Abstract
The present study was carried out to estimate genetic and phenotypic parameters for growth rate and efficiency-related traits in Dorper crossbred sheep population. Data on body weight collected from 2012 to 2021 at Debre Birhan Agricultural Research Center, Amhara Regional State, Ethiopia, were used to estimate phenotypic and genetic parameters for daily gain from birth to weaning (DG0-3), daily gain from weaning to 6 months (DG3-6), and daily gain from 6 months to yearling (DG6-12) and corresponding Kleiber ratios (KR0-3, KR3-6, KR6-12), efficiency of growth (GE0-3, GE3-6, GE6-12), and relative growth rate (RG0-3, RG3-6, RG6-12). Genetic parameters were estimated by restricted maximum likelihood (REML) procedure fitting six different univariate animal models and the most appropriate model for each trait was determined by log-likelihood ratio test. Multivariate analysis was carried out to estimate correlations between traits. Year and season of birth had a significant effect (p<0.001) in all studied traits. Direct heritability estimates for DG0-3, DG3-6, DG6-12, KR0-3, KR3-6, KR6-12, GE0-3, GE3-6, GE6-12, GR0-3, GR3-6, and GR6-12 were 0.45±0.15, 0.04±0.06, 0.15±0.11, 0.30±0.08, 0.13±0.11, 0.14±0.12, 0.34±0.15, 0.39±0.17, 0.31±0.14, 0.25±0.08, 0.23±0.13, and 0.23±0.13 respectively. Genetic correlation estimates between DG3-6 and other traits were positive and high in magnitude to their respective growth phase (0.95, 0.86, and 0.91 for KR3-6, GE3-6, and GR3-6 respectively). As the Dorper crossbred sheep are reaches market weight at about 6 months of age, focusing on improving traits measured during weaning to 6 months of age is more feasible. Selection based on DG3-6 is recommended to improve efficiency-related traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethiopia holds the largest sheep population with a constantly growing of 42.91 million heads (CSA 2021). Despite the huge number and large importance, productivity of the flock is very low. The carcass yield per animal slaughter is estimated to be 10 kg which is lower than the sub Saharan African sheep breeds (13 kg) (Abdi et al. 2019; Legese and Fadiga 2014). Although the indigenous sheep breeds are adapted to the existing environmental situation, they have limited genetic capacity to sustaining the fast growing demand for animal product (Getachew et al. 2016). To this end, to meet the ever-increasing demand for animal product, crossbreeding program based on exotic sires (mainly Awassi and Dorper) has been implemented since the early 1980s.
The pure Dorper nucleus flock was established at Debre Birhan Agricultural Research Center (DBARC) to be used as an improver breed in a crossbreeding program in 2011, while Dorper-based crossbreeding program has been implemented to improve the growth rate and carcass quality of indigenous sheep population in 2012. The research on Dorper-based crossbreeding program was focused on the development of synthetic breed from Dorper-local sheep crossing, production of 50% crossbred rams, and utilization of the crossbred ram in village-based crossbreeding program. The synthetic breed development and 50% crossbred ram production has been implemented at DBARC sheep research station and a new breed-to-be is in the making, while the village-based crossbreeding program has been implemented in pre-defined demarcated area of Amhara region in order to prevent the indigenous breeds from unnecessary genetic dilution. The rapid weight gain, high carcass quality, and non-selective grazing ability of the crossbred lambs under farmer management condition make them one of the preferred breed for the genetic improvement of the local populations in the central highland of Ethiopia (Abebe et al. 2016; Mekonen et al. 2018)
Besides, focusing on improving productivity through genetic improvement, improving the feed utilization efficiency of the breed is vital for efficient and sustaining the breeding program. Currently, due to rapid population growth and urbanization, grazing pastures are shrinking and shifting into arable land. As a result, sheep production in the central highland of Ethiopia is being shifting from extensive system to semi-intensive system in which feed cost comprises most of the production costs. Therefore, improving efficiency of meat production by reducing the cost of mutton production is crucial to improve the efficiency of genetic improvement program (Ghafouri-Kesbi and Gholizadeh 2017). To include such traits in the selection index, knowledge on genetic parameters regarding both growth rate and efficiency-related traits is vital. While information on genetic parameters for growth traits and Kleiber ratio is available (Besufkad et al. 2022; Tesema et al. 2020), there is no information regarding genetic parameter for efficiency of growth and relative growth rate in Dorper × local sheep in Ethiopia. Therefore, this study was conducted to evaluate the on-ongoing Dorper × local sheep crossbreeding program and generate information to optimize the breeding program by evaluating the genetic parameters for growth rate and efficiency-related traits of Dorper crossbred sheep population.
Materials and methods
The breeding flock and management
Data used in this study were obtained from the sheep research station of Debre Birhan Agricultural Research Center (DBARC), Amhara Regional State, Ethiopia, which is located 120 km North-east of Addis Ababa at a latitude of 09°36′23′′N and longitude of 39°39′10′′E. The altitude is approximately 2765 m.a.s.l. The area is characterized by a bi-modal rainfall pattern, where the main rainy season is from June to September and unreliable short rainy season is expected from February/March to April. Since 2012, local ewes were mated with pure Dorper rams to produce 50% crossbred lambs at the research station. Then the crossbred ewe lambs produced in the first cross were mated with the best 50% crossbred rams (Inter se mating) to develop synthetic breed through successive crossing. However, the third-generation (F3) mating has been started since 2019. In this regard, about 1350 (634 first generation, 673 second generation, and 43 third generation) crossbred lambs descended from 46 sires (25 pure Dorper to produce F1 crossbred lambs and 21 crossbred Dorper rams (50%) for successive crossing) and 586 dams (265 local and 321 Dorper 50%) were produced from 2012 to 2021. Breeding rams were selected based on estimated breeding value (EBV) for 6-month weight and other morphological traits such as color, absence of horn, and body conformations. Ewes were first exposed to ram at about 14 months of age. Controlled mating was practiced, and one selected breeding rams was allowed to mate with 25 to 30 ewes and mating were lasted on average of 60 days. Since 2019, MateSel software has been applied to make mating group in order to control inbreeding and to maximize genetic gain across generation. New born lambs were weighed and ear-tagged at the time of birth or 24 h afterwards and animal’s parent, date of birth, sex of lamb, birth litter size, and color were recorded. Lambs were usually separated from their dam at 3 months of age.
The animal were allowed to graze on natural pasture during the day for 6 to 7 h and penned at night during dry and short rainy seasons (from September to June). On the other hand, because of high occurrence of mortality due to fasciolosis outbreak in 2014, all animals were forced to kept indoors day and night during the main rainy season (from July to September) and fed dry hay as a basal diet without any supplementation of vitamins and minerals premix. However, since 2019, the animals were allowed to feed green forage and grass during this season with a cut and carry feeding system. The experimental animal were supplemented with 200 to 400 g/head/day mixed concentrate depending up on status, age category, and availability of grazing feed. The mixed concentrate had 19.9% crude protein and 79% total digestive nutrient. The animal has free access to fresh water twice a day. As a routine flock health management practice of the research center, the experimental animals were drenched against internal parasites and were vaccinated against common viral disease of the area.
Data collection, management, and analyses
Traits considered in this study were birth weight (WT0), weaning weight (WT3), 6-month weight (WT6), yearling weight (WT12), daily gain from birth to weaning (DG0-3), daily gain from weaning to 6 months (DG3-6), daily gain from 6 months to yearling (DG6-12), Kleiber ratio from birth to weaning (KR0-3), Kleiber ratio from weaning to 6 months (KR3-6), Kleiber ratio from 6 months to yearling (KR6-12), growth efficiency from birth to weaning (GE0-3), growth efficiency from weaning to 6 months (GE3-6), growth efficiency from 6 months to yearling (GE6-12), relative growth rate from birth to weaning (GR0-3), relative growth rate from weaning to 6 months (GR3-6), and relative growth rate from 6 months to yearling (GR6-12). Birth weight was taken within 24 h of the birth of lamb. Weaning, 6-month, and yearling weights were taken by synchronized lambing occurred within 5-day interval from the actual weighted dates and adjusted to the exact days of 90, 180, and 365 respectively. In meat type breed, daily gain is an important component of market lamb production and affects the economic success of producing slaughter lambs (Eskandarinasab et al. 2010). Daily gain was calculated as DG0-3 = ((WT3−WT0)/90) × 1000, DG3-6 = ((WT6−WT3)/90) × 1000, and DG6-12 = ((WT12−WT6)/180) × 1000. The Kleiber ratio has been proposed as an efficient criterion for feed efficiency under low-input range conditions which provides a good indication of how economically an animal grows (Mohammadi et al. 2011). Kleiber ratio was computed as KR0-3 = ADG0-3/WT30.75, KR3-6 = ADG3-6/WT60.75, and KR6-12 = ADG6-12/WT120.75, accordingly to Kleiber (1947). Efficiency of growth was calculated as GE0-3 = ((WT3−WT0)/WT0) × 100, GE3-6 = ((WT6−WT3)/WT3) × 100, and GE6-12 = ((WT12−WT6)/WT6) × 100. Relative growth rate was computed as GE0-3 = (Loge (WT3) − Loge (WT0))/90) × 100, GE3-6 = (Loge (WT6) − Loge (WT3))/90) × 100, and GE6-12 = (Loge (WT12) − Loge (WT6))/180) × 100. Additive coefficient of variation (CVA) allows us to have an insight into the genetic variability of traits as well as to compare precisely traits measured at different times or in different population (Ghafouri-Kesbi and Gholizadeh 2017). Additive coefficient of variance estimated as (CVA) = \(\frac{\sqrt{\upsigma_{\textrm{a}}^2}}{\overline{x}}\times 100\) where σ2a is additive genetic variance and \(\overline{x}\) is the sample mean.
Fixed effects for growth rate and efficiency-related traits were estimated using the GLM procedure of SAS 9.1 software (SAS 2002). The considered fixed effects were generation of lambs in three classes (1st, 2nd, and 3rd), sex of lambs in two classes (male and female), birth litter size in two classes (single and twin), parity of dam in six classes, year of lambing in 10 classes (2012–2021), and season of lambing in three classes (rainy, dry, and short rainy season). Means were compared using Tukey-kramers test. The model used for the analysis of fixed effects was:
where Yijklm is an observation; μ is overall mean; Gri is fixed effects of lamb generation; Yrj is fixed effects of year of birth; Btk is fixed effects of birth type; Bsl is fixed effects of birth season; Pm is fixed effects of parity; Sn is fixed effects of sex of lamb; and eijklmn is residual error.
(Co)variance components and genetic parameters were estimated using restricted maximum likelihood (REML) method fitting univariate animal model using WOMBAT software (Meyer 2007). Multivariate analysis was applied for genetic and phenotypic correlation estimates. By excluding or including permanent environmental or maternal genetics effects, the following six univariate animal models were fitted for each trait.
Model (1) y = Xβ + Zaα + e
Model (2) y = Xβ + Zaα + Zpepe + e
Model (3) y = Xβ + Zaα + Zmm + e Cov(α, m) = 0
Model (4) y = Xβ + Zaα + Zmm + e Cov(α, m) ≠ 0
Model (5) y = Xβ + Zaα + Zmm + Zpepe + e Cov(α, m) = 0
Model (6) y = Xβ + Zaα + Zmm + Zpepe + e Cov(α, m) ≠ 0
where y is a vector of observations on the considered traits and β, α, m, pe, and e are vectors of significant fixed effects, direct additive genetic effects, maternal genetic effects, permanent environment effects, and the residual effects, respectively, whereas X, Za, Zm, and Zpe are corresponding incidence matrices relating the fixed effects, direct additive genetic effects, maternal additive genetic effects, and permanent environmental effects of the dam. Total heritability (h2t) was estimated using the formula (σ2a + 0.5σ2m + 1.5σam)/σ2p (Willham 1972). Where σ2a is additive variance, σ2m is maternal variance, σam is covariance between direct and maternal additive genetic effect, and σ2p is total phenotypic variance. Direct heritability (h2a), maternal heritability (h2m), and relative permanent maternal environmental effects (c2) were calculated as ratios of estimates of σ2a, σ2m, and σ2c respectively, to the phenotypic variance σ2p. Likelihood ratio tests were conducted, to choose the best model and to test the significance of random effects for each trait (Meyer 2006).
Results and discussion
Phenotypic performance
Mean, standard deviation (SD), coefficient of variation (CV), and pedigree structure of studied traits are presented in Table 1. Crossbred lambs had remarkably higher daily gain, Kleiber ratio, efficiency of growth, and relative growth rate during the pre-weaning phase compared to the post-weaning growth phases. The result is in close agreement with the findings of Eskandarinasab et al. (2010); Ghafouri-Kesbi et al. (2011); Ghafouri-Kesbi and Gholizadeh (2017); and Mohammadi et al. (2012) in different sheep breeds. In addition, traits measured during the pre-weaning period had smallest phenotypic coefficient of variation (CV). This difference can be explained by during the pre-weaning period, lambs are less affected by environmental factors owing to maternal support. However, after weaning as the lambs lose maternal support, they exposed to environmental stress, which decrease growth of lambs. Moreover, traits measured from weaning to 6 months of age had higher phenotypic coefficient of variation (CV) compared to other growth phases. On the other hand, lambs had higher daily gain and efficiency of growth during 6 months to yearling growth phase than weaning to 6 months. This result revealed that lambs were highly influenced by environmental factors after separating from their dam than other growth phases. This implies that special attention is required during pre-weaning growth phase to produce productive replacement flock and sustaining the breeding program.
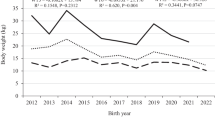
Least-squares means (+SE) for the traits studied are presented in Tables 2 and 3. Year of birth had significant effect (p<0.001) in all studied traits. The significant effect of birth year on studied traits has been reported by Baneh and Hafezian (2009); Ghafouri-Kesbi et al. (2011); and Ghafouri-Kesbi and Gholizadeh (2017) for different sheep breeds. The effect of birth year arises from difference in climatic and environmental condition, and animal management in different years (Bakhshalizadeh et al. 2016; Mohammadi et al. 2010). Climate and environmental changes have an effect on the quality and quantity of grazing pasture, which affect the provision of feed and other requirements for animals. Phenotypic performance for all studied traits showed a declined trend over years (Figs. 1, 2, 3, and 4). This may be happened due to the deliberate differences in animal management over the years. Because of high occurrence of mass mortality in the research center due to fasciolosis outbreak in 2014, all animals were not allowed to graze on natural pasture during winter season, kept indoors, and fed only dry hay as a basal diet and supplemented with 200g head/day mixed concentrate without any supplementation of vitamins and mineral premix. This management strategy could expose the animals to vitamin E deficiency. Vitamin E deficiency is often associated with feeding growing lambs for long period (2–3 months) with little or no access to green feed. Moreover, selenium and vitamin E are stored for a short period in the body, and a continual dietary supply is essential. According to Ramírez-Bribiesca et al. (2005) and Ziaei (2015), deficiency of either or both selenium and vitamin E can reduce growth, reproductive performance, and immune response of the animals.
Generation of lambs significantly influenced all studied traits in post-weaning growth phases except GE3-6 (p<0.05) but not significant effects in all traits measured during pre-weaning growth period. Second-generation lambs had significantly higher (p<0.001) DG3-6, KR3-6, and GR3-6 compared to third-generation lambs. This difference can be explained by higher heterosis effects in second-generation than third-generation lambs (due to loss of heterosis effects across generation). The result is in close agreement with results reported by Boujenane et al. (1999) in inter se mating between D’man-Sardi sheep crosses. Contrary to the present finding, Gizaw et al. (2012) reported that performance of third-generation lambs were higher than first- and second-generation lambs in Awassi-Menz sheep crosses. However, from 6 months to yearling growth period, third-generation lambs had significantly higher (p<0.001) in all studied traits compared to second-generation lambs. This can be due to the small data size in third-generation lambs. Generation × year interaction had significant effect (p<0.05) in DG3-6, DG6-12, KR3-6, KR6-12, GE6-12, and GR6-12, but not in other studied traits. In this regard, first-generation lambs had higher (p<0.01) DG3-6 and KR3-6 in 2018 and DG6-12, KR6-12, GE6-12, and GR6-12 in 2019 compared to second-generation lambs.
Sex of lambs had significant effect (p<0.01) in DG6-12, but not in other studied traits. Male lambs had significantly higher DG6-12 compared to females (p<0.01). The effect of sex on DG6-12 can be arise from difference in endocrine system in which estrogen hormone has a limiting effect on growth of long bones in female (Baneh and Hafezian 2009). The result is contrary to the report of Eskandarinasab et al. (2010); Ghafouri-Kesbi and Gholizadeh (2017); and Mohammadi et al. (2012) who report significant effect of sex in all studied traits. Single lambs had significantly higher daily gain and Kleiber ratio than twin lambs in pre-weaning growth phase (p<0.001). This difference can be explained by competition between twins to feed with their dam’s milk causes them to receive less milk than singles. Significant effect of birth type on daily weight gain and Kleiber ratio was in agreement with other reports (Eskandarinasab et al. 2010; Ghafouri-Kesbi and Gholizadeh 2017; Mohammadi et al. 2012).
The effect of parity was significant on DG0-3, DG6-12, KR6-12, GE6-12, and GR6-12 (p<0.05). In the current result, the effect of parity on studied traits is not much important. This result is contrary to the reports of Eskandarinasab et al. (2010); Ghafouri-Kesbi and Gholizadeh (2017); and Kholghi et al. (2014) for different sheep breeds. Birth season had significant source of variation in all studied traits (p<0.001). Lambs born during short rainy season had higher (p<0.001) daily gain, Kleiber ratio, efficiency of growth, and relative growth rate during the pre-weaning growth phase. During the short rainy season, lambs were allowed to graze on natural pasture (they feed green forages) but during main rainy season, lambs were restricted from grazing of green pasture and fed dry hay as a basal diet without any supplementation of vitamins and minerals premix. This practice is not enough to cover the nutritional requirements of the growing lamb. On the other hand, lambs born during main rainy and dry seasons had higher phenotypic performance during post-weaning growth period in all studied traits. The higher post-weaning phenotypic performance of lambs born during main rainy and dry seasons is attributed to the higher abundance of grazing pasture following the main rain and during short rainy seasons. This can be also due to the effect of compensatory growth which is a period of accelerated growth following a period of restricted development (Kesbi and Tari 2015).
Genetic parameter estimates
The estimates of (co)variance components and corresponding genetic parameters are presented in Table 4. The most appropriate model for pre-weaning daily gain and efficiency of growth was models 6 and 4 respectively. Fitting a permanent environmental and maternal genetic effect substantially increased the log-likelihood values, indicating a significant effect of permanent environmental and maternal genetic effect on pre-weaning daily gain. Moreover, including maternal genetic effect (model 4) significantly increased the log-likelihood values over other models, indicating that the covariance between direct and maternal effect was significant for pre-weaning efficiency of growth. On the other hand, the most appropriate model for pre-weaning Kleiber ratio and relative growth rate was model included only direct additive genetic effects (model 1). Based on the most appropriate model, direct heritability estimates for pre-weaning daily gain, Kleiber ratio, efficiency of growth, and relative growth rate were 0.45±0.15, 0.30±0.08, 0.34±0.15, and 0.25±0.08 respectively. Estimated values of CVA for pre-weaning daily gain, Kleiber ratio, efficiency of growth, and relative growth rate were 18.85%, 7.26%, 20.07%, and 9.0% respectively. Direct heritability estimates and CVA recorded in the pre-weaning growth period were higher than other findings (Eskandarinasab et al. 2010; Ghafouri-Kesbi and Gholizadeh 2017) in different sheep breeds. Based on the appropriate model, the estimates of maternal heritability for daily gain and efficiency of growth were 0.14±0.14 and 0.19±0.10 respectively.
σ 2 P, phenotypic variance; σ2a, additive variance; σ2m, maternal variance; σ2c, common environment variance; σ2e, error variance; h2a, direct heritability; h2m, maternal heritability; h2c, ration of common environment variance to the total phenotypic variance; h2t, total heritability; ram, genetic correlation between direct and maternal additive heritability; σam, covariance between direct and maternal additive genetic effect; CVA, additive coefficient of variance; Log (L), log likelihood; DG0-3, daily gain from birth to weaning; DG3-6, daily gain from weaning to 6 months; DG6-12, daily gain from 6 months to yearling; KR0-3, Kleiber ratio from birth to weaning; KR3-6, Kleiber ratio from weaning to 6 months; KR6-12, Kleiber ratio from 6 months to yearling; GE0-3, growth efficiency from birth to weaning; GE3-6, growth efficiency from weaning to 6 months; GE6-12, growth efficiency from 6 months to yearling; GR0-3, relative growth rate from birth to weaning; GR3-6, relative growth rate from weaning to 6 months; GR6-12, relative growth rate from 6 months to yearling
Fitting only direct genetic effect (model 1) resulted in significantly (p<0.05) higher log-likelihood for DG3-6 in comparison to the model which included other effects. Fitting permanent environmental and maternal genetic effect (model 6) improved the log-likelihood for KR3-6, indicating that covariance between direct and maternal genetic effect was significant for these traits, while model 4 was the best model for GE3-6 and GR3-6. Based on the appropriate model, direct genetic heritability estimates for DG3-6, KR3-6, GE3-6, and GR3-6 were 0.04±0.06, 0.13±0.11, 0.39±0.17, and 0.23±0.13 respectively. Estimates of CVA were 13.07%, 23.73%, 48.45%, and 31.94% for DG3-6, KR3-6, GE3-6, and GR3-6 respectively. Maternal genetic heritability estimates for KR3-6, GE3-6, and GR3-6 were 0.16±0.14, 0.30±0.12, and 0.24±0.11 respectively. Model included only direct genetic effects (model 1) was the most appropriate model for DG6-12, KR6-12, GE6-12, and GR6-12 respectively. Direct heritability estimates were 0.15±0.11, 0.14±0.12, 0.31±0.14, and 0.23±0.13 for DG6-12, KR6-12, GE6-12, and GR6-12 respectively and estimates of CVA for the corresponding trait were 15.15%, 13.55%, 25.46%, and 19.44% respectively. The estimates of CVA, direct, and maternal genetic heritability in this study are remarkably higher than the estimate by Ghafouri-Kesbi and Gholizadeh (2017) for Baluchi sheep in all growth periods. There are few previous reports on estimates of genetic parameters for efficiency-related traits in sheep. This study can help to include efficiency-related traits in the selection index to improve the efficiency of the ongoing genetic improvement program. Medium to higher estimates of direct heritability and CVA in the current study indicate higher potential for genetic improvement of studied traits in the different growth periods.
Correlation estimates
The estimates of genetic and phenotypic correlations between studied traits are presented in Table 5. The genetic and phenotypic correlation between studied traits ranged from low to high in magnitude (−0.48 to 0.98). Traits measured during the pre-weaning growth phase had negative and weak genetic correlations with traits measured during the post-weaning growth phases (ranged from −0.34 to −0.08). It indicates that lambs with higher growth rate and efficiency-related traits in pre-weaning period have less efficient during the post-weaning period and vice versa. The negative and weak genetic correlations between those traits imply that different genetic mechanisms are involved in expressing those traits at different stage of growth (Mohammadi et al. 2011; Mohammadi et al. 2015). Similarly, Mohammadi et al. (2010) find similar correlation estimates to the current study. In agreement to genetic correlations, phenotypic correlation between traits measured during pre-weaning growth phase had negative and weak correlation estimates with traits measured during post-weaning growth phases. Similar findings have been reported by Ghafouri-Kesbi and Gholizadeh (2017) for Baluchi sheep. Compensatory growth phenomenon may played an important role; lambs with poor early growth possibly due to poor pre-weaning environment tended to have higher post-weaning growth than lambs in better pre-weaning conditions (Afolayan et al. 2007).
Dorper crossbred sheep are a meat type and attains market weight at about 6 months of age in the central highland of Ethiopia. Furthermore, since genetic correlation between traits measured during pre-weaning growth period had negative and weak correlation estimates with traits measured during post-weaning growth period. As a result, improving traits measured during weaning to 6 months of age is more feasible. The genetic correlation estimates between DG3-6 and other traits were positive and high in magnitude to their corresponding growth phase (0.95, 0.86, and 0.91 for KR3-6, GE3-6, and GR3-6 respectively). These positive and strong genetic correlations between DG3-6 and other traits imply no negative genetic change in efficiency-related traits due to selection for growth rate (Bakhshalizadeh et al. 2016; Ghafouri-Kesbi and Gholizadeh 2017; Gowane et al. 2011). Moreover, strong and positive genetic correlations between DG3-6 and other traits measured during the same growth period indicated that selection for or against one trait would result in concomitant genetic changes in other traits. Genetic correlation between efficiency-related traits during weaning to 6 months of growth period was positive and strong in magnitude (0.92 for KR3-6-GE3-6) which means that all the efficiency traits measured during this period can be improved simultaneously; conclude that the same genes affect the two traits.
Conclusion
Phenotypic performance for all studied traits showed a declined trend over the years. This may be because of deliberate differences in animal management over the years. The genetic parameters estimated for efficiency-related traits in Dorper crossbred sheep population show that efficiency-related traits have medium to high CVA and heritabilities and possibility to improving though selection. The genetic correlation between traits measured during weaning to 6 months of age had negative and weak correlation estimates with traits measured during pre-weaning growth period. Thus, as the Dorper sheep reaches market weight at about 6 months of age, improving traits measured during weaning to 6 months of age is more feasible. As the genetic correlation estimates between DG3-6 and other traits were positive and high in magnitude to their respective growth phase, selection based on DG3-6 is recommended.
Data availability
Data will be made available on request.
References
Abdi, Y., Birhanu, A., & Eyob, E. (2019). Evaluation of Carcass Yield Characteristic of Sheep and Goat at ELFORA Export Abattoir, Bishoftu town, Ethiopia. Advances in Biological Research, 13, 46.
Abebe, A., Gizaw, S., Getachew, T., Lemma, S., Bisrat, A., Goshme, S., Besufekad, S., Abebe, A., Mekonen, T., Zewudie, T., and Chaniyalew, Y. (2016). Growth Performance of Dorper and its F1 Crossbreds at Debre-Birhan Agricultural Research Center, Ethiopia 1. Proceedings of the 8th Annual Regional Conference on Completed Research Activities of Livestock, 1–21
Afolayan, R. A., Pitchford, W. S., Deland, M. P. B., & McKiernan, W. A. (2007). Breed variation and genetic parameters for growth and body development in diverse beef cattle genotypes. Animal, 1(1), 13–20. https://doi.org/10.1017/S1751731107257933
Bakhshalizadeh, S., Hashemi, A., Gaffari, M., Jafari, S., & Farhadian, M. (2016). Estimation of genetic parameters and genetic trends for biometric traits in Moghani sheep breed. Small Ruminant Research, 134, 79–83. https://doi.org/10.1016/j.smallrumres.2015.12.030
Baneh, H., & Hafezian, S. H. (2009). Effects of environmental factors on growth traits in Ghezel sheep. African Journal of Biotechnology, 8(12).
Besufkad, S., Goshime, S., Abebe, A., Bisrat, A., Abebe, A., Getachew, G., Zewudie, T., Lemma, S., Areaya, A., & Gizzaw, S. (2022). Estimates of genetic parameters for growth traits and Kleiber ratio in Dorper x Local crossbred sheep population. Proceedings of the 14th Annual Regional Conference on Completed Livestock Research Activities, Bahir Dar, Ethiopia.
Boujenane, B. I., Chafik, A., & Benbihi, M. (1999). Heterosis retained in different generations of inter se mating between D’man and Sardi sheep. Journal of Animal Breeding and Genetics, 116(2), 151-159.
CSA. (2021). Federal democratic republic of Ethiopia. Central statistical agency. Agricultural sample survey, Volume II, Report on livestock and livestock. Central Statistical Agency (CSA), Addis Ababa, Ethiopi, 2(March), 34–35. https://www.scirp.org/journal/articles.aspx?searchcode=CSA+(Central+Statistics+Agency)+(2015)+Agricultural+Sample+Survey+2014%252F15.+Volume+II+Report+on+Livestock+and+Livestock+Characteristics+(Private+Peasant+Holdings)%252C+Central+Statistical+Agency+(C. Accessed 30 Sept 2022
Eskandarinasab, M., Ghafouri-Kesbi, F., & Abbasi, M. A. (2010). Different models for evaluation of growth traits and Kleiber ratio in an experimental flock of Iranian fat-tailed Afshari sheep. Journal of Animal Breeding and Genetics, 127(1), 26–33. https://doi.org/10.1111/j.1439-0388.2008.00789.x
Getachew, T., Haile, A., Wurzinger, M., Rischkowsky, B., Gizaw, S., Abebe, A., & Sölkner, J. (2016). Review of sheep crossbreeding based on exotic sires and among indigenous breeds in the tropics: An Ethiopian perspective. African Journal of Agricultural Research, 11(11), 901-911.
Gizaw, S., Lemma, S., Getachew, T. G., & Abebe, A. (2012). Development of a synthetic Awassi-Menz sheep breed. Proceedings of Livestock Research
Ghafouri-Kesbi, F., & Gholizadeh, M. (2017). Genetic and phenotypic aspects of growth rate and efficiency-related traits in sheep. Small Ruminant Research, 149, 181–187. https://doi.org/10.1016/j.smallrumres.2017.02.006
Ghafouri-Kesbi, F., Abbasi, M. A., Afraz, F., Babaei, M., Baneh, H., & Arpanahi, R. A. (2011). Genetic analysis of growth rate and Kleiber ratio in Zandi sheep. Tropical Animal Health and Production, 43(6), 1153–1159. https://doi.org/10.1007/s11250-011-9816-2
Gowane, G. R., Chopra, A., Prince, L. L. L., Mishra, A. K., & Arora, A. L. (2011). Genetic analysis for growth traits of prolific Garole × Malpura (GM) sheep. Tropical Animal Health and Production, 43(2), 299–303. https://doi.org/10.1007/s11250-010-9718-8
Kesbi, F. G., & Tari, A. R. (2015). Relative growth rate in sheep: Heritability and relationship with absolute growth rate and body weight. Songklanakarin Journal of Science and Technology, 37(1), 20–27.
Kholghi, M., Shahrebabak, M. M., Moradi, H., & Mohammadi, H. (2014). Model comparisons and genetic parameter estimates of growth traits in baluchi sheep. Slovak Journal of Animal Science 2014(1), 12–18.
Kleiber M. 1947. Body size and metabolic rate. Physiological Reviews 27: 511-541.
Legese, G., & Fadiga, M. (2014). Small ruminant value chain development in Ethiopia: Situation analysis and trends. ICARDA/ILRI Project Report. Nairobi, Kenya: International Center for Agricultural Research in the Dry Areas/International Livestock Research Institute. ICARDA/ILRI Project Report.
Mekonen, T., Alemayehu, L., Abebe, A., Bisrat, A., Yitagesu, E. (2018). Body Weight and Carcass Characterstics of 25% Dorper x Local Crossbred Sheep Supplemented with Legumes and Mungbean Halum in North Shewa, Ethiopia. Proceeding of the 10th Annual Conference on Completed Livestock Research Activities, Bahirdar, Ethiopia.
Meyer, K. (2006). WOMBAT-A program for mixed model analyses by restricted maximum likelihood. Animal Genetics and Breeding Unit. Armidale, 55.
Meyer, K., 2007. WOMBAT—A tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). Journal of Zhejiang University Science 8, 815–821. https://doi.org/10.1631/jzus.2007.B0815
Mohammadi, Y., Rashidi, A., Mokhtari, M. S., & Esmailizadeh, A. K. (2010). Quantitative genetic analysis of growth traits and Kleiber ratios in Sanjabi sheep. Small Ruminant Research, 93(2–3), 88–93. /https://doi.org/10.1016/j.smallrumres.2010.05.005
Mohammadi, K., Rashidi, A., Mokhtari, M. S., & Nassiri, M. T. B. (2011). The estimation of (co)variance components for growth traits and Kleiber ratios in Zandi sheep. Small Ruminant Research, 99(2–3), 116–121. https://doi.org/10.1016/j.smallrumres.2011.03.058
Mohammadi, H., Shahrebabak, M. M., & Shahrebabak, H. M. (2012). Genetic parameter estimates for growth traits and prolificacy in Raeini Cashmere goats. Tropical Animal Health and Production, 44(6), 1213–1220. https://doi.org/10.1007/s11250-011-0059-z
Mohammadi, K., Abdollahi-Arpanahi, R., Amraei, F., Mohamadi, E. M., & Rashidi, A. (2015). Genetic parameter estimates for growth and reproductive traits in Lori sheep. Small Ruminant Research, 131, 35–42. https://doi.org/10.1016/j.smallrumres.2015.07.029
Ramírez-Bribiesca, J. E., Tortora, J. L., Huerta, M., Hernández, L. M., López, R., & Crosby, M. M. (2005). Effect of selenium-vitamin E injection in selenium-deficient dairy goats and kids on the Mexican plateau. Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 57(1), 77–84. https://doi.org/10.1590/s0102-09352005000100011
SAS, 2002. SAS user’s guide version 9.1: Statistics. SAS Institute Inc., Cary, NC, USA.
Tesema, Z., Deribe, B., Lakew, M., Zegeye, Z., Kefale, A., Shibeshi, M., Yizengaw, L., Belayneh, N. (2020). Growth performance of Dorper x indigenous crossbred sheep under smallholder management system. Proceedings of the 13th Annual Regional Conference on Completed Livestock Research Activities, Bahir Dar, Ethiopia.
Willham, R.L., 1972. The role of maternal effects in animal breeding: III:biometrical aspects of maternal effects in animals. J. Anim. Sci. 35, 1288–1293.
Ziaei, N. (2015). Effect of selenium and vitamin E supplementation on reproductive indices and biochemical metabolites in Raieni goats. Journal of Applied Animal Research, 43(4), 426–430. https://doi.org/10.1080/09712119.2014.980415
Acknowledgements
The Dorper sheep experiment was financially supported by Amhara Agricultural Research Institute (ARARI) and Ethiopian Institute of Agricultural Research (EIAR). We would like to acknowledge all livestock breeding and management staff specially Mr. Deribew Bekele and Mr. Alemayehu H/Mikael, who over the years have assisted in managing the experimental flock and in data collection.
Funding
This work was supported by Debre Birhan Agricultural Research Center (DBARC); Ethiopian Institute of Agricultural Research (EIAR).
Author information
Authors and Affiliations
Contributions
S. Besufkad contributed in designing of the breeding program, data analysis, and writing the result of the research. S. Goshme, A. Abebe, A. Bisrat, A. Abebe, and T. Getachew contributed in designing the breeding program, animal management data collection, and implementation of the breeding program. T. Zewudie, A. Areaya, L. Alemayehu, and A. Kebede contributed in data collection and management of the experimental animals.
Corresponding author
Ethics declarations
Ethical statements
We do not conduct animal experiment. We only used data collected from animals used for breeding purposes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Debre Birhan Agricultural Research Center is one of the research centers under Amhara Regional Agricultural Research Institute.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besufkad, S., Goshme, S., Abebe, A. et al. Genetic evaluation of growth rate and efficiency-related traits in Dorper × local crossbred sheep population. Trop Anim Health Prod 55, 249 (2023). https://doi.org/10.1007/s11250-023-03669-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03669-2