Abstract
A cross-sectional study was conducted in three counties (Damxung, Maizhokunggar and Yadong) in Tibet in April and May 2015. A total of 1,523 yaks owned by 181 herders were randomly selected and blood sampled. Sera were tested using the rose bengal test (RBT) and a competitive immune-enzymatic assay (C-ELISA) and the test results interpreted in parallel. The individual yak prevalence was 2.8% (95% CI 2.0–3.7) with a herd prevalence of 18.2% (95% CI 12.9–24.6). At the individual level, two predictor variables, age and production system, were significantly associated with seropositivity by a binary logistic regression analysis. The odds of Brucella infection were significantly higher in older Yaks (3–5 years old, OR = 4.51; 95% CI 1.53–19.29; ≥6 years old, OR = 3.89; 95% CI 1.23–17.21) compared to those of younger yaks (≤2 years old). The odds of seropositivity for yaks managed under an agro-pastoral production system were 2.9 (95% CI 1.48–5.86) times higher compared to those managed under a pastoral production system. At the herd level, an association between the infection with Brucella and a history of abortions in the herd was observed (OR = 4.98, 95% CI 1.48–16.62). Surprisingly, vaccination was not associated with a lower level of infection (p = 0.49 and p = 0.99 for individual and herd level data, respectively). The results of the survey indicate that bovine brucellosis is endemic among the yak population in the plateau region of China, and the risk factors identified in the study should be considered in the epidemiology of the disease and when developing control programs for the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine brucellosis is principally caused by Brucella abortus, although B. melitensis and B. suis can occasionally cause disease if animals are grazed and/or managed with sheep and goats, or pigs, respectively (Praud et al. 2016). Although it has been eradicated in some countries, such as Australia and New Zealand, it remains endemic in many countries over the world including China (Díaz 2013, Sun et al. 2016).
The Tibet Autonomous Region is one of the largest pastoral provinces in China, with an average altitude of more than 4000 m above sea level. In most areas, the average annual temperature is below 5 °C although the temperature may rise to above 25 °C in summer (Gerald et al. 2003). The annual precipitation is below 400 mm with more rain in the southeast area than the northwest area (Zuo et al. 2009). Agriculture and animal husbandry have been the predominant industry supporting the Tibetan economy, although tourism is expanding. The total output from the agriculture, livestock and fishery industries provides almost 20% of the GDP despite the fragile ecological environment (Anonymous 2013). Yaks (Bos grunniens) have been playing an important role in the livelihood of the pastoralists through the production of meat, milk, fibre and hides (Gerald et al. 2003). In Tibet, there are approximately 5 million yaks, representing for 34% of the world’s yak population (Ma et al. 2014).
Brucellosis was recognised in Tibet in the 1980s as a disease of both livestock and humans. Through the implementation of vaccination and slaughter of affected animals, the disease was reported to have been controlled (Anonymous 1985); however, recently, human infection in Tibet has re-emerged (ZhanDui et al. 2008). As infection in humans always arises from contact with domestic or wild animal reservoirs and their products (Godfroid et al. 2005), in order to effectively control the disease, China initiated a surveillance programme of livestock in 2009 (Bureau of Veterinary 2009). As a result of this surveillance, the prevalence of brucellosis in livestock, including yaks, cattle, sheep and goats, from three counties of Tibet was reported to be 1.25% (Wu et al. 2011). However, there is little up-to-date information about the disease on the Tibetan plateau. Consequently, the current study was designed to determine the seroprevalence, distribution and risk factors for brucellosis in yaks.
Materials and methods
Study areas and design
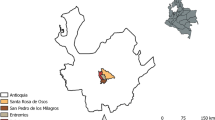
The study was conducted in the counties of Damxung, Maizhokunggar and Yadong in the Tibet Autonomous Region (Fig. 1). There are four livestock production systems adopted in Tibet: pastoral; agro-pastoral; crop-based livestock and agro-forestry-pastoral mixed production systems (Nyima et al. 2005). In the pastoral production system, livestock are usually grazed on rangeland pastures under a nomadic management structure, while in a crop-based livestock production system, animals are fed crop residues, such as straw during winter and spring, are grazed during summer and are managed by integration of the two methods during autumn. The agro-pastoral production system involves a mixture of these two systems with part grazing on rangelands and some supplementary feeding of crop residues. The agro-forestry-pastoral mixed production system is a blend of livestock, crop and forestry production. Yaks are primarily raised by subsistence farmers and are the dominant livestock in the three counties included in this study. Pali township, located in Yadong County, is exclusively a pastoral area, situated south of Lhasa and borders Bhutan and India. Maizhokunggar is an agro-pastoral area which has the national highway 318 traversing it linking Lhasa with the Sichuang Province. Damxung to the north of Lhasa is another pastoral area of Tibet.
A cross-sectional survey was conducted from April to May 2015. The locations of the sampled counties in Tibet were displayed by generating two choropleth maps using ArcGIS 10.3.
Yak sampling and sample size
Based on the distribution of yaks, yaks from three counties were randomly selected for sampling. Four townships were randomly selected from the eight townships present in both Maizhokunggar and Damxung Counties. Pali was selected from Yadong County as most yaks in this county are raised in this township. In Pali, all yak calves are vaccinated with S2 Brucella vaccine (Qianyuanhao Biological Co, China), which imparts immunity for approximately 2 years in cattle. A total number of 181 households in 30 villages were randomly selected from the townships. At least 40 yaks in each village were randomly selected for sampling. Herders located in remote areas were excluded from the sampling frame because of the logistical challenges in sampling them. Herds with at least four yaks aged more than 1 year were eligible for inclusion in the study.
The sample size was calculated using Epitools (Sergeant 2015) to estimate the prevalence using a design prevalence of 10% (Gao et al. 2013), with 95% confidence and 5% precision.
Serological tests
Approximately 8 ml of whole blood was collected from the jugular vein of the selected yaks. The samples were left at room temperature for a few hours and then centrifuged at 3000 rpm for 10 min. The sera were decanted and stored at −20 °C until testing. Each serum sample was tested with a commercial rose bengal test (RBT) (Institute Pourquir, IDEXX Montpellier, France) and a C-ELISA (INGEZIM BRUCELLA Compac 2.0, SPAIN) using the protocol outlined by the manufacturer and OIE (2009). The reported sensitivity (Se) and specificity (Sp) in Bos taurus of the C-ELISA were 98 and 99.9%, respectively, and for the RBT, 81.2 and 86.3%, respectively (Gall and Nielsen 2004). The tests were interpreted in parallel where a sample was classified as positive if one or more of the tests was positive. When tests were interpreted in parallel, the calculated sensitivity and specificity of the combined tests were 99.6 and 86.2%, respectively. For a herd to be classed as positive, at least one seropositive yak was required to be detected in the sampled herd.
Statistical analyses
Data were transferred to Microsoft Excel (Microsoft Excel 2010, Redmond, USA). Seroprevalence, together with 95% CI, was calculated and further analysed using the statistical software R (R Development Core Team 2013).
As yak calves from Pali township had a history of vaccination, analyses to identify risk factors were restricted to data from yaks from Maizhokunggar and Damxung counties. Variables with a p ≤ 0.20 on the univariable analyses were offered to a multivariable logistic regression analysis. A multivariable logistic regression model was generated using a backward stepwise process to control for confounding and test for effect modification. Variables with a p < 0.05 were retained in the final model. The model was evaluated through conducting the likelihood ratio test and the model with the smallest AIC (Akaike Information Criterion) value was regarded as the most appropriate model (Boukary et al. 2013). The model was then assessed by the Hosmer-Lemeshow goodness-of-fit test. Additionally, a receiver operating characteristic (ROC) curve was generated to display the predictive accuracy of the model (Sing et al. 2005).
Results
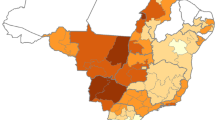
A total of 1523 yaks belonging to 181 herders originating from 30 villages were sampled in the three selected counties. Overall, the seroprevalence on the RBT was 1.8% (95% CI 1.2–2.6) which was similar to the C-ELISA result of 1.5% (95% CI 1.0–2.3) (p = 0.67, χ 2 = 0.183). When the tests were interpreted in parallel, the seroprevalence of antibodies against brucellosis was 2.8% (95% CI 2.0–3.7). 18.2% (95% CI12.9–24.6) of herds contained one or more seropositive animals. There was no significant difference in the seroprevalence between vaccinated and unvaccinated yaks at both the individual and herd level (p = 0.49, χ 2 = 0.48; p = 0.99, χ 2 = 1, respectively). The geographical distribution of the results is displayed in Fig. 2.
For the risk factor analyses, data were restricted to the 1283 yaks in 159 herds from the counties of Maizhokunggar and Damxung. The four factors age, herd size, county and production system were all significant on the univariable analyses (p < 0.20) and were selected for inclusion in the saturated multivariable logistic regression model (Table 1). In the final model, only the production system and age of animals were significantly associated with seropositivity in yaks at the animal level. Yaks in the agro-pastoral production system (OR = 2.90, 95% CI 1.48–5.86) as well as aged ≥6 years old (OR = 3.89, 95% CI 1.23–17.21) and between 3 and 5 years old (OR = 4.51, 95% CI 1.53–19.29) had significantly higher odds of seropositivity compared to yaks in the pastoral production system and aged ≤2 years old, respectively (Table 2). The likelihood ratio test, χ 2 = 16.37 (p < 0.001), AIC value of the final model of 326.95 and a Hosmer-Lemeshow goodness-of-fit value (p = 0.46), indicated that the model was a good fit of the data. The area under the ROC curve was 0.61, indicating that the model had moderate to good predictive ability.
Herds with a history of abortion in the preceding year to the survey were more likely to be seropositive (OR = 4.98, 95% CI 1.48, 16.62) than herds without abortions. No other management or husbandry variables produced a significant association with seropositive herds (all p > 0.20).
Discussion
Although there is an official bovine brucellosis surveillance strategy in Tibet, this study is one of the first epidemiological studies on the disease investigating the prevalence in yaks managed under different production systems in the region. The study showed that the seroprevalence in individual yaks was relatively low (2.8%), although the herd prevalence was reasonably high (18.2%). A higher animal level prevalence in yaks has been reported in India (21.1%) and Nepal (22%) (Bandyopadhyay et al. 2009, Jackson et al. 2014). In China, the prevalence reported in yaks has varied, with a range of 6.5 to 13.4% in animals on Qing-Tibet plateau (Gao et al. 2013, He et al. 2015), reaching 12.8% in Xinjiang Autonomous Region (Yuan et al. 2014).
In order to relieve the pressure on degraded grasslands, Tibet has implemented regulations to restrict grazing on pasture. Pasture has been fenced and allocated to different households (Wang et al. 2014) and the number of livestock owned by individual households has been limited. These measures may restrict livestock movement with the potential to reduce potential contact between herds, and hence likely disease transmission of brucellosis between herds in the region.
It is apparent from this study that the disease is endemic in yaks in the Tibetan plateau. It is possible that the introduction of yaks to improve the local genetics and through movement for trade and nomadic grazing purposes have resulted in disease spread (Boukary et al. 2013). Yaks in the study areas are recognised as of superior quality and are frequently exchanged and exported to other areas for breeding purposes. This movement of animals has the potential to play a key role in the distribution of the disease. In addition, two national highways traverse the counties of Damxung and Maizhokunggar, as well as a railway line passing through Damxung, resulting in more trade between Tibet and other regions of China. Furthermore, Yadong County borders Bhutan and India allowing for potential cross-border movement of yaks. Tibet also has an overall low temperature, due to its high altitude, allowing extended environmental survival of Brucella organisms (Aune et al. 2012). Customs may also play a role in the disease, as herders still raise livestock in a traditional manner. Herders slaughter animals themselves when the livestock are old, and the higher prevalence in older animals is likely to lead to a greater risk of infection for the herders and their families. Finally, with the development of Tibet, nomadic practices have been replaced by an agropastoralist system which has significantly changed people’s diet and lifestyle resulting in more unhygienic living conditions, degraded quality of fodder, consumption of dirty water and improved road transportation. These changes could increase the public health risk for zoonotic diseases (Jackson et al. 2014) and the potential for the further spread of brucellosis.
At the individual animal level, age and production system were significant in the logistic regression model. This is likely to be associated with an increased opportunity of exposure to the bacterium through environmental exposure to contaminated materials such as aborted foetuses, fluids and milk. In the current study, animals between the age of 3 and 5 years, which coincides with sexual maturity, had the highest prevalence. Others have similarly shown an increased risk of infection with Brucella spp. at this time (Matope et al. 2011).
The production system was identified as a risk factor for seropositivity in the current study, as has also been reported by others (Jergefa et al. 2009). The lower population density associated with a nomadic system would decrease potential exposure to Brucella, in contrast the higher stocking density in an agro-pastoral system would provide a greater opportunity for non-infected animals to have contact with infected yaks.
Others have reported a higher seroprevalence in larger herds (Jackson et al. 2014) which was in contrast to that found in the current study where smaller herds (<50 head) were more likely to be infected in the univariable analysis (p < 0.05, OR = 2.26, 95% CI 1.14–4.71). This finding may be confounded by the area of land owned/managed and stocking density may be a more appropriate method of determining risk of infection in future studies.
In this study, a history of abortions in the herd in the 12 months preceding the survey was strongly linked to seropositivity (p < 0.05). This is consistent with other studies and is not unexpected given the association between infection and abortion (Alhaji et al. 2016).
In the present study, the RBT and C-ELISA were adopted as diagnostic tests since they were convenient. However, the diagnostic tests used have not been validated in yaks and this should be undertaken to ensure these tests are suitable for use in this species, even though they have been used in yaks in other studies (He et al. 2015). In addition, as agglutinating antibodies induced by the B. suis strain 2, vaccine are reported to last for approximately 4 months post-vaccination (Han et al. 2009), in Pali, all yaks sampled were older than 2 years of age and consequently, it is highly likely that the positive results indicate natural infection rather than a vaccination induced one.
In conclusion, this study demonstrated that the animal level seroprevalence to brucellosis in domestic yaks (B. grunniens) was low (2.8%) in Tibet, although the herd level seroprevalence was higher (18.2%). To minimise future spread of infection, it is recommended that an annual vaccination programme should be implemented, as has previously been adopted for cattle (Baghiyan and Shirvanyan 2016). There is a need for educational material to be developed for yak herders to minimise infection of humans who are in close association with these animals or who consume unpasteurised milk and milk products.
References
Alhaji, N.B., Wungak, Y.S., and Bertu, W.J., 2016. Serological survey of bovine brucellosis in Fulani nomadic cattle breeds (Bos indicus) of North-central Nigeria: potential risk factors and zoonotic implications, Acta Tropica, 153, 28–35
Anonymous, 1985. Comments of preventing Brucellosis in Tibet, Tibetan Journal of Science and Technology, 4, 47–49. (In Chinese)
Anonymous, 2013. Accelerating development of agriculture and animal husbandry in China, Process of Agricutural Products, 6, 16–19. (In Chinese)
Aune, K., Rhyan, J.C., Russell, R., Roffe, T.J., and Corso, B., 2012. Environmental persistence of Brucella abortus in the Greater Yellowstone Area, The Journal of Wildlife Management, 76, 253–261
Baghiyan, G., and Shirvanyan, A.Y., 2016. Evaluation of the effectiveness of experimental vaccination of large and small cattle against brucellosis in the Republic of Armenia, Annals of Agrarian Science, 14, 283–287
Bandyopadhyay, S., Sasmal, D., Dutta, T.K., Ghosh, M.K., Sarkar, M., Sasmal, N.K., and Bhattacharya, M., 2009. Seroprevalence of brucellosis in yaks (Poephagus grunniens) in India and evaluation of protective immunity to S19 vaccine, Tropical Animal Health and Production, 41, 587–592
Boukary, A.R., Saegerman, C., Abatih, E., Fretin, D., Bada, R.A., De Deken, R., Harouna, H.A., Yenikoye, A., and Thys, E., 2013. Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of niger, PloS one, 8, e83175
Bureau of Veterinary, M.O.A., P.R.China, 2009. Animal Health in China, (China Agriculture Press, Beijing)
Díaz, A.E., 2013. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus, Revue Scientifique et Technique, 32, 43–51, 53–60
Gall, D., and Nielsen, K., 2004. Serological diagnosis of bovine brucellosis: a review of test performance and cost comparison, Revue Scientifique et Technique, 23, 989–1002
Gao, J., Zhang, K., Shahzad, M., Zhang, D., Han, Z., and Li, J., 2013. Seroprevalence of Brucella infection in yaks (Bos grunniens ) in Tibet, China, Cattle practice, 21, 109–111
Gerald, W., Han, J., and Long, R., 2003. The yak, (FAO Regional Office for Asia and the Pacific, Bangkok)
Godfroid, J., Cloeckaert, A., Liautard, J.P., Kohler, S., Fretin, D., Walravens, K., Garin-Bastuji, B., and Letesson, J.J., 2005. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis, Veterinary Research, 36, 313–326
Han, L., Fu, C.X., Jin, X.J., Yuan, L., Li, G., Fang, X., Ji, W.H., and Wang, Y.T., 2009. Change of antibody after vaccinated by S2 among dairy, Chinese Journal of Veterinary Medicine, 45, 82–83. (In Chinese)
He, S.Z., Song, R.D., Niu, Y.J., Game, J.Y., Zhang, Q.L., and Li, J.K., 2015. Serological detection against brucellosis of yak in Yushu area, China Cattle Science, 41, 94–95. (In Chinese)
Jackson, D.S., Nydam, D.V., and Altier, C., 2014. Prevalence and risk factors for brucellosis in domestic yak Bos grunniens and their herders in a transhumant pastoralist system of Dolpo, Nepal, Preventive Veterinary Medicine, 113, 47–58
Jergefa, T., Kelay, B., Bekana, M., Teshale, S., Gustafson, H., and Kindahl, H., 2009. Epidemiological study of bovine brucellosis in three agro-ecological areas of central Oromiya, Ethiopia, Revue Scientifique Et Technique, 28, 933–943
Ma, J.Y., Bao, Y.H., Xia, C. Y, and Song, T. Z, 2014. Situation, problems and developmental strategies of cultivation industry of Tibetan yak, China Cattle Science, 40, 74–74. (In Chinese)
Matope, G., Bhebhe, E., Muma, J.B., Oloya, J., Madekurozwa, R.L., Lund, A., and Skjerve, E., 2011. Seroprevalence of brucellosis and its associated risk factors in cattle from smallholder dairy farms in Zimbabwe, Tropical Animal Health and Production, 43, 975–982
Nyima, T., Luo, X.G., Yu, S.X., and Judson, G., 2005. A survey of the mineral status of livestock in the Tibet Autonomous Region of China, (Australian Centre for International Agricultural Research, Canberra)
OIE, 2009. Bovine brucellosis. In Manuals of Diagnostic Tests and Vaccines for Terrestrial animals (Chapter.2.4.3).2009. http://www.oie.int. Accessed 15 Sep 2016
Praud, A., Durán-Ferrer, M., Fretin, D., Jaÿ, M., O'Connor, M., Stournara, A., Tittarelli, M., Travassos Dias, I., and Garin-Bastuji, B., 2016. Evaluation of three competitive ELISAs and a fluorescence polarisation assay for the diagnosis of bovine brucellosis, The Veterinary Journal, 216, 38–44
R Development Core Team, 2013. R:A language and environment for statistical computing.Vienna, Austria:R Foundation for Statistical Computing.2013. http://www.r-project.org. Accessed 2 Mar 2016
Sergeant, E., Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease.2015. http://epitools.ausvet.com.au. Accessed 5 Feb 2015
Sing, T., Sander, O., Beerenwinkel, N., and Lengauer, T., 2005. ROCR: visualizing classifier performance in R, Bioinformatics, 21, 3940–3941
Sun, M.J., Di, D.D., Li, Y., Zhang, Z.C., Yan, H., Tian, L.L., Jing, Z.G., Li, J.P., Jiang, H., and Fan, W.X., 2016. Genotyping of Brucella melitensis and Brucella abortus strains currently circulating in Xinjiang, China, Infection, Genetics and Evolution, 44, 522–529
Wang, Y., Wang, J., Li, S., and Qin, D., 2014. Vulnerability of the Tibetan pastoral systems to climate and global change, Ecology and Society, 19, 8
Wu, G.Z., Zhong, Z., Pingcuo, Z., Bai, J., La, Z., Laqiong, Deji, Dawa, Dawa, C., Laba, Z., Dazhen, W., and Dawa, C., 2011. Epidemiological survey of brucellosis on cattle and sheep, Tibetan Journal of Animal Husbandry and Veterinary, 2, 15–17. (In Chinese)
Yuan, L.G., Shi, Q., and Pu, J.W., 2014. Investigation of brucellosis infection of ruminants on natural pastures, Chinese Journal of Veterinary Medicine, 50, 84–85. (In Chinese)
ZhanDui, Ruan, S.L., Gongsang, Q., and Cui, B.Y., 2008. Epidemiological analysis of brucellosis in Naqu and Qamdo, Tibet in 2006, Disease Surveillance 23, 285–286. (In Chinese)
Zuo, H.L., Fu, P.S., and Du, J., 2009. Countermeasure and change of environment and climate in Tibet, Tibetan Journal of Science and Technology, 6, 55–58. (In Chinese)
Acknowledgements
We would like to thank the John Allwright Fellowship from ACIAR (Australian Centre for International Agricultural Research) and Murdoch University for sponsoring this study as part of a PhD studentship. The Tibet Livestock Research Institute and Veterinary stations in the three sampled counties are acknowledged for their field assistances during sample collection. Also, we acknowledge the staff of the China Animal Health and Epidemiology Center for their laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, J., Duoji, C., Yuan, Z. et al. Seroprevalence and risk factors for bovine brucellosis in domestic yaks (Bos grunniens) in Tibet, China. Trop Anim Health Prod 49, 1339–1344 (2017). https://doi.org/10.1007/s11250-017-1331-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1331-7