Abstract
The aim of this study was to determine if ejaculation modifies the testicular and accessory sex glands’ blood flow after ejaculation, and if those changes differ according to the process that leads to ejaculation. Twelve adult Corriedale rams were used and assigned at random to the four procedures that lead to ejaculation: (G1) electroejaculation; (G2) artificial vagina; (G3) transrectal ultrasound-guided massage of the accessory sex glands; (G4) natural mating. Hemodynamic characteristics evaluation of the male reproductive system was conducted immediately before and at 30 and 90 min after ejaculation. The internal iliac artery peak systolic velocity (PSV) decreased (P=0.01) and supratesticular artery PSV increased (P=0.042) 90 min after ejaculation in all groups. In conclusion, ejaculation modifies the reproductive system’s blood flow, with slight variations depending on the studied ejaculation methods. Additionally, ejaculation altered the internal iliac and supratesticular arteries PSV, and the supratesticular artery end-diastolic velocity (EDV) in rams. The supratesticular artery PSV was the only studied variable that differed according to the procedure that triggered the ejaculation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive systems’ blood supply is of great importance in the evaluation of the reproductive potential of males since it is the main route through which nutrients, oxygen, and hormones arrive and regulate its functions (Dyce et al. 2017; Samir et al. 2021). Moreover, blood flow is the main source of heat in the testis, which, on the other hand, can affect testis function and spermatogenesis (Kastelic et al. 2018). Although it has been reported that sexual stimulation increases fluid content in rams’ testis (Ungerfeld and Fila 2011), there is scarce information available on the effects of sexual arousal and/or post-ejaculation refractoriness on blood flow arriving at the reproductive system, particularly in farm animals. It should be considered that ejaculation triggers important changes in the cardiovascular system, as immediately after ejaculation, there is a peak in heart rate in rams (Orihuela and Ungerfeld 2016), accompanied by a concomitant increase in cardiac output. The process that triggers ejaculation might also influence changes in blood flow, as natural mating involves an important increase in the heart’s workload due to intense muscular activity (Too et al. 1973), in addition to the local rise in blood pressure required for penile erection (Sjaastad et al. 2010).
The process of erection and ejaculation is complex and depends on the interaction of multiple systems, including the nervous, endocrine, muscular, and vascular systems (Watson 1964). In small ruminants, semen can be collected through different techniques, each with different emphases on the structures that are stimulated. In effect, while semen collection using an artificial vagina (AV) involves processes similar to mating and ejaculation, the collection with electroejaculation (EE) entails the application of low-intensity electrical pulses with a transrectal probe, eliciting penis erection, semen emission, and ejaculation (see review: Abril-Sánchez et al. 2019). Electrical discharges stimulate most nerves and muscles of the region, including branches of the hypogastric plexus that innervate the seminal vesicle, prostate gland, and vas deferens, the pudendal nerve, which has branches that innervate the proximal region of the urethra, but also induces strong contractions of all the muscles surrounding these areas (see review: Abril-Sánchez et al. 2019). These stimuli elicit vigorous muscular contractions, leading to significant increases in creatine kinase concentration that persist elevated for several hours (Abril-Sánchez et al. 2017). Notably, changes in heart rate during semen collection using electroejaculation (EE) are similar in both anesthetized and non-anesthetized rams (Orihuela et al. 2009), suggesting that the stimuli elicit an intense cardiovascular response independent of the animal’s consciousness.
Recently, an alternative technique has been developed for semen collection in small ruminants: transrectal ultrasound-guided massage of the accessory sex glands (TUMASG) (Santiago-Moreno et al. 2013). This technique involves inserting an ultrasound probe to the rectum to locate and massage the ampulla of the vas deferens and the bulbourethral gland. It also includes pressing it against the symphysis pubis, and massaging the penile, perineal, and pelvic parts of the urethra (Santiago-Moreno et al. 2013). In certain species and individuals, TUMASG may require the administration of some electrical pulses with the EE probe, albeit to a lesser extent than when semen is collected exclusively with EE (Ungerfeld et al. 2015). Consequently, the utilization of different techniques could lead to variations in changes in blood flow to the reproductive system.
For instance, in bucks, Guerrero-Gutierrez et al. (2021) reported a tendency for an increase in blood flow velocity in the seminal vesicle after EE and TUMASG. Similarly, Alonge et al. (2018) observed an increase in prostatic vascular flow after ejaculation induced by digital manipulation of the penis in dogs. In contrast, Shafik et al. (2007) could not record changes in canine testicular blood flow after ejaculation induced by prostatic electrovibration. In humans, orgasm induced through deep prostatic massage elicits greater muscular contractions compared to direct penile stimulation, resulting it more intense and diffuse (Alwaal et al. 2015).
Regarding the above-mentioned reports, Doppler ultrasound could be an interesting tool to study changes in blood flow because it is a non-invasive and effective method to evaluate the hemodynamics of the male reproductive system that has been used in several species (Samir et al. 2021). Employing the use of spectral Doppler mode, it is possible to determine the extent of blood perfusion of an organ of the reproductive system by assessing indices and velocities of blood flow within the corresponding supplying artery. A decrease in these indices (resistance or pulsatility index) indicates an increase in vascular perfusion of the tissues evaluated. These measurements provide relevant information for the andrological examinations, infertility diagnosis, and follow-up evaluations (Ginther 2007). Indeed, evaluating the hemodynamic characteristics of the cranialis prostatic artery in dogs by spectral Doppler ultrasonography, Alonge et al. (2018) found that the resistance index decreases significantly following ejaculation. Similarly, recent research in goats showed that blood flow velocities from the ampulla branch to the vesicular gland increase after ejaculation (Guerrero-Gutierrez et al. 2021).
Considering all this information, we hypothesized that the blood flow received by the different organs of the reproductive system of rams increases temporarily after ejaculation and differs according to the process that leads to ejaculation (natural mating, AV, EE, or TUMASG). Hence, the main objective of the present study was to determine if the processes that lead to ejaculation modify the supratesticular and internal iliac arteries’ blood flow as estimations of the blood that arrive at the testes, epididymis, and accessory glands, and if those changes differ after natural mating, AV, EE, or TUMASG in rams.
Materials and methods
Animals and general handling
This study was conducted in the Facultad de Veterinaria, Montevideo, Uruguay (latitude: 34°79′S, longitude: 56°06′W). The procedures used and animal care approaches were approved by the Comisión de Ética en el Uso de Animales (CEUA) of the Universidad de la República (UdelaR, Uruguay). Twelve adult Corriedale rams (3 to 6 years old; 78.4 ± 10.2 kg of body weight, and 2.5 to 3.5 of body condition score (range 1–5) (mean ± SD)) andrologically evaluated were used in this study. All animals were maintained in a sheepfold (approximately 300 m2), with easy access to sheds, fed with hay and mixed with a commercial concentrate (~200 g/animal/day of concentrate that covered maintenance requirements; Adrelin, Young, Río Negro, Uruguay), and free access to mineral salt licks and water. All the animals were trained for semen collection with AV and were also habituated to EE and TUMASG as were involved before in other experimental studies that required the application of these techniques. Four days before the beginning of the study, semen was collected from all the animals with AV to homogenize their spermatic reserve.
Experimental design
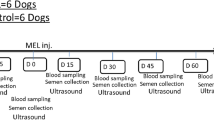
All the rams were subjected to the four procedures that lead to ejaculation, including the three procedures of semen collection (AV, EE, and TUMASG) and natural mating as a control in a Latin square design, so overall, semen was collected from the 12 animals with the four procedures (overall = 48 collections from 12 animals). For this, semen was collected from six rams/day, applying each procedure to 1–2 rams each day, alternating the procedures and the sequence, therefore applying all the procedures to all the rams in 8 days.
Semen collection procedures
Rams were individually moved to a pen located 50 m from their pen, without visual connection with their pen for collecting semen. For natural mating and for collecting semen with an AV, an estrous ewe was located in a 4 m × 2 m pen. Receptivity was induced with the application of intravaginal sponges impregnated with 30 mg of medroxyprogesterone acetate for 6 days, followed by the administration of an analogue of PGF2alpha (Delprostenate, Glandinex, Universal, Montevideo, Uruguay) and the administration of 0.5 mg of estradiol cypionate (Cipiosyn, Zoetis, Montevideo, Uruguay) at sponge withdrawal, followed by a second and third administration of 0.25 mg of estradiol cypionate. Receptiveness was determined with another teaser ram not used in the study.
Electroejaculation was performed using an automatic electroejaculator equipment provided with a probe of 16 cm length × 2.5 cm width, with three longitudinal electrodes (ePorvac, e325 model, Argentina). The rams were stimulated with 3 pulses of 3 V, increasing 1 V and 1 pulse in each series, until ejaculation. Only one animal did not ejaculate and the maximum voltage applied was 7 V with 15 pulses (16.8 ± 9.7 pulses, range 3–33 (mean ± SD)). The equipment had manual control of pulses, which were applied for 3 s alternating rest periods of approximately 3 s.
The TUMASG procedure was performed using the MyLab One Vet ultrasound scanner equipped (Esaote, Genoa, Italy) according to Santiago-Moreno et al. (2013). Briefly, the accessory sex glands were observed by real-time transrectal ultrasonography using a 6–10 MHz linear-array multi-frequency transducer with a plastic extension to allow its external manipulation. The transducer was placed in the region of the ampulla of the vas deferens to begin massaging the gland with vigorous back-and-forth movements, in the cranial and caudal directions, to promote ejaculation. The procedure was performed for 3 min, and if ejaculation did not occur, two or three pulses of 4 V of EE were applied with the same equipment as for the EE procedure, followed by another massage cycle for 3 min, and if still the ram did not ejaculate, EE was used again with the same pulse and voltage sequence (3.2 ± 2.6 pulses, range 3–9) until the animal ejaculated or completed 10 min of the entire procedure. Nine animals ejaculated, and three of them did not require the application of EE.
Blood flow recordings
Doppler ultrasound evaluations were performed immediately before, and 30 and 90 min after each procedure with the MyLab One Vet ultrasound scanner equipped. Hemodynamic characteristics evaluation of the supratesticular (Fig. 1A) and internal iliac arteries (Figure 1B) was performed using pulsed wave Doppler ultrasound to obtain spectral tracing and vascular indices. A minimum of three waves from each scanning was used for the evaluation. After the correction of the insonation angle (maximum 60°) and artifact-free tracing, the caliper was placed into the lumen of the internal iliac and supratesticular arteries, the images were frozen, and a wave analysis was performed to determine the peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistance index (RI = [PSV−EDV]/PSV). All the velocimetric indices were determined automatically (Rodriguez et al. 2020).
A Spectral Doppler ultrasonography of the supratesticular artery to obtain blood flow indices and velocities (PSV, EDV, RI) of rams. EDV, end-diastolic velocity; PSV, peak systolic velocity; RI, resistance index. B Spectral Doppler ultrasonography of the internal iliac artery to obtain blood flow indices and velocities (PSV, EDV, RI) of rams. EDV, end-diastolic velocity; PSV, peak systolic velocity; RI, resistance index
Internal iliac artery spectral Doppler ultrasonography was studied with a linear-array multi-frequency transducer (6 to 8 MHz) and a plastic extension to allow external manipulation. The supratesticular artery was evaluated using a microconvex multi-frequency transducer (5 to 10 MHz) for the trans scrotal exam at the location of the right pampiniform plexus to assess the supratesticular artery.
Statistical analysis
The experimental design used was a Latin square. The residual normality and variances homoscedasticity were initially tested using the Shapiro-Wilk and Bartlett tests, respectively. All the procedures and variables that were normally distributed, or normalized with adequate transformations, were compared between treatments with a mixed model (proc mixed; SAS OnDemand for Academics) including the treatment, time to the procedure, and their interaction as main effects, and the day of evaluation as a random effect. The main results are presented as LS mean ± pooled SEM. Differences were considered significant when P ≤ 0.05.
Results
The hemodynamic indices and velocities of the supratesticular and the internal iliac arteries, and the P values of the main effects, are presented in Table 1. The procedures per se did not modify any variable. However, the internal iliac and supratesticular arteries PSV and, supratesticular artery EDV changed with time. The internal iliac artery PSV decreased 90 min after ejaculation in relation to initial values (P=0.01, Fig. 2A), while supratesticular artery PSV increased gradually independently of the procedure used (P=0.04), reaching maximum values 90 min after ejaculation. The supratesticular artery EDV increased 90 min after ejaculation (P=0.01, Fig. 2B).
A Internal iliac artery systolic peak velocity (PSV) and B supratesticular artery end-diastolic velocity (EDV), before (BE), 30 and 90 min after ejaculation with electroejaculation (EE), artificial vagina (AV), transrectal ultrasound-guided massage of the accessory sex glands (TUMASG), and natural mating (NM) in rams. Values are shown in a single line as there were no differences among treatments in these variables. Letters denote significant differences with time (P < 0.05)
There was also a significant interaction between time and procedures in the supratesticular artery PSV (P=0.046): 30 min after ejaculation values were greater in TUMASG and EE than in NM and AV; and 90 min after ejaculation values were greater in AV than TUMASG and NM, with no difference in EE with the other groups (Fig. 3). Furthermore, the internal iliac artery EDV and the internal iliac and supratesticular arteries RI did not differ with time, treatments, or their interaction.
Supratesticular artery systolic peak velocity (PSV), before (BE), 30 and 90 min after ejaculation with electroejaculation (EE), artificial vagina (AV), transrectal ultrasound-guided massage of the accessory sex glands (TUMASG), and natural mating (NM) in rams. Letters denote significant differences with time, and asterisks show differences between treatments in specific times (interaction between treatment and time): 30 min: TUMASG and EE × AV and NM, and 90 min: AV × NM and TUMASG (P < 0.05)
Discussion
To the best of our knowledge, this is the first study to describe the general changes in the blood flow that irrigates the reproductive system induced by ejaculation in farm animals. Furthermore, this study also provides a wider overview, demonstrating that the procedure by which the ram ejaculates also influences some of these changes. The results of the present study demonstrate that ejaculation affects the blood supply of the reproductive system of rams. While a previous study described blood flow patterns following semen collection using artificial vagina in rams (Ntemka et al. 2021), another recent study reported the effect of ejaculation induced by TUMASG and EE on the seminal vesicle blood flow in bucks (Guerrero-Gutierrez et al. 2021). After ejaculation, an increase in scrotal surface temperature has been observed in cattle (Kastelic et al. 1996), which may also explain the increase in PSV and EDV of the supratesticular artery after ejaculation in the present study, as the blood flow achieving by the testicular vascularization is the main source of testicular heat (Kastelic and Rizzoto 2020).
The responses to EE and TUMASG were similar despite the stimulation of different organs with different emphases. In general, ejaculation depends on the complex interaction of sympathetic and parasympathetic pathways as well as hormonal response, and may consequently affect the cardiovascular system, reflected by alterations of systolic blood pressure during ejaculation in man; this may be attributed to the involvement of oxytocin in the central control of blood pressure (Alwaal et al. 2015; Carmichael et al. 1994; Graber et al. 1985).
In rams, it has been previously demonstrated that mating, even an estrous or a non-estrous ewe, triggers an important increase in their heart rate, reaching maximum values immediately after ejaculating (Orihuela and Ungerfeld 2016). Similarly, in humans, there is an increase in norepinephrine in the bloodstream after ejaculation, which promotes an increase in the amplitude of the QRS complex (a combination of the Q wave, R wave and S wave that represents ventricular depolarization) and blood pressure (Banerjea and Sen 1976). These observations provide an explanation for the postejaculatory increases in the supratesticular artery hemodynamic velocities observed in our study.
Furthermore, it is important to consider that sexual stimulation and mating increase cortisol secretion in rams (Gonzalez et al. 1988), leading to an increase in heart rate. Similarly, both EE and TUMASG are stressful procedures, and thus, also can trigger increases in cortisol concentrations (Abril-Sánchez et al. 2018; Damian and Ungerfeld 2011), which may contribute to the similar responses between procedures. On the other hand, the massage used during TUMASG was not as effective to induce ejaculation as in other species (Abril-Sánchez et al. 2019) and most rams received electrical pulses, which might be enough to trigger changes similar to those provoked by EE. Therefore, it would be interesting to investigate whether changes in blood flow are similar after TUMASG and EE in species in which males ejaculate only with the massage without requiring electrical pulses, as observed in Chamois (Rupicapra pyrenaica) (Abril-Sánchez et al. 2019).
Ejaculation did not result in changes in the resistance index, with any of the procedures, reflected by the extent of vascular flow that arrives at both the accessory sex glands and the testes remained consistent across all procedures, corroborating previous studies in rams that also reported no difference in hemodynamics and testicular ultrasound patterns following ejaculation in rams (Ntemka et al. 2021; Gouletsou et al. 2003). However, there were differences in the PSV between the procedures at certain times after ejaculation, suggesting that the procedure modifies the increase in blood flow to the reproductive system at different times post-ejaculation. This agrees with Guerrero-Gutierrez et al. (2021), who observed an increase in blood flow to the accessory sex glands after ejaculation using both EE and TUMASG. The variations in the timing of changes in PSV, and thus, in the blood flow among the procedures may be attributed to the specific pathways involved in initiating ejaculation in each case, as well as the intensity of the stimulation required in different species. In this sense, PSV for NM and AV differs 90 min after ejaculation, suggesting that the mechanisms leading to ejaculation do not provoke similar changes as supposed.
This study also provides evidence of changes produced during the refractory period, which refers to the period following ejaculation when the male is unresponsive to further sexual stimuli, which is not yet completely understood (Levin 2009). In rams, when given the opportunity to engage in sexual behavior again spontaneously, the increase in the heart rate recorded during the subsequent matings is similar to that recorded after the first mating (Orihuela and Ungerfeld 2016), indicating that the ram’s general physiology recovers quickly. Moreover, although there is an increase in PCO2 and lactic acid concentration after the first ejaculation, normal values are recovered 5 min later (A. Orihuela and R. Ungerfeld, unpublished data). However, in contrast, changes in the blood flow of the reproductive system, including the internal iliac and supratesticular arteries PSV and the supratesticular artery EDV, were even greater 90 min than 30 min after ejaculation. It is interesting that although reactive homeostasis triggers responses that quickly recover the general equilibrium, the reproductive system still did not return to the basal state even after the refractory period ended, indicating that the local and the systemic requirements dissociate the responses. Although this study did not directly investigate the end of the refractory period in relation to local blood flow, it opens an interesting area for future studies.
Additionally, the findings of this study underscore the value of spectral Doppler ultrasonography as a valuable tool for monitoring the hemodynamics and functionality of the reproductive organs in rams, and can be used to improve knowledge of ejaculation vascular physiology in the clinical context, for example, to evaluate hyperemia and/or ejaculatory dysfunction in patients with suspected pathologies, as reported in previous studies (Keener et al. 2000; Park et al. 2006; Hara et al. 2015; Alonge et al. 2018).
Conclusions
Ejaculation induces changes in the ram reproductive system’s blood flow, with slight differences depending to the processes studied that lead to ejaculation. In particular, ejaculation modified the internal iliac and supratesticular arteries PSV, and the supratesticular artery EDV in rams. The supratesticular artery PSV was the only studied variable that differed according to the procedure that triggered the ejaculation.
Data availability
The datasets of this study are available from the corresponding author upon request.
References
Abril-Sánchez, S., Crosignani, N., Freitas-de-Melo, A., Terrazas, A., Damián, J.P., Beracochea, F., Silveira, P., Ungerfeld, R., 2018. Sedation or anaesthesia decrease the stress response to electroejaculation and improve the quality of the collected semen in goat bucks. Animal, 12, 2598-2608. https://doi.org/10.1017/s1751731118000320.
Abril-Sánchez, S., Freitas-De-Melo, A., Beracochea, F., Damián, J.P., Giriboni, J., Santiago-Moreno, J., Ungerfeld, R., 2017. Sperm collection by transrectal ultrasound-guided massage of the accessory sex glands is less stressful than electroejaculation without altering sperm characteristics in conscious goat bucks. Theriogenology, 98, 82-87. https://doi.org/10.1016/j.theriogenology.2017.05.006.
Abril-Sánchez, S., Freitas-de-Melo, A., Giriboni, J., Santiago-Moreno, J., Ungerfeld, R., 2019. Sperm collection by electroejaculation in small ruminants: A review on welfare problems and alternative techniques. Animal Reproduction Science, 205, 1-9. https://doi.org/10.1016/j.anireprosci.2019.03.02.
Alonge, S., Melandri, M., Fanciullo, G., Lacalandra, G.M., Aiudi, G., 2018. Prostate vascular flow: The effect of the ejaculation on the power doppler ultrasonographic examination. Reproduction in Domestic Animals, 53, 110–115. https://doi.org/10.1111/rda.13078.
Alwaal, A., Breyer, B.N., Lue, T.F., 2015. Normal male sexual function: emphasis on orgasm and ejaculation. Fertility and Sterility, 0015-0282. https://doi.org/10.1016/j.fertnstert.2015.08.033.
Banerjea, B.K., Sen, S.C., 1976. Electrocardiographic study of the effect of masturbation on normal individuals. Indian Journal of Physiology and Pharmacology, 20, 4, 226–230.
Carmichael, M.S., Warburton, V.L., Dixen, J., Davidson, J.M., 1994. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Archives of Sexual Behavior, 23, 59–79. https://doi.org/10.1007/bf01541618.
Damian, J.P., Ungerfeld, R., 2011. The stress response of frequently electroejaculated rams to electroejaculation: hormonal, physiological, biochemical, haematological and behavioural parameters. Reproduction in Domestic Animals, 46, 646-650. https://doi.org/10.1111/j.1439-0531.2010.01722.x.
Dyce, K.M., Sack, W.O., Wensing, C.J.G., 2017. Textbook of veterinary anatomy, fifth Elsevier, London.
Ginther OJ, 2007. Ultrasonic imaging and animal reproduction: color-doppler ultrasonography, Book 4. EEUU, Equiservices Publishing 258.
Gonzalez, R., Orgeur, P., Signoret, J.P., 1988. Luteinizing hormone, testosterone and cortisol responses in rams upon presentation of estrous females in the nonbreeding season. Theriogenology, 30, 1075–1086. https://doi.org/10.1016/0093-691x(88)90282-8.
Gouletsou, P.G. Amiridis, G.S., Cripps, P.J., Lainas, T., Deligiannis, K., Saratsis P., Fthenakis, G.C., 2003. Ultrasonographic appearance of clinically healthy testicles and epididymides of rams. Theriogenology, 59, 1959–1972. https://doi.org/10.1016/s0093-691x(02)01259-1.
Graber, B., Rohrbaugh, J.W., Newlin, D.B., Varner, J.L., Ellingson, R.J., 1985. EEG during Masturbation and Ejaculation. Archives of Sexual Behavior, 14. https://doi.org/0004-0002/85/1200-0491504.50/0.
Guerrero-Gutierrez, M., Ungerfeld, R., Rodriguez, M.G.K., Santiago-Moreno, J., Giriboni, J., 2021. Using transrectal ultrasound-guided massage of the accessory sex glands for buck semen collection yields semen with greater cryoresistance than electroejaculation alone during the breeding season. Theriogenology, 172, 42-149. https://doi.org/10.1016/j.theriogenology.2021.06.016.
Hara, R., Nagai, A. Fujii, T, Fukumoto, K., Ohira, S., Yoshimasa, J., Yokoyama T., Miyaji, Y., 2015. Case report practical application of color doppler ultrasonography in patients with ejaculatory dysfunction. International Journal of Urology, 22, 609—611. https://doi.org/10.1111/iju.12754.
Kastelic, J., Rizzoto, G., 2020. Termorregulación testicular, in: Ungerfeld, R. (Ed), Reproducción de los animales domésticos. Edra-Asís, Zaragoza,. 70-74.
Kastelic, J.P., Cook, R.B., Coulter, G.H., Saacke, R.G., 1996. Ejaculation increases scrotal surface temperature in bulls with intact epidymides. Theriogenology, 46, 889-892. https://doi.org/10.1016/s0093-691x(96)00246-4.
Kastelic, J.P., Rizzoto, G., Thundathil, J., 2018. Review: Testicular vascular cone development and its association with scrotal thermoregulation, semen quality and sperm production in bulls. Animal, 12, 133–s141. https://doi.org/10.1017/S1751731118001167.
Keener, T.S., Winter, T.C., Berger, R., Krieger, J.N., Nodell, C., Rothman, I., Nghiem, H.V., 2000. Prostate Vascular Flow: The effect of ejaculation as revealed on transrectal power doppler sonography. American Journal of Roentgenology, 175, 1169–1172. https://doi.org/10.2214/ajr.175.4.1751169.
Levin, R. J., 2009. Revisiting post-ejaculation refractory time—what we know and what we do not know in males and in females. Journal of Sexual Medicine, 6, 2376–2389. https://doi.org/10.1111/j.1743-6109.2009.01350.x.
Ntemka, A., Kiossis, E., Boscos, C., Theodoridis, A., Patsikas, M., Tsakmakidis, I., 2021. Chios ram testicular blood flow and echotexture changes depending on age, season and ejaculation process. Polish Journal of Veterinary Sciences. 24, 579–587. https://doi.org/10.24425/pjvs.2021.139983.
Orihuela, A., Ungerfeld, R., 2016. Heart rate patterns during courtship and mating in rams and estrous and non-estrous ewes (Ovis aries). Journal of Animal Science, 94, 556-562. https://doi.org/10.2527/jas.2015-9425.
Orihuela, A.V., Aguirre, C., Hernández, I., Flores-Pérez, Vázquez I, 2009. Effect of anesthesia on welfare aspects of hair sheep (Ovis aries) during electro-ejaculation. Journal of Animal Veterinary Advances, 8, 305–308.
Park, S.Y., Lim, J.W., Kim, H.J., Lee, D.H., Ko, Y.T., 2006. The effect of ejaculation on prostate vascular flow: assessment by transrectal color doppler sonography. Ultrasound in Medicine and Biology, 32, 96–97. https://doi.org/10.1016/j.ultrasmedbio.2006.02.351.
Rodriguez, M.G.K., Santos, V.J.C., Uscategui, R.A.R., Mariano, R.S.G., Simões, A.P.R., Da Silva, P.D.A, Maronezi, M.C., Padilha-Nakaghi, L.C., Avante, M.L., Bartlewski, P.M., Feliciano, M.A.R., 2020. Maternal and fetal ultrasonographic characteristics, vulvar temperature, and vaginal mucous impedance as variables associated with the onset of parturition in term and induced pre-term ewes. Animal Reproduction Science, 223, 106647. https://doi.org/10.1016/j.anireprosci.2020.106647.
Samir, H., Radwan, F., Watanabe, G., 2021. Advances in applications of color Doppler ultrasonography in the andrological assessment of domestic animals: A review. Theriogenology, 16, 252-261. https://doi.org/10.1016/j.theriogenology.2020.12.002.
Santiago-Moreno, J., Castaño, C., Toledano-Díaz, A., Esteso, M.C., López-Sebastián, A., Guerra, R., Ruiz, M.J., Mendoza, N., Luna, C., Cebrián-Pérez, J.A., Hildebrandt, T.B., 2013. Cryopreservation of aoudad (Ammotragus lervia sahariensis) sperm obtained by transrectal ultrasound-guided massage of the accessory sex glands and electroejaculation. Theriogenology, 79, 383–391. https://doi.org/10.1016/j.theriogenology.2012.10.011.
Shafik, A., Shafik, A.A., Shafik, I.A., El Sibai, O., 2007. Physiological considerations of the morphologic changes of the testicles during erection and ejaculation: a canine study. Urologia Internationalis, 79, 262–266. https://doi.org/10.1159/000107960.
Sjaastad, O.V., Hove, K., Sand, O., 2010. Physiology of domestic animals, second Scandinavian Veterinary Press, Oslo.
Too, K., Kanagawa, H., Ishikawa, T., 1973. Radiotelemetry of electrocardiogram during copulation in animals. Advances in Animal Electrocardiography, 6, 9–16. https://doi.org/10.11276/jsvc1968.6.1.
Ungerfeld, R., Fila, D., 2011. Testicular fluid content evaluated by ultrasound image computer assisted analysis increases with small-dose. Reproduction in Domesic Animals, 46, 720-3. https://doi.org/10.1111/j.1439-0531.2010.01735.x.
Ungerfeld, R., López-Sebastián, A., Esteso, M., Pradiee, J., Toledano-Díaz, A., Castaño, C., Labrador, B., Santiago-Moreno, J., 2015. Physiological responses and characteristics of sperm collected after electroejaculation or transrectal ultrasound-guided massage of the accessory sex glands in anesthetized mouflons (Ovis musimon) and Iberian ibexes (Capra pyrenaica). Theriogenology, 84, 1067-1074. https://doi.org/10.1016/j.theriogenology.2015.06.009.
Watson, J.W., 1964. Mechanism of Erection and Ejaculation in the Bull and Ram. Nature, 204, 95–96.
Acknowledgements
The authors acknowledge Fabio Cardoso, Madeleine Guerrero-Gutierrez, Victoria Fernández, Lucía Porto, Federica Salomone, and Valentina Marrone for their help with the data recording.
Funding
The research that gives rise to the results presented in this publication received funding from the Agencia Nacional de Investigación e Innovación and the Universidad de la República (UdelaR), under the code PD_NAC_2021_1_166524.
Author information
Authors and Affiliations
Contributions
The designed study was performed by M.G.K.R. and R.U. In the experimental period, the samples were collected by M.G.K.R., M.I.V., D.F., and J.G. The results obtained were analyzed by M.G.K.R. and R.U. The preparation of the manuscript and its correction were carried out by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez, M.G.K., Vázquez, M.I., Giriboni, J. et al. Semen collection and ejaculation trigger changes in the blood flow of the reproductive system in rams. Trop Anim Health Prod 55, 318 (2023). https://doi.org/10.1007/s11250-023-03724-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03724-y