, Lakew M, Getachew T, Tilahun M, Belayneh N, Kefale A, Shibesh M, Zegeye A, Yizengaw L, Getachew WA, Tiruneh S, Kiros S, Asfaw M, Molla B (2022) Genetic and non-genetic parameter estimates for growth traits and Kleiber ratios in Dorper× indigenous sheep. Animal 16(6):100533
Willham R (1972) The role of maternal effects in animal breeding: III. Biometrical aspects of maternal effects in animals. J Anim Sci 35:1288–1293
Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH (2010) An ecologist’s guide to the animal model. J Anim Ecol 79:13–26. https://doi.org/10.1111/j.1365-2656.2009.01639.x
Ziaei N (2015) Effect of selenium and vitamin E supplementation on reproductive indices and biochemical metabolites in Raieni goats. J Appl Anim Res 43(4):426–430. https://doi.org/10.1080/09712119.2014.980415
Acknowledgements
We would like to acknowledge all livestock breeding and management staff specially Mr. Deribew Bekele and Mr. Alemayehu H/Mikael, who over the years have assisted in managing the experimental flock and in data collection. We also acknowledge the international Center for Agricultural Research in the Dry Ares (ICARDA) for their invaluable assistance and provision of technical expertise and materials support in data analysis and running breeding programs.
Funding
The Dorper sheep experiment was financially supported by government budget of Amhara Agricultural Research Institute (ARARI) and Ethiopian Institute of Agricultural Research (EIAR).
Author information
Authors and Affiliations
Contributions
S. Besufkad, contributed in designing of the breeding program, data analysis, writing the result of the research. S. Goshme, A. Abebe, A. Bisrat, S. Lemma, S. Gizaw and T. Getachew contributed in designing the breeding program, animal management data collection and implementation of the breeding program. T. Zewudie and A. Areaya contributed in data collection and management of the experimental animals.
Corresponding author
Ethics declarations
Ethical approval
We do not conduct animal experiment. We only used data collected from animals used for breeding purposes.
Conflict of interest
‘Declaration of interest: none’.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Debre Birhan Agricultural Research Center is one of the research center under Amhara Agricultural Research Institute (ARARI).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besufkad, S., Goshme, S., Abebe, A. et al. Estimates of genetic parameters for growth traits in dorper crossbred sheep population. Trop Anim Health Prod 56, 264 (2024). https://doi.org/10.1007/s11250-024-04170-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-024-04170-0