Abstract
Seroprevalence and risk factors of bovine brucellosis (Brucella abortus) in herds and cattle were estimated by a cross-sectional study in the state of Paraná, Brazil. The state was divided into seven regions and a random, two-stage sampling was performed on properties and cattle from each region between 2018 and 2019. Serum samples were collected from 11,592 cows over 24 months from 1,757 properties and a questionnaire was applied to identify potential risk factors. As recommended by the National Program for the Control and Eradication of Animal Brucellosis and Tuberculosis (PNCEBT), serological testing for the detection of anti-Brucella antibodies included the buffered plate agglutination test (screening test) and the fluorescence polarization assay (confirmatory test). The seroprevalence of bovine brucellosis on properties and in cattle was 4.87% (95% confidence interval [CI]: 3.98–5.93%) and 2.24% (95% CI: 1.47–3.41%), respectively. Multiple logistic regression analysis identified larger herd size and failure to test for brucellosis as risk factors for the presence of anti-B. abortus antibodies. These results demonstrate no change in the prevalence when comparing initial studies conducted in 2002. Given our findings, it is recommended that policies for brucellosis control include a widespread vaccination program for higher prevalence areas and eradication approach to lower prevalence areas. All steps related to correct immunization of the herds should be verified and improved by training and education. Health education action must be carried out informing farmers about the risks of introducing animals not tested for brucellosis into their herds and the benefits of testing their herds regularly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine brucellosis is a zoonosis caused by Brucella abortus. The main clinical signs of bovine brucellosis are related to reproductive disorders in males and females (Neta et al., 2010; Poester et al., 2013). Other signs include interstitial mastitis and arthritis (Lage et al., 2008; Poester et al., 2013).
The disease has a worldwide distribution, even though several countries in Western and Northern Europe, Canada, Japan, Australia, and New Zealand are believed to be free from the causal agent (OIE 2020). This was achieved by animal health programs using different combinations of strategies (Zhang et al., 2018).
In Brazil, the mean economic impact of bovine brucellosis was estimated at US$206 per dairy cow infected and $108 per beef cow infected, taking into account costs associated with reproductive losses, decreased milk and beef production, and costs of veterinary interventions (Santos et al., 2013). According to that study, every 1% increase or decrease in prevalence in Brazil is expected to increase or decrease the economic burden of brucellosis by approximately $76 million per year. Economic impact of brucellosis has been described in detail by McDermott et al. (2013).
The Programa Nacional de Controle e Erradicação da Brucelose e Tuberculose Animal — PNCEBT (National Program for the Control and Eradication of Animal Brucellosis and Tuberculosis) was developed in 2001 in Brazil by the national animal health authority, Ministério da Agricultura Pecuária e Abastecimento — MAPA (Ministry of Agriculture, Livestock and Supply) (Brasil, 2001). The main objective of the PNCEBT is to reduce the prevalence and incidence of both diseases. To achieve this objective, sound evidence is necessary and thus many states performed brucellosis prevalence studies with a standard methodological approach. Reported prevalence of herds in the different states of Brazil varied between 0.32 and 41.5% and heterogenicity was evident throughout the country (Alves et al., 2009; Marvulo et al., 2009; Negreiros et al., 2009; Ogata et al., 2009; Rocha et al., 2009; Sikusawa et al., 2009; Silva et al., 2009; Villar et al., 2009; Azevedo et al., 2009; Chate et al., 2009; Dias et al., 2009; Gonçalves et al. 2009a; Gonçalves et al. 2009b; Klein-Gunnewiek et al., 2009; Borba et al., 2013; Almeida et al., 2016; Clementino et al., 2016).
Seven states of Brazil reported a second prevalence study. Of those, only Mato Grosso, Mato Grosso do Sul, and Rondônia described reduction of prevalence of seropositive herds due to vaccination (Anzai et al., 2016; Barddal et al., 2016; Baumgartem et al., 2016; Dias et al., 2016; Guedes et al., 2016; Inlamea et al., 2016; Silva et al., 2016). Herd prevalence in several states of Brazil was obtained between 2003 and 2014 and can be seen in Fig. 1.
Dias et al. (2009) estimated brucellosis prevalence in Paraná in 2002. They reported a herd prevalence of 4% [CI 3.2–4.8%] and animal prevalence of 1.7% [CI 1.1–2.4%] and identified the purchase of breeding cattle and sharing of pastures as risk factors.
As per PNCEBT, control of brucellosis in Brazil is based on vaccination of female calves between 3 and 8 months of age, testing of cattle prior to their movements when animals are destinated for reproduction or agglomeration, elimination of all positive animals in abattoirs, voluntary certification of free herds, and surveillance and culling in areas with low prevalence. Female calves are mainly vaccinated with B. abortus strain S19, although strain RB51 may be used instead according to PNCEBT (Brasil, 2017b). Also, the latter can be used in a revaccination program at the farmer will.
Considering the importance of understanding the results achieved under the PNCEBT actions in the state of Paraná, Brazil, we aimed in this study to assess current risk factors and the variation in prevalence of brucellosis in cattle herds in Paraná, Brazil. Furthermore, data obtained would allow the evaluation of the efficacy of control measures implemented since the inception of PNCEBT 18 years ago.
Materials and methods
Study area and population
This epidemiological study was performed in the state of Paraná, positioned in the south region of Brazil. The target population was all its domestic female bovines and buffalos (in this text generically referenced as cattle) over 24 months of age. The region has a geographical area of 199.305 km2 and a cattle population of 8,397,219 individuals (IBGE 2017), which represents 4.8% of the national herd. Its bovine population is the eighth largest in the nation. Paraná has the third highest milk production in the country (IBGE 2017).
The state is divided into seven regions to characterize the different regional production profiles. This regional partitioning is the same as was used in a previous study (Dias et al., 2009), to allow comparisons (Fig. 2).
Sampling
A two-level sampling was applied to estimate seroprevalence of herds and cattle seropositive for anti-Brucella antibodies. The first step consisted to choose a defined number of farms, followed by the random selection of cows over 24 months of age within those farms. We limited the scope of the study to this category in order to focus on reproductive age and reduce false positive due to immunization, considering that female calves are vaccinated between 3 and 8 months of age in Brazil, according to PNCEBT (Brasil, 2017b). Collection of samples and their analysis in the laboratory were accomplished from September 2018 to April 2019.
The sample size of properties to be accessed in each of the seven regions was estimated in accordance with the formula for simple random samples (Thrusfield, 2018):
where n denotes the sample size, Z is the normal distribution value at α level of confidence, P is expected prevalence, and d is the absolute error.
Thus, we defined the parameters to define sample size of properties as degree of confidence of 0.95, expected apparent prevalence of 5%, and absolute error of 0.03.
An exhaustive alphabetically sorted list of primary units was used to apply a systematic random sampling. When the selected unit needed to be replaced, it was substituted by the next available propriety in the list. To select the number of animals to be tested in each farm, we calculated the minimum number of cows that should have been tested to reveal the brucellosis status of the herd, considering the sensitivity and specificity of the test protocols (animal level) as 95% and 99.5%, respectively (Gall and Nielsen, 2004), 20% of intra-herd prevalence, and a sensitivity and specificity greater than or equal to 90% (herd level). Thus, 10 animals were sampled in properties with up to 99 cows older than 24 months and 15 animals were sampled in properties with 100 or more cows older than 24 months. Table 1 provides information regarding the target population and samples per region.
Within the properties, cows were selected randomly. Cows in the peripartum period, i.e., approximately 15 days before and after delivery, were excluded from the selection, since it can affect diagnosis results.
Serologic tests
A serial testing scheme was performed as described in national regulation (Brasil, 2017a). Each serum sample was submitted to buffered plate agglutination test (BPAT) and reactors (positive samples) were subsequently submitted for fluorescence polarization assay (FPA), both tests accomplished as described in OIE standards (OIE 2019). BPAT has sensitivity of 95.4% and 98.4%, while those values are 97.5% and 98.9% for FPA (Gall and Nielsen, 2004). Samples that reacted to both assays were considered seropositive. All tests were performed by the Centro de Diagnóstico Marcos Enrietti (Diagnosis Centre Marcos Enrietti) in 2019.
Apparent prevalence in herds and animals
Apparent prevalence in herds was calculated for each region as the direct proportion of tested herds that were seropositive. A herd was considered positive when one or more of its cows tested positive.
Herd prevalence in the state of Paraná was based on the relative weight of each region (HWS) as follows:
Thus, when calculating herd prevalence in Paraná, each farm weight was applied based on what region this farm is located and regions with larger total number of properties contributed more to state level herd prevalence.
The weight of each animal in the calculation of the prevalence of infected animals within the regions (AWR) was given by the following equation:
To compute apparent animal prevalence within the state of Paraná, each sampled cow had its weight (AWS) calculated by the following expression (DOHOO et al., 2003):
Therefore, herds contributed according to their size to animal prevalence in each region and in the state level.
The final weight of each farm and animal can be conferred in the data made available below in “Availability of data and material” section.
Also, a spatial analysis using a kernel density estimate map was produced to represent clustering of positive herds. The model was design to consider and correct the differences of sampling density across the state of Paraná.
Study of risk factors
A questionnaire to access potential risk factors was fulfilled based solely on the owner or farm manager answers. All herds sampled provided one set of answers each. Data were collected in loco, in a face-to-face interview during the same visit to collect samples, using an electronic device, and then uploaded into an electronic spreadsheet.
Questions included information regarding the number of bovines and buffalos reared by age and sex category, presence of other domestic and wild species, management and operation type, breeds, trade of animals, placental waste disposal, veterinary attention, vaccination, and history of abortions on the property (Appendix I).
Statistical analysis
Statistical analyses were performed using R Core Team (2018). Confidence intervals (CIs) were obtained by binomial logistic regression model.
Variables with more than two possible answers (not dichotomic) were regrouped in some cases for a more meaningful analysis. The number of cows was used as a measure of herd size and was categorized based on percentiles.
All potential risk factors were submitted to univariate analysis regarding whether the respective farm was classified as brucellosis infected herd or not. Techniques used to achieve this were the chi-squared test or Fischer test. A conservative p value of ≤ 0.2 was used as criteria to select which variables would be used in a multivariate logistic regression (Silva Abreu et al., 2009). The construction of a statistical model was based in a backward elimination of variables until all the variables were statistically significant in the model (p < 0.05). When necessary, models fitting was compared using Akaike information criterion (AIC).
Results
Apparent herd prevalence per region and for the entire state is presented in Table 2. Of 1,757 herds, 95 had one or more positive cows. Prevalence at state level was calculated applying weight as described in “Materials and methods.”
Table 3 shows the apparent prevalence in cows aged over 24 months. Of 11,592 animals, 137 were seropositive (2.24%).
Table 4 shows the apparent prevalence in herds stratified by type of production. Beef herds had higher prevalence, except in region 4, while mixed-type herds had lower levels, except in region 7; when considering the 95% confidence intervals, the only difference observed was between mixed-type and beef herds in region 2.
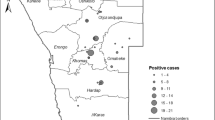
Based in the geolocation of each of the farms sampled, a kernel density estimate was produced for positive herds in Paraná (Fig. 3). It shows that seropositive farms are unevenly distributed across the area, with higher density in the north and northwest regions. The model considers the sampling density of the different areas of the state.
Herd and cattle prevalence obtained in a similar cross-sectional study developed in 2002 (Dias et al., 2009) was compared to the present study (Fig. 4). No evident differences in terms of prevalence were noticed.
As result of univariate analysis, the following variables had p ≤ 0.2 when confronted with the herd outcome for brucellosis and were included in a multivariate logistic regression analysis: purchase (p < 0.001) and sale (p < 0.001) of cattle, introduction of breeding cattle (p = 0.02), number of cows aged over 24 months (p < 0.001), brucellosis vaccination (p = 0.01), correct administration of vaccine (p < 0.001), slaughter of animals on the farm (p < 0.001), presence of equines (p < 0.001), breed (p < 0.001), presence of domestic birds (p = 0.01), presence of domestic feline (p = 0.04), presence of tapir (p = 0.05), presence of swine (p = 0.05), presence of wild feline (p = 0.08), presence of flooded areas on the farm (p = 0.08), presence of wild canine (p = 0.12), concentration of cattle in some areas of the farm (p = 0.14), presence of resting areas to other herds within the farm (p = 0.15), system intensity — confined, semi-confined, or extensive (p = 0.15) — and presence of caprine or ovine (p = 0.18).
In the multivariate logistic regression, only two variables had a significative result (p ≤ 0.05) and were included in the final model: number of cows aged over 24 months (3 levels: small herds (≤ 8 cows — 50th percentile), medium herds (≥ 9 to ≤ 88 cows — 51st to 95th percentiles), and large herds (≥ 89 cows — 96th to 100th percentiles)) and if cattle were tested for brucellosis (Table 5).
Discussion
The results revealed an overall herd prevalence of 4.87% (CI 3.98–6.00), distributed heterogeneously in the state of Paraná. Regions 1. Noroeste (13.14%) and 2. Centro-Oeste-Norte (9.6%) had the highest prevalence in the area while regions 6. Leste-Sul (1.19%) and 7. Sudoeste (2.4%) had the lowest (Table 2). The differences can be partially attributed to different production systems and practices within each region, as discussed below. Also, a kernel density model (Fig. 3) has shown an intense variation of infected farms across the areas of the state of Paraná.
The geographical pattern of higher herd prevalence of brucellosis in the northern areas of the state of Paraná when compared to its southern areas mirrors the prevalence of their bordering states, that is, São Paulo and Santa Catarina, respectively. In São Paulo, the bordering state in the north, herd prevalence was estimated at 10.2% (Dias et al., 2016), comparable to regions 1. Noroeste and 2. Centro-Oeste-Norte of Paraná, while a herd prevalence of 1.2% was reported by the bordering state in the south, Santa Catarina (Baumgartem et al., 2016), comparable to regions 6. Leste-Sul and 7. Sudoeste of Paraná. Bordering regions share some features as same climate and types of production and hold a higher rate of animals’ trade within its area, which may explain those spatial patterns. Argentina and Paraguay reported similar overall cattle prevalence — 2.1% and 3.15%, respectively (Aznar et al., 2012), compared with 2.24% in Paraná.
Animal prevalence (Table 3) tends to follow the same distribution seen in herd prevalence (Table 2). However, its lower values do not allow confirmation that differences observed between regions are statistically significant.
Herd prevalence at the state level was 4.87 (CI 95% 3.98–5.93) and animal prevalence was 2.24 (CI 95% 1.47–3.41). Thus, no major change was detected when comparing that figures to those of a similar study conducted in 2002 (Dias et al., 2009) when those authors reported herd prevalence being 4.02 (CI 95% 3.23–4.80) and animal prevalence 1.73 (CI 95% 1.10–2.36), as shown in Fig. 4. Since 2001, the PNCEBT has introduced a range of measures to control cattle brucellosis in Brazil (Brasil, 2001), including mandatory vaccination of all female calves. Regardless of this, the vaccination coverage has grown slowly in most states of Brazil (Brasil, 2020). In Paraná, vaccination levels in female calves increased from 64.8 to 79.7% between 2014 and 2018 (Fig. 5). Even considering this increasing pattern, limited immunization at the population level may partially explain why no decrease in brucellosis prevalence was observed in this period. Different levels of vaccination will lead to different intensity in herd prevalence decrease (Amaku et al., 2009).This is of particular relevance to areas with high prevalence, in which successful immunoprotection tends to reduce prevalence levels (Barddal et al., 2016; Inlamea et al., 2016). To better understand the role of vaccination, further analysis of prevalence of brucellosis and vaccination coverage in each area of the state is recommended. Also, it is worthwhile to consider that higher levels of vaccination may produce a slightly higher number of false positive when using BPAT and FPA testing protocol (Nielsen et al., 1996; Gall and Nielsen, 2004).
Brucellosis vaccination coverage of female calves in the state of Paraná between 2014 and 2018 (Brasil, 2020)
Conversely, the southern area of Paraná (regions 6 and 7) will benefit much less from a reliable vaccination program due its low prevalence of brucellosis. In these areas, prevalence is as low as that observed in the state of Santa Catarina (Baumgartem et al., 2016), where brucellosis vaccines are forbidden as a general rule and a surveillance program aimed at eradicating the disease is the current strategy. The network surveillance in this state includes slaughterhouse serum sampling, milk pool sampling in every dairy herd, and investigation of epidemiological links of infected herds. Each infected herd is fully tested twice, and positive animals are eliminated before restrictions may be lift. A test-and-slaughter strategy has been used worldwide in countries that successfully eradicated the disease (Zhang et al., 2018).
Risk factors identified in multivariate logistic regression were herd size and frequency of testing animals for brucellosis (Table 4). Herd size seems to play an important role in brucellosis transmission as larger herds are concentrated in areas with higher prevalence. While herd size is a relative concept, its association with brucellosis has been demonstrated over a diverse range of situations (Crawford et al., 1990). Although animals within large herds are not intrinsically more susceptible to infection, there are aspects associated with large-sized herds which may facilitate the transmission of brucellosis. Farms running larger herds are more likely to source replacement animals externally and from different origin, which increases the risk of introducing B. abortus. Also, more complex and intense relationship between animals tends to occur within larger herd size, resulting in wider opportunities to transmission events occur (Ossada et al., 2013). Herd size has been described as a major risk factor in many other states in Brazil. Using massive datasets from different areas of Brazil, de Alencar Mota et al. (2016) concluded that larger herds that purchased replacement stock pose greater risk regarding brucellosis.
Routine testing of animals for brucellosis allows assessment of herd status and control of the disease. Our data indicated that of the 778 farms where brucellosis is tested regularly, 33 (4.29%) were classified as positive, while in the 979 farms where testing was not regularly performed, 60 (6.12%) were classified as positive. The state of Paraná accounts for 30% of all brucellosis tests in Brazil, which corresponds to roughly 3 million tests performed between 2014 and 2018 (Brasil, 2020). Nevertheless, it is recommended that further analysis is undertaken to ascertain how the current testing protocol impacts on disease control. The adequate identification of positive animals and their culling should be followed by a consistent surveillance program in their farm of origin. All dairy herds must be annually tested for brucellosis following a policy enforced by authorities (PARANA, 2017) and in our sample, 572/769 (74.38%) dairy herds reported regular animal testing, while only 54/505 (10.69%) beef herds reported regular testing for brucellosis. Accordingly, beef cattle had a higher herd prevalence (Table 4) and this is in line with reports from Argentina (Caione et al., 2010) and other states of Brazil (de Alencar Mota et al., 2016).
In a previous study of risk factors for brucellosis in Paraná, purchase of breeding cattle and pasture rental were described as risk factors (Dias et al., 2009), and in their univariate analysis, the authors reported that the herd size had a high correlation (p < 0.001) with the disease. However, the influence of brucellosis testing was not assessed in this previous study. Our data did not show any relationship to prevalence of brucellosis for the variable pasture rental (p = 0.78).
Given the limitation of cross-sectional studies to detect risk factors (DOHOO et al., 2003), it is our recommendation that longitudinal studies are conducted to further explore risk and causal effects. As well, consideration should be given to the fact that transversal studies will present limitations in detecting eventual reduction of brucellosis prevalence in regions 3, 4, 5, 6, and 7, due to their low level of disease prevalence and their respective confidence intervals.
In conclusion, it is recommended that policies for brucellosis control include a consistent vaccination program, especially in areas of high risk and/or higher prevalence, thus including beef and larger herds. All steps related to immunization of the herds should be verified and improved by training and education, from the adequate maintenance of cold chain to the correct inoculation of cattle. Furthermore, a strong health education action must be implemented, informing farmers about the risks of introducing animals into their herds without testing. In areas exhibiting low levels of disease prevalence (regions 6 and 7), the state should assess the possibility of implementing eradication strategies. New approaches should be adapted to access eventual changes in the pattern of this, including those based on surveillance system.
Data availability
The following raw data are available online at https://data.mendeley.com/datasets/3ntpkdvscc/2, Table S1: Brucellosis_Parana_Animal_Prevalence, Table S2: Brucellosis_Parana_Herd_Prevalence, Table S3: Risk Factors Brucellosis—NoGeo.xlsx, and Table S4: Variables key.pdf.
References
Almeida, E.C., Freitas, A.A., Pontual, K.A.Q., Souza, M.M.A., Amaku, M., Dias, R.A., Ferreira, F., Telles, E.O., Heinemann, M.B., Gonçalves, V.S.P., Neto, J.E., Marvulo, M.F.V., Filho, J.H.H.G., Neto, J.S.F. and Silva, J.C.R., 2016. Prevalence and associated risk factors for bovine brucellosis in the state of Pernambuco, Brazil Semina:Ciencias Agrarias, 37, 3413–3424
Alves, A.J.S., Amaku, M., Ferreira, F., Ferreira Neto, J.S., Dias, R.A., Gonçalves, V.S.P., Figueiredo, V.C.F., Lôbo, J.R. and Bahiense, L., 2009. Epidemiological situation of bovine brucellosis in the State of Bahia, Brazil Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 6–13
Amaku, M., Dias, R.A., Ferreira Neto, J.S. and Ferreira, F., 2009. Modelagem matemática do controle de brucelose bovina por vacinação Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 135–141 (Escola de Veterinária UFMG)
Anzai, E.K., Costa, D., Said, A.L.P.R., Grisi-Filho, J.H.H., Amaku, M., Dias, R.A., Ferreira, F., Galvis, J.O.A., Gonçalves, V.S.P., Heinemann, M.B., Telles, E.O. and Neto, J.S.F., 2016. An update on the epidemiologic situation of bovine brucellosis in the state of Espírito Santo, Brazil Semina:Ciencias Agrarias, 37, 3437–3448
Azevedo, S.S., Ferreira Neto, J.S., Dias, R.A., Ferreira, F., Amaku, M., Figueiredo, V.C.F., Lôbo, J.R., Gonçalves, V.S.P., Souza, A.C. and Vasconcellos, S.A., 2009. Situação epidemiológica da brucelose bovina no Estado do Espírito Santo Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 19–26
Aznar, M.N., Samartino, L.E., Humblet, M.-F. and Saegerman, C., 2012. Bovine Brucellosis in Argentina and Bordering Countries: Update , doi: https://doi.org/10.1111/tbed.12018
Barddal, J.E.I., Quixabeira-Santos, J.C., Lopes, I.F., Neto, J.S.F., Ferreira, F., Amaku, M., Dias, R.A., Telles, E.O., Filho, J.H.H.G., Heinemann, M.B., Gonçalves, V.S.P. and Aguiar, D.M., 2016. Effect of vaccination in lowering the prevalence of bovine brucellosis in the state of Mato Grosso, Brazil Semina:Ciencias Agrarias, 37, 3479–3492
Baumgartem, K.D., Veloso, F.P., Filho, J.H.H.G., Ferreira, F., Amaku, M., Dias, R.A., Telles, E.O., Heinemann, M.B., Gonçalves, V.S.P. and Neto, J.S.F., 2016. Prevalence and risk factors for bovine brucellosis in the State of Santa Catarina, Brazil Semina:Ciencias Agrarias, 37, 3425–3436
Borba, M.R., Stevenson, M.A., Gonçalves, V.S.P., Neto, J.S.F., Ferreira, F., Amaku, M., Telles, E.O., Santana, S.S., Ferreira, J.C.A., Lôbo, J.R., Figueiredo, V.C.F. and Dias, R.A., 2013. Prevalence and risk-mapping of bovine brucellosis in Maranhão State, Brazil Preventive Veterinary Medicine, 110, 169–176
Brasil, 2020. Diagnostico situacional do PNCEBT - Programa Nacional de Controle e Erradicacao da Brucelose e Tuberculose Animal,
Brasil, 2017a. IN 34, de 9 de setembro de 2017 Requisitos para diagnóstico de brucelose por laboratórios da Rede Mapa. DOU de 25/09/2017 (no 184, Seção 1, pág. 2)
Brasil, 2017b. Ministério da Agricultura Pecuária e Abastecimento. Secretária de Defesa Agropecuária. Instrução normativa n. 10, de 03 março de 2017. Diário Oficial da União, no116 , Brasília, DF, 20 jun. 2017. Seção 1, p. 4-8.
Brasil, 2001. Ministério da Agricultura Pecuária e Abastecimento. Secretária de Defesa Agropecuária. Instrução normativa n. 2, de 10 de janeiro de 2001. Diário Oficial da União, no11 , Brasília, DF, 16 jan. 2001. Seção 1, p. 11.
Caione, J.., Carraro, M.C., Vanina, L., Tellechea, J., de Olavarrieta, M. and Spath, E., 2010. Prevalencia de Brucelosis en el partido de Nueve de Julio Rev. Med. Vet. (B. Aires), 91
Chate, S.C., Dias, R.A., Amaku, M., Ferreira, F., Moraes, G.M., Costa Neto, A.A., Monteiro, L.A.R.C., Lôbo, J.R., Figueiredo, V.C.F., Gonçalves, V.S.P. and Ferreira Neto, J.S., 2009. Situação epidemiológica da brucelose bovina no Estado do Mato Grosso do Sul Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 46–55
Clementino, I.J., Dias, R.A., Amaku, M., Ferreira, F., Telles, E.O., Heinemann, M.B., Gonçalves, V.S.P., Grisi Filho, J.H.H., Neto, J.S.F., Alves, C.J., Batista Santos, C.D.S.A. and De Azevedo, S.S., 2016. Epidemiological situation of bovine brucellosis in the state of Paraiba, Brazil Semina:Ciencias Agrarias, 37, 3403–3412
Crawford, R.P., Huber, J.D. and Adams, B.S., 1990. EPIDEMIOLOGY AND SURVEILLANCE In:, Animal Brucellosis, (CRC Press), 131–151
de Alencar Mota, A.L.A., Ferreira, F., Ferreira Neto, J.S., Dias, R.A., Amaku, M., Hildebrand Grisi-Filho, J.H., Telles, E.O. and Picão Gonçalves, V.S., 2016. Large-scale study of herd-level risk factors for bovine brucellosis in Brazil Acta Tropica, 164, 226–232 (Elsevier B.V.)
Dias, J.A., Müller, E.E., Dias, R.A., Freitas, J.C., Amaku, M., Ferreira, F., Silva, M.C.P., Lôbo, J.R., Figueiredo, V.C.F., Gonçalves, V.S.P. and Ferreira Neto, J.S., 2009. Situação epidemiológica da brucelose bovina no Estado do Paraná [Epidemiological situation of bovine brucellosis in the State of Paraná, Brazil] Arq. Bras. Med. Vet. Zootec, 61, 66–76
Dias, R.A., Belchior, A.P.C., Ferreira, R.D.S., Gonçalves, R.C., Barão, R.S.C., Sousa, P.D.R., Santos, A.M.A., Amaku, M., Ferreira, F., Telles, E.O., Filho, J.H.H.G., Heinemann, M.B., Gonçalves, V.S.P. and Neto, J.S.F., 2016. Controlling bovine brucellosis in the state of São Paulo, Brazil: Results after ten years of a vaccination program Semina:Ciencias Agrarias, 37, 3505–3518
DOHOO, I., MARTIN, W. and STRYHN, H., 2003. Veterinary Epidemiology Research, (University of Prince Edward Island: Charlottetown)
Gall, D. and Nielsen, K., 2004. Serological diagnosis of bovine brucellosis: A review of test performance and cost comparison (Office International des Epizootes)
Gonçalves, V.S.P., Delphino, M.K.V.C., Dias, R.A., Ferreira, F., Amaku, M., Ferreira Neto, J.S., Porto, T.B., Alves, C.M., Figueiredo, V.C.F. and Lôbo, J.R., 2009. Situação epidemiológica da brucelose bovina no Estado de Minas Gerais Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 35–45
Gonçalves, V.S.P., Ribeiro, L.A., Caldas, R.A., Francisco, P.F.C., Dias, R.A., Ferreira, F., Amaku, M., Ferreira Neto, J.S., Figueiredo, V.C.F., Lôbo, J.R. and Borges, J.R.J., 2009. Situação epidemiológica da brucelose bovina no Distrito Federal Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 4–18
Guedes, I.B., Bottene, I.F.N., Monteiro, L.A.R.C., Filho, J.M.L., Heinemann, M.B., Amaku, M., Filho, J.H.H.G., Dias, R.A., Ferreira, F., Telles, E.O., Gonçalves, V.S.P. and Neto, J.S.F., 2016. Prevalence and risk factors for bovine tuberculosis in the State of Mato Grosso do Sul, Brazil Semina:Ciencias Agrarias, 37, 3579–3588
IBGE - Instituto Brasileiro de Geografia e Estatística, 2017. Censo Agro 2017
Inlamea, O.F., Da Rocha, A.B., Ferreira, F., Filho, J.H.H.G., Heinemann, M.B., Dias, R.A., Telles, E.O., Gonçalves, V.S.P., Amaku, M. and Neto, J.S.F., 2016. Effect of vaccination in lowering bovine brucellosis in the state of Rondônia, Brazil Semina:Ciencias Agrarias, 37, 3493–3504
Klein-Gunnewiek, M.F.C., Amaku, M., Dias, R.A., Ferreira, F., Gitti, C.B., Pereira, L.A., Figueiredo, V.C.F., Lobo, J.R., Gonçalves, V.S.P. and Ferreira Neto, J.S., 2009. Situação epidemiológica da brucelose bovina no Estado do Rio de Janeiro Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 77–84
Lage, A.P., Poester, F.P., Paixão, T.A., Silva, T.M.A., Xavier, M.N., Minharro, S., Miranda, K.L., Alves, C.M., Mol, J.P.S. and Santos, R.L., 2008. Rev Bras Reprod Anim,
Marvulo, M.F.V., Ferreira, F., Dias, R.A., Amaku, M., Groff, A.C.M., Gonçalves, V.S.P., Figueiredo, V.C.F., Lôbo, J.R. and Ferreira Neto, J.S., 2009. Situação epidemiológica da brucelose bovina no Estado do Rio Grande do Sul Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 93–102
Negreiros, R.L., Dias, R.A., Ferreira, F., Ferreira Neto, J.S., Gonçalves, V.S.P., Silva, M.C.P., Figueiredo, V.C.F., Lôbo, J.R., Freitas, J. and Amaku, M., 2009. Situação epidemiológica da brucelose bovina no Estado de Mato Grosso Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 56–65
Neta, A.V.C., Mol, J.P.S., Xavier, M.N., Paixão, T.A., Lage, A.P. and Santos, R.L., 2010. Pathogenesis of bovine brucellosis (W.B. Saunders)
Nielsen, K., Gall, D., Jolley, M., Leishman, G., Balsevicius, S., Smith, P., Nicoletti, P. and Thomas, F., 1996. A homogeneous fluorescence polarization assay for detection of antibody to Brucella abortus Journal of Immunological Methods, 195, 161–168 (Elsevier B.V.)
Ogata, R.A., Gonçalves, V.S.P., Figueiredo, V.C.F., Lôbo, J.R., Rodrigues, A.L., Amaku, M., Ferreira, F., Ferreira Neto, J.S. and Dias, R.A., 2009. Situação epidemiológica da brucelose bovina no Estado do Tocantins Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 126–134
OIE - World Organisation for Animal Health, 2019. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019,
OIE - World Organisation for Animal Health 2020.
Ossada, R., Grisi-Filho, J.H.H., Ferreira, F. and Amaku, M., 2013. Modeling the Dynamics of Infectious Diseases in Different Scale-Free Networks with the Same Degree Distribution Adv. Studies Theor. Phys, 7, 759–771
PARANA, 2017. Portaria N 132, de 26 de maio de 2017. Disciplina os critérios para o recebimento de leite “in natura”, em estabelecimentos sob inspeção oficial localizados no estado do Paraná, em apoio ao Programa Estadual de Controle e Erradicação da Brucelose e da Tub,
Poester, F.P., Samartino, L.E. and Santos, R.L., 2013. Pathogenesis and pathobiology of brucellosis in livestock,
Rocha, W. V., Gonçalves, V.S.P., Coelho, C.G.N.F.L., Brito, W.M.E.D., Dias, R.A., Delphino, M.K.V.C., Ferreira, F., Amaku, M., Ferreira Neto, J.S., Figueiredo, V.C.F., Lôbo, J.R. and Brito, L.A.B., 2009. Situação epidemiológica da brucelose bovina no Estado de Goiás Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 27–34
Santos, R.L., Martins, T.M., Borges, Á.M. and Paixão, T.A., 2013. Economic losses due to bovine brucellosis in Brazil Pesquisa Veterinária Brasileira, 33, 759–764
Sikusawa, S., Amaku, M., Dias, R.A., Ferreira Neto, J.S., Martins, C., Gonçalves, V.S.P., Figueiredo, V.C.F., Lôbo, J.R. and Ferreira, F., 2009. Situação epidemiológica da brucelose bovina no estado de santa catarina Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 103–108
Silva, N.D.S., Groff, A.C.M., Vidor, A.C.M., Filho, J.H.H.G., Heinemann, M.B., Dias, R.A., Telles, E.O., Gonçalves, V.S.P., Amaku, M., Ferreira, F. and Neto, J.S.F., 2016. Epidemiological situation of bovine brucellosis after implementation of a vaccination program in Rio Grande do Sul State, Brazil Semina:Ciencias Agrarias, 37, 3519–3530
Silva, V.G.S.O., Dias, R.A., Ferreira, F., Amaku, M. and Neto, J.S.F., 2009. Situação Epidemiológica da Brucelose Bovina no Estado de Sergipe.pdf 109–117
Villar, K.S., Amaku, M., Dias, R.A., Ferreira Neto, J.S., Benitez, F., Gonçalves, V.S.P., Figueiredo, V.C.F., Lôbo, J.R. and Ferreira, F., 2009. Situação epidemiológica da brucelose bovina no Estado de Rondônia Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 61, 85–92
Zhang, N., Huang, D., Wu, W., Liu, J., Liang, F., Zhou, B. and Guan, P., 2018. Animal brucellosis control or eradication programs worldwide: A systematic review of experiences and lessons learned (Elsevier B.V.)
Acknowledgements
The authors wish to thank all field workers from Paraná Agribusiness Defense Agency (ADAPAR) who contribute to this research.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.M., O.S.B., J.H.H.G.F., R.A.D., M.B.H., E.O.T., V.S.P.G., J.S.F.N., and F.F.; analyzed data: D.L.R.; performed research: D.L.R. and E.A.A..; wrote paper: D.L.R..; review and editing: J.S.F.N., V.S.P.G., and C.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The manuscript does not contain clinical studies or patient data and the research was approved by the Ethic Committee in the Use of Animals of School of Veterinary Medicine and Animal Science under protocol number CEUA 3253011018.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues, D.L., Amorim, E.A., Ferreira, F. et al. Seroprevalence and risk factors for bovine brucellosis in the state of Paraná, Brazil: an analysis after 18 years of ongoing control measures. Trop Anim Health Prod 53, 503 (2021). https://doi.org/10.1007/s11250-021-02945-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-02945-3