Abstract
The seroprevalence of Coxiella burnetii in goats and sheep in the UAE was evaluated by ELISA testing of 437 small ruminant sera samples from livestock farms (LivF) and 478 from the Al Ain livestock market (AALM), and the data from the pilot serosurvey were analyzed using the z-test of two proportions and Fisher’s method. The overall proportion of C. burnetii-seropositive goats of 32.1% was significantly higher than 24.7% for seropositive sheep (p < 0.05). On the other hand, the difference in the proportions of C. burnetii-seropositive sheep from the LivF (27.9%) and AALM (21.7%) was not statistically significant (p > 0.05). By comparison, the proportion of C. burnetii-seropositive goats from LivFs was 31.6% compared to 32.5% for the goats from the AALM (p > 0.05). In addition, there was variability in the proportions of C. burnetii-seropositive goats and sheep from the different Emirates, but none of differences was statistically significant (p > 0.05). These data provide the first evidence of apparent C. burnetii infections in goat and sheep herds in the UAE with seropositivity rate that is significantly higher in goats than sheep. The epidemiology and animal and public health implications of this pathogen need to be thoroughly evaluated in the UAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the greater Gulf region, sheep and goats are a greatly favored source of meat and other animal products. Despite this, however, the local and regional livestock production is still unable to meet the domestic demands for red meat (Sherif et al., 2014, pp. 236–241). While several factors may be responsible for the shortcoming, lack of strong animal disease surveillance systems in the region is a significant bottleneck to livestock production. In an OIE report cited by Shimshony and Enomides (2006), for example, gaps in early detection and reporting of animal diseases were cited as being partially responsible for the high prevalence of infectious livestock diseases in the region. In respect to animal disease research in the greater Gulf region, on the other hand, the few studies reported in the literature are mainly focused on regionally endemic transboundary diseases like contagious caprine pleuropneumonia (Thiaucourt and Bolske, 1996, pp. 1397–1414), peste de petits ruminants (Moustafa, 1993, pp. 857–863), and foot and mouth disease (Moustafa and El Gadir, 1993, pp. 831–837). With such skewed research efforts, and particularly so in the UAE, there exists a range of “neglected livestock diseases” that warrant study and documentation. In respect to coxiellosis, for example, there is critical lack of baseline data making any evidence-based disease intervention strategy difficult. Interestingly, the UAE government has been working towards a national policy for improving the economic efficiency of livestock production enterprises (Sherif et al., 2014, pp. 236–241). While these efforts will potentially resolve some of the livestock health and production bottlenecks, there is still lack of a specific national policy that addresses poor reproductive performance in livestock.
Elsewhere, several studies have consistently linked suboptimal profitability of livestock enterprises to poor reproductive performance of herds (Cabrera, 2014, pp. 211–221; Gädicke et al., 2010, pp. 9–19; Singh et al., 2015, pp. 211–215; Van Asseldonk et al., 2015, pp. 115–122). Moreover, several studies have also attributed a significant proportion of preventable reproductive failure cases in sheep and goats to a variety of abortifacient pathogens like Coxiella burnetii (Angelakis et al., 2000, 297–309; Rousset et al., 2012; Scringeour et al., 2000, pp. 74–76), Brucella spp. (Rahman et al., 2017, pp. 394–399, Chlamydia abortus (Barati et al., 2017, pp. 288–294), Campylobacter jejuni (Sahin et al., 2012, pp. 680–687), Leptospira spp. (Tagliabue et al., 2016, pp. 129–138; Topazio et al., 2015, pp. 53–57), Listeria monocytogenes (Lotfollahi et al., 2017, pp. 425–429), and Toxoplasma gondii (Kalambhe et al., 2017, pp. 35–38; Liassides et al., 2016, 359–366) among others.
With the exception of Brucella spp. (Mohammed et al., 2013, pp. 82–86), the presence of the above listed abortigenic pathogens of livestock remains largely unstudied in the UAE. Indeed, while C. burnetii has been linked to reproductive failure in small ruminants in many countries (Angelakis et al., 2000, 297–309; Rousset et al., 2012; Scringeour et al., 2000, pp. 74–76), similar reports on the UAE are lacking in the literature. Coxiellosis studies in the UAE have been limited to serological detection of C. burnetii antibodies in cattle (Barigye et al., 2021, pp. 112), semi-free ranging wild ungulates (Chaber et al., 2012, pp. 220–222), and racing camels (Afzal et al. 1994, pp. 787–792). In a study done in Saudi Arabia, C. burnetii infection was confirmed by PCR testing of biological specimens derived from camels and other livestock species (Mohammed et al., 1995, pp. 715–719). In fact, clinical Q-fever, which is caused by C. burnetii, has also been reported in humans in Saudi Arabia (Angelakis et al., 2000, pp. 297–309) as well as in Oman (Scringeour et al., 2000, pp. 74–76) as they have been across the globe. No similar studies have been reported in the literature for the UAE. The present pilot study was therefore done to collect baseline data on the seroprevalence of C. burnetii in goats and sheep in the UAE as a prelude to more detailed epidemiological investigations and studies in future that will characterize the animal and public health significance of the pathogen in the country.
Materials and methods
Study area, sample size, and blood samples
A total of 915 small ruminant blood samples were tested during this study of which 437 were from livestock farms (LivF) distributed across four Emirates within the UAE and 478 from the Al Ain livestock market (AALM) located in the Emirate of Abu Dhabi. The validity of the sample size for LivF and AALM was determined using the formula for sample size estimation, \(n={z}_{\alpha }^{2}pq/{L}^{2}\) (Thrusfield, 2007, 3rd Edition, pp. 283–240), where \(n\) is the sample size, \({z}_{\alpha }\) is the normal deviate (1.96) at 5% level of significance, \(p\) is the estimated prevalence, \(\mathrm{q}=1-\mathrm{p}\), and \(\mathrm{L}\) is the precision of estimate usually at 5%. Using a priori overall seroprevalence of 27.2% for C. burnetii reported for goats in a study done in Iran (Asadi et al., 2013, pp. 163–168), the formula was used to calculate the ideal sample sizes:
To adjust for potential non-compliance and design effect, n = 437 was used for the LivF and 478 for the AALM. The majority of the studied farms is small to medium in size with animal populations ranging from ~ 100 to 120 animals. These farms were conveniently selected on the basis of belonging to nuclear and/or extended families of third-year veterinary students from the Department of Veterinary Medicine, United Arab Emirates University (UAEU), that were willing to participate in the survey. The goats and sheep raised at these farms are predominantly of local breed commonly encountered in the UAE.
To implement the study, 20 third-year undergraduate veterinary students from UAEU were recruited and trained in the basics of animal restraint and blood collection by jugular venipuncture. The students were then provided blood collection kits and dispatched to their family farms where they collected jugular vein blood from randomly selected goats and sheep. Within hours of collection, the blood samples were brought back to the veterinary pathology laboratory at the Falaj Hazza campus, UAEU, where sera samples were separated by centrifugation at 1000 × g, 10 min, and aliquots of sera stored at \({-20 }^{\mathrm{o}}\mathrm{C}\) until testing was done. Of the 437 blood samples from LivFs, 222 were from sheep and 215 from goats. The research assistant, a qualified veterinarian, also worked with groups of selected third-year veterinary students to collect blood samples from the AALM. The randomly selected adult sheep and goats of both sexes were properly restrained and bled from the jugular vein into vacutainers. The predominantly indigenous and regionally sourced goats and sheep sold at the AALM are mostly used as a source of meat. The blood samples were then allowed to clot for about 10 min at environmental temperatures, packed in cool boxes, and then carried back to the laboratory. Serum samples were separated by centrifugation at 1000 × g, 10 min, and aliquots of sera kept at \({-20 }^{\mathrm{o}}\mathrm{C}\) until testing was done within a month of collection. Of the 478 blood samples collected from the AALM, 244 were from sheep and 234 from goats.
Coxiella burnetii indirect ELISA test
The screening for C. burnetii antibodies was done using an indirect ELISA (Q fever C. burnetii antibody test kit, IDEXX Laboratories, Liebefeldt-Bern, Switzerland) according to the kit manufacturer’s instructions. After reading the plates in a spectrophotometer at 450 nm, the results were expressed as a percentage of the ratio of the test sample OD450 to the positive control OD450 (S/P %). As per the kit manufacturer instructions, test samples with S/P % ≥ 40% were determined to positive and those with S/P% < 30% were deemed negative.
Statistical data analysis
The z-test for two proportions, which is identical to the chi-square test with one degree of freedom (Chen et al., 2015, pp. 525–538), was used to test for differences in the proportions of C. burnetii-seropositive animals in any two animal groups. The decision to reject the null hypothesis of no difference between the two proportions (hypothesized difference = 0) was based on the calculated p value, which is commonly compared to a significance threshold level of α = 0.05 (Ioannidis, 2018, pp. 1429–1430). Moreover, a 95% confidence interval for the corresponding difference between the two proportions was also calculated. *In situations where the absolute number of incidences in either group of animals was less than 5 or when the overall percentage of incidence was less than 20%, the Fisher-exact method provided a valid alternative to the z-test for two proportions (Agresti, 1992, pp. 131–153).
Results
Coxiella burnetii seroprevalence in sheep and goats
Overall, of the 915 serum samples tested, 28.3% (259/915) were seropositive for C. burnetii antibodies. When the data are segregated by animal species as presented in Table 1, the proportion of C. burnetii-seropositive goats (32.1%) is significantly higher than the seropositive sheep (24.7%) (p < 0.05).
It is noteworthy that of the 915 sera that were tested, 437 were from LivFs and 478 from the AALM. From this study, the proportion of C. burnetii-seropositive sheep from the LivF was 27.9% (62/222) compared to 21.7% (53/244) of the sheep from AALM (Table 1). The difference between the two proportions of seropositive sheep is not statistically significant (p > 0.05). On the other hand, the proportion of C. burnetii-seropositive goats from the LivF was 31.6% (68/215) compared to 32.5% (76/234) for the AALM. Similar to the case of the sheep, the difference between the two proportions of C. burnetii-seropositive goats is not statistically significant (p > 0.05).
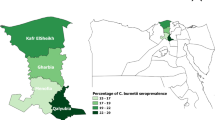
As presented in Table 2, there is variability in the proportions of C. burnetii-seropositive sheep and goats from the four study Emirates. In respect to sheep, Abu Dhabi registered the highest proportion of C. burnetti-seropositive animals (45.8%) followed by Dubai (33.3%), Sharjah (9.9%), and RAK (7.1%). For goats, on the other hand, the highest proportion of seropositive animals was recorded in Abu Dhabi (50.0%), followed by Dubai (18.3%) and RAK (11.8%). In all these cases, there were no statistically significant differences between the proportions of C. burnetii-seropositive sheep and goats in the different Emirates (p > 0.05; Table 2). As no caprine samples were collected from Sharjah due to absence of goats on the studied farms, no species-specific data are presented for the Emirate.
Discussion
On the basis of the serological data collected during the pilot serosurvey, we hereby report apparent C. burnetii infections in goat and sheep herds in the UAE. As vaccination of small ruminants against coxiellosis is not usually practiced in the UAE, these data are reliably consistent with apparent natural C. burnetii infections. The latter is an important abortigenic and zoonotic pathogen of small ruminants with potential animal and public health implications in the country. Interestingly, the overall proportion of C. burnetii-seropositive goats (32.1%) is significantly higher than for seropositive sheep (24.7%) (p < 0.05) suggesting an apparently higher C. burnetii infection rate in goats than sheep. Similar findings were reported in Iran (Asadi et al., 2013; pp. 163–168) and elsewhere. It will be important to carry out studies on the different risk factors of infection in the two species to better understand the epidemiology of the disease in small ruminants under UAE climatic and animal husbandry conditions. Previously, C. burnetii antibodies were detected in dairy cattle (Barigye et al., 2021; pp. 112), semi-free ranging wild ungulates (Chaber et al., 2012, pp. 220–222; Lloyd et al., 2010, pp. 83–89), and racing camels (Afzal and Sakkir, 1994, pp. 787–792) in the UAE. When the present data are interpreted against the background of these reports, C. burnetii may be widespread across multiple animal species in the country including goats and sheep. As coxiellosis may be associated with abortion and other forms of reproductive failure in small ruminants (Angelakis et al., 2000, pp. 297–309; Rousset et al., 2012; Scrimgeour et al., 2000, pp. 74–76), these data also warrant additional research to further assess the possible role this pathogen may be having in reproductive failure in goat and sheep herds in the UAE. Besides, C. burnetii is an important zoonotic pathogen that has previously been reported in humans in some countries neighboring the UAE like Saudi Arabia (Angelakis et al., 2000, pp. 297–309) and Oman (Georgiev et al., 2013, pp. 20,407; Scringeour et al., 2000, pp. 74–76). Studies are also needed to assess the public health risks to humans particularly in occupational groups that work closely with animals like veterinarians and farm workers.
It should be noted that with the exception of Sharjah where no goat samples were collected, C. burnetii antibodies were detected in both goat and sheep samples from the LivFs and AALM located in all the study Emirates. However, the differences in the proportions of seropositive goats and sheep from LivFs and AALM were not statistically significant. Since there is no coxiellosis surveillance in the UAE, the culling of animals on the basis of C. burnetii seropositivity should not have been expected. Therefore, the comparatively similar seropostivity for animals sampled at the LivFs and AALM should not be surprising.
In conclusion, the present serosurvey has adduced serological evidence on the apparent coxiellosis problem in goat and sheep herds in the UAE. While most cases of abortion cases in domesticated small ruminants in the UAE have previously been largely attributed to brucellosis, it is possible that coxiellosis may be a contributory factor in reproductive failure in goats and sheep in the country. As C. burnetii antibodies were detected in small ruminant samples collected from study LivF and the AALM, there exists an apparent zoonotic risk to farm workers and other at-risk occupational human groups. “One health” studies will be needed to clarify the dynamics of possible zoonotic transmission between animal and human populations in the country. Moreover, molecular epidemiological studies will also be needed to generate definitive data on the prevalence of infections caused by this pathogen. The additional data will inform development of evidence-based veterinary protocols for investigating cases of abortion and other forms of reproductive failure in small ruminants in the country.
Availability of data
All documents containing the raw data for this research project are available and will be provided in a timely manner if requested.
Code availability
(software application or custom code).
Not applicable.
References
Afzal, M., Sakkir, M., 1994. “Survey of antibodies against various infectious disease agents in racing camels in Abu Dhabi, United Arab Emirates.” Revue scientifique et technique, 13, 787–792; https://doi.org/10.20506/rst.13.3.794.
Agresti, A., 1992. "A Survey of Exact Inference for Contingency Tables". Statistical Science. 7, 131–153.
Alhamada, A.G., Habib, I., Barnes, A., Robertson, I., 2017. Risk factors associated with Brucella seropositivity in sheep and goats in Duhok Province, Iraq. Veterinary Sciences, 4, 65, https://doi.org/10.3390/vetsci4040065.
Angelakis, E., Raoult, E., 2010. Q fever. Veterinary Microbiology, 140, 297-309; https://doi.org/10.1016/j.vetmic.2009.07.016.
Asadi, J., Kafi, M., Khalili, M., 2013. Seroprevalence of Q fever in sheep and goat flocks with a history of abortion in Iran between 2011 and 2012. Veterinaria Italiana. 49, 163-168.
Van Asseldonk, M.A.P.M., Bontje, D.M., Backer, J.A., Van Roermund, H.J.W., Bergevoet, R.H.M., 2015. Economic aspects of Q fever control in dairy goats. Preventive Veterinary Medicine. 121, 115 -122; https://doi.org/10.1016/j.prevetmed.2015.06.010.
Barati, S., Moori-Bakhtiari, N., Najafabadi, M.G., Momtaz, H., Shokuhizadeh, L., 2017. The role of zoonotic chlamydial agents in ruminant abortion. Iranian Journal of Microbiology, 9, 288-294.
Barigye, R., Hassan, N.A.D., Abdalla-Alfaki, I.M., Barongo, M.B., Mohamed, M.E.H., Mohteshamuddin, K., 2021. Seroprevalence of Coxiella burnetii in a dairy cattle herd from the Al Ain region, United Arab Emirates. Tropical Animal Health and Production, 53, 112. https://doi.org/10.1007/s11250-021-02570-0.
Cabrera, V.E., 2014. Economics of fertility in high-yielding dairy cows on confined TMR systems. Animal, 8, 211-221; https://doi.org/10.1017/S1751731114000512.
Chaber., A.L, Lloyd, C., O’Donovan, D., Mckeown, S., Werney, U., Bailey, T., 2012. A serologic survey for Coxiella burnetii in semi-wild ungulates in the Emirate of Dubai, United Arab Emirates. Journal of Wildlife Diseases, 48: 220-222; https://doi.org/10.7589/0090-3558-48.1.220.
Chen, Y.F., Yabes, J.G., Brooks, M.M., Singh, S., Weissfeld, L.A., 2015. A likelihood ratio test for nested proportions. Statistics in Medicine, 34, 525–538; https://doi.org/10.1002/sim.6363
Gadicke, P., Vidal, R., Monti, G., 2010. Economic effect of bovine abortion syndrome in commercial dairy herds in Southern Chile. Preventive Veterinary Medicine, 97, 9-19. https://doi.org/10.1016/j.prevetmed.2010.07.008.
Gebremedhin, E.Z., Agonafir, A., Tessema, T.S., Tilahun, G., Medhin, G., Vitale, M., Marco, V.D., Cox, E., Vercuysse, J., Dorny, P., 2013. Seroepidemiological study of ovine toxoplasmosis in east and west Shewa zones of Oromia Regional State, Central Ethiopia. BMC Veterinary Research, 9, 117; https://doi.org/10.1186/1746-6148-9-117.
Georgiev, M., Afonso, A., Neubauer, H., Needham, H., Thiery, R., Rodolakis, A., Roest, H., Stark, K., Stegeman, J., Vellema, P., van der Hoek, W., More, S., 2013. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveillance: bulletin Europeen sur les maladies transmissibles. 18, 20407;
Hassan, N.A.D., Mohteshamuddin, K., Al Aiyan, A., Mohamed, M.E.H., Biffa, D.A., Barigye, R., 2018. Serological evidence of Brucella spp, Coxiella burnetii, Chlamydophila abortus, and Toxoplasma gondii infections in sheep and goat herds in the United Arab Emirates. Proc. Neglected Tropical Diseases Congress: The Future challenges. December 05–06, 2018 Dubai, UAE.
Ioannidis, J.P., 2018. The proposal to lower P value thresholds to. 005. JAMA, 319, 1429–1430; https://doi.org/10.1001/jama.2018.1536.
Kalambhe, D., Gill, G.P.S., Singh, B.B., 2017. Molecular detection of Toxoplasma gondii in the slaughter sheep and goats from the North India. Veterinary Parasitology. 241, 35–38;https://doi.org/10.1016/j.vetpar.2017.05.009
Liassides, M., Christodoulou, V., Moschandreas, J., Karagiannis, C., Mitis, G., Koliou, M., Antoniou, M., 2016. Toxoplasmosis in female high school students, pregnant women and ruminants in Cyprus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 110, 359–366; https://doi.org/10.1093/trstmh/trw038.
Lloyd, C., Stidworthy, M.F., Ulrich, W., 2010. Coxiella burnetti abortion in captive dama gazelle (Gazella dama) in the United Arab Emirates. Journal of Zoo and Wildlife Medicine. 41, 83–89; https://doi.org/10.1638/2009-0005.1.
Lotfollahi, L., Chaharbalesh, A., Ahangarzadeh Rezaee, M., Hasani, A., 2017. Prevalence, antimicrobial susceptibility and multiplex PCR-serotyping of Listeria monocytogenes isolated from humans, foods and livestock in Iran. Microbial Pathogenesis. 107, 425–429; https://doi.org/10.1016/j.micpath.2017.04.029.
Moeller, R.B., Jr., 2001. Causes of caprine abortion: diagnostic assessment of 211 Cases (1991-1998). Journal of Veterinary Diagnostic Investigations. 13:265-270. https://doi.org/10.1177/104063870101300317.
Mohammed, M.A., Shigidy, M.T., Al-Juboori, A.Y., 2013. Seroprevalence and epidemiology of brucellosis in camels, sheep and goats in Abu Dhabi Emirates. International Journal of Animal and Veterinary Advances. 5, 82-86.
Mohammed, O.B., Jarelnabi, A.A., Aljumaah, R.S., Alshaikh, M.A., Bakhiet, A.O., Omer, S.A., Alagaili, A.N., Hussein, M.F., 1995. Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: molecular detection from camel and other domestic livestock. Asian Pacific Journal of Tropical Medicine. 7, 715–719; DOI.org/https://doi.org/10.1016/S1995-7645(14)60122-X.
Moustafa, T., El-Gadir, F. 1993. Studies on foot and mouth disease in the eastern region of Abu Dhabi, United Arab Emirates. Revue Scientifique et Technique. 12: 831–837; https://doi.org/10.20506/rst.12.3.719
Moustafa, T., 1993. Rinderpest and peste des petits ruminants-like disease in the Al-Ain region of the United Arab Emirates. Revue Scientifique et Technique. 12: 857–863; https://doi.org/10.20506/rst.12.3.716.
Nietfeld, J.C., 2001. Chlamydial infections in small ruminants. The Veterinary Clinics of North America. Food Animal Practice. 17:301–314; https://doi.org/10.1016/s0749-0720(15)30030-x.
Rousset, E., Sidi-Boumedine, K., Thiery, R., 2012. In: OIE Manual of diagnostic tests and vaccines for terrestrial animals, 7th ed. OIE Press, Paris.
Sahin, O., Fitzgerald, C., Stroika, S., Zhao, S., Sippy, R.J., Kwan, P., Plummer, P.J., Han, J., Yaeger, M.J., Zhang, Q., 2012. Molecular evidence for zoonotic transmission of an emergent, highly pathogenic Campylobacter jejuni clone in the United States. Journal of Clinical Microbiology 50, 680-687; https://doi.org/10.1128/JCM.06167-11.
Scrimgeour, E.M., Johnston, W.J., Al Dhary, S.I.I., El-Khatim, H.S., John, V., Musa, M., 2000. First report of Q. fever in Oman. Emerging Infectious Diseases. 6, 74–76; https://doi.org/10.3201/eid0601.000114.
Sherif, S., Al-Shorepy, S., Al-Juboori, A., Fathelrahman, E., 2014. Sustainability of sheep and goat production systems under the United Arab Emirates’ arid land constraints. APCBEE Procedia 8: 236-241.
Shimshony, A., Economides, P., 2006. Disease prevention and preparedness for animal health emergencies in the Middle East. Revue Scientifique et Technique. 25, 253–269; https://doi.org/10.20506/rst.25.1.1667.
Singh, B.B., Dhand, N.K., Gill, J.P.S., (2015). Economic losses occurring due to brucellosis in Indian-livestock populations. Preventive Veterinary Medicine. 199, 211–215; https://doi.org/10.1016/j.prevetmed.2015.03.013.
Tagliabue, S., Figarolli, B.M., D'Incau, M., Foschi, G., Gennero, M.S., Giordani, R., Giordani, R., Natale, A., Papa, P., Ponti, N., Scaltrito, D., Spadari, L., Vesco, G., Ruocco, L., 2016. Serological surveillance of leptospirosis in Italy: two‑year national data (2010‑2011). Veterinaria Italiana. 52, 129–38; https://doi.org/10.12834/VetIt.58.169.2.
Thiaucourt, F., Bolske, G., 1996. Contagious caprine pleuripneumonia and other mycoplasmoses of sheep and goats. Revue Scientifique et Technique (International Office of Epizootics). 15, 1397-1414.
Thrusfield, M., 2007. Veterinary epidemiology. 3rd ed. Hoboken, USA: Wiley-Blackwell, 230, 238–240.
Topazio, J., Tonin, A.A., Machado, G., Noll, J.C., Ribeiro, A., Moura, A.B., Carmo, G.M., Grosskopf, H.M., Martins, J.L., Badke, M.R., Stefani, L.M., Lopes, L.S., Da Silva, A.S., 2015. Antibodies to Leptospira interrogans in goats and risk factors of the disease in Santa Catarina (West side), Brazil. Research in veterinary science. 99, 53–57; https://doi.org/10.1016/j.rvsc.2015.01.014.
Funding
This study was funded by financial support provided by the United Arab Emirates University Research Office through a Startup Grant No. 31F099.
Author information
Authors and Affiliations
Contributions
Robert Barigye was PI of the research project, contributed to the study design and project implementation, supervised the field and laboratory work, and led the writing of the manuscript.
Nabeeha Abdelgaleel D. Hassan was the research assistant and participated in the field work and laboratory testing of the blood samples.
Ibrahim M. Abdalla-Alfaki was Co-PI and the project statistician that did the statistical data analysis and contributed to the writing of the manuscript.
Mohamed Elfatih H. Mohamed and Khaja Mohteshamuddin were Co-PI and contributed to the study design and writing of the manuscript,
Co-authors: Hamda Khalfan Khamis Al Alawi, Afra Mohammed Balhayema Aldhaheri, Fatma Mohammed Ghanim, Maryam Ali Sumail Alkhateri, and Nouf Saeed Ali Alalawi were third-year veterinary students that participated in the field collection of blood samples and participated in the ELISA testing of the serum samples.
Corresponding author
Ethics declarations
Human and animal rights consent
Note that this manuscript was not based on clinical studies and that animal restraint and blood collection by venipuncture was done in compliance with animal welfare standards.
Consent for publication
All persons included as co-authors on this manuscript have provided their consent and agree to be herein included.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barigye, R., Hassan, N.A.D., Abdalla-Alfaki, I.M. et al. Pilot serosurvey of Coxiella burnetii in domesticated small ruminants in the United Arab Emirates. Trop Anim Health Prod 54, 156 (2022). https://doi.org/10.1007/s11250-022-03150-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03150-6