Abstract
A study was carried out to characterize and differentiate four West African Dwarf goat populations on the basis of morphometric traits and also predict their body weights using linear body measurements. A total of 384 goats were sampled from four agro-ecological zones from the middle belt and southern part of Ghana. The morphometric traits examined for phenotypic differentiation using univariate and multivariate analysis were body weight, body length, withers height, chest girth, chest depth, shoulder point width, rump length, rump width, head length, head width, shin circumference, horn length, ear length, tail length, and hair length. Results obtained showed that agro-ecological zone and age significantly (p < 0.05) influenced the performance of most traits. The phenotypic correlations among the traits were low to high (0.06–0.67) indicating linear relationships among these traits which may be caused by both genetic and environmental factors. The regression analysis also revealed that a single trait which may be used to predict the body weight of male West African Dwarf goats of ages 1–4 years is chest/heart girth and that of female counterparts is withers height. All the populations were significantly (p < 0.0001) distant (differentiated) from each other, but the largest (4.62) estimated Mahalanobis distance (D2) was between West African Dwarf goat populations in the transitional zone P1 and the rainforest P3 while the least (1.61) was observed between transitional zone P1 and semi-deciduous forest zone P2. The highest similarity between individuals within population was found in the rainforest P3 (79.76%) and the lowest in the transitional zone P1 (47.13%). The three canonical components (Can1, Can2, and Can3) explained the total (100%) between-population variation in morphometric traits. However, in partitioning of the variance, the first two canonical components (Can1 and Can2) accounted for cumulative proportion of 84.35% of between-population variance. In order of importance, tail length, head length, ear length, shin circumference, head width, and shoulder point width were the most discriminating variables responsible for the variations among the four goat populations. In conclusion, morphometric variations exist among West African Dwarf goat populations of Ghana. Yet, further studies on molecular genetic diversity of the populations are recommended to support a sustainable breed improvement strategy. Also, equations have been developed that can be used to reliably predict the body weight of WAD goats and future research should also focus on developing single variable weight estimator measuring tapes based only on either the chest girth, withers height, or body length prediction equations for farmers to use in the absence of weighing scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to FAO (2014) Africa has approximately 35% of the world’s goat population. This is probably due to the critical roles goats play in supporting families in most parts of rural Africa, including contribution to nutrition and food security, employment, income, and manure for soil fertility management (Ndao et al. 2019). The African Goat Improvement Network (AGIN) of the USAID’s Feed the Future Initiative has identified 46 breeds of goat in Africa, of which 14 are indigenous breeds and one of these native breeds is the West African Dwarf (WAD) goat found in most West African countries (Huson et al. 2014). In Ghana, the most predominant small ruminant livestock genetic resource is the West African Dwarf (WAD) goat with an estimated population of about 6,044,000 as against 4,335,000 for sheep according to the Ghana Livestock Development Policy and Strategy (MoFA 2016; Ofori and Hagan 2020). The wide acceptance of the WAD goat in the country makes the breed an integral part of the mixed farming systems of most rural households, serving as a coping mechanism against crop failure among other benefits. The breed possesses desirable traits such as hardiness/adaptability, relatively high prolificacy, precociousness, and trypanotolerance (MoFA 2016). Despite the significance of the WAD goat in the country, the breed is yet to be phenotypically characterized in order to harness its full potential.
According to Verma et al. (2016), linear body measurements are important because they reflect the breed standards and are also important in describing the morphological structure and developmental ability of the animals. These measurements are valuable and helpful in developing suitable selection criteria (Islam et al. 1991). Linear body measurements have been used in predicting body weight and carcass trait in sheep (Sowande and Sobola 2008; Tadesse and Gebremariam 2010; Birteeb et al. 2012) and therefore forms an important aspect of phenotypic characterization (FAO 2012). There is however very little research work done on goats and especially on the West African Dwarf (WAD) goat in Ghana. There is therefore the need to establish the relationship between live body weight and some linear body measurements in the WAD goats as a step towards establishing prediction equation to estimate live body weight of goats under field conditions without using weighing scales.
The realization of the full potential of the WAD depends on the phenotypic information which will subsequently help in the selection of individuals with better trait values to be parents of the next generation (Oldenbroek and van der Waaij 2015). A previous work on characterization of the WAD goats looked at only the morphological characteristics in only two ecological zones of the country. This current study was carried out to characterize four WAD goat populations under four agro-ecological zones on morphometric traits and also predict their body weights using linear body measurements.
Materials and methods
Description of study area
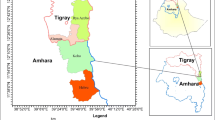
The study was conducted in four (4) agro-ecological zones (transitional, semi-deciduous forest, rainforest, and coastal savannah) of Ghana (as described in Table 1) because the WAD goat is known to be concentrated and adapted to the middle belt and southern part of the country as opposed to the Sudan and Guinea savannah eco-zones where cross-breeding of the WAD goat and the Sahelian has being on-going over the past few years.
Sampling and sample size
A multi-stage purposive sampling technique involving four (4) stages were used as suggested in the phenotypic characterization guidelines (FAO 2012): agro-ecological zone, district, community, and household. There were 4 agro-ecological zones, 8 districts—2 in each agro-ecological zone, 4 communities in each district, 4 households per community, and 3 WAD goats (2 females and 1 male) from each household. Hence, 128 households were sampled and a predetermined sample size (n) of 384 WAD goats (256 females and 128 males) examined. The number of goats measured was as follows: each ecological zone (96), each district (48), each community (12), and each household (3). The number in each age group was as follows: < 1 year (50), ≥ 1 < 2 years (56), ≥ 2 < 3 years (73), ≥ 3 < 4 years (91), and ≥ 4 < 5 years (114). The sample size was estimated using the FAO (2012) generic method for determining sample size as follows:
where:
- n:
-
sample size
- z:
-
the z value for 95% confidence level = 1.96
- m:
-
the margin of error (the confidence interval of + or − 5%) = 0.05
- p:
-
the estimated value for 50% proportion of the sample that will respond a given way to a survey question, p = 0.50
Animal management
The WAD goats sampled were managed under both semi-intensive and extensive systems in individual households where the animals are allowed to go and feed on grasses, browse plants, and kitchen waste and return to pens in the afternoon or evening for shelter in the case of the former but for the latter, goats roam about to fend for themselves with little or no shelter.
Data collection
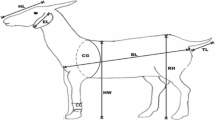
The quantitative (morphometric) traits measured were the body weight and linear body traits which were taken with a weighing scale and measuring tape respectively as shown in Fig. 1 as follows:
-
Body weight (BW): the fasted live body weight (kg) of the goat taken with a hanging mini crane scale of 300 kg capacity.
-
Body length (BL): the horizontal distance (cm) from the point of shoulder to the pin bone.
-
Withers height (WH): the (vertical) height (cm) from the bottom of the front foot to the highest point of the shoulder between the withers.
-
Chest girth (CG): the circumference of the body (cm) immediately behind the shoulder blades in a vertical plane, perpendicular to the long axis of the body.
-
Chest depth (CD): vertical plane distance/deepness (cm) of the chest, perpendicular to the long axis of the body.
-
Shoulder point width (SPW): the horizontal distance (cm) between the left and right upper arm.
-
Rump length (RL): measured (cm) from the hip bone to pin bone
-
Rump width (RW): the horizontal distance (cm) between the left and right hip bones.
-
Head length (HL): distance (cm) between the highest points of the parietals to the middle of the rostral margin of the incisive bone
-
Head width (HW): distance (cm) between the two zygomatic arches
-
Shin circumference (SC): the circumference of the front cannon bone (cm) between the front pastern and the knee of the foreleg.
-
Horn length (HoL): length of horn (cm) on its exterior side from its root at the poll to the tip.
-
Ear length (EL): length (cm) of the external ear from its root on the poll to the tip.
-
Tail length (TL): distance (cm) from the base of the tail to the tip.
-
Hair length (HaL): length (cm) of hair from the base of the hair on the skin to the tip (on the backline, at the rump).
Data analysis
Univariate analysis (ANOVA)
Data obtained for morphometric/quantitative traits were verified for normality using the Shapiro-Wilk test for normality and then subjected to the analysis of variance (ANOVA); both using GenStat (Discovery Edition 12) (VSNI 2012) and where there was a significant difference between means in the ANOVA, the least significant difference (LDS) was used to separate such means. The effects of agro-ecological zone, sex, and age on body weight and linear body traits were tested using the model:
where:
- Yijk:
-
individual observation of each body trait
- μ:
-
overall mean
- Si:
-
effect of ith sex (i = male, female)
- Aj:
-
effect of jth age (j = 1, 2, 3, 4, and 5 years old)
- Ek:
-
effect of kth agro-ecological zone (k = transitional zone 1; semi-deciduous forest 2; rainforest 3; and coastal savannah 4)
- eijk:
-
random error associated with each observation
The correlation analysis was performed using IBM Corp SPSS Statistics (Version 21.0), 2012 predictive analytics software, based on the general equation:
where:
- rxy:
-
the correlation coefficient of the linear relationship between the traits x and y
- xi:
-
the measured values of trait x
- \( \overline{x} \):
-
the mean of the values of trait x
- yi:
-
the measured values of trait y
- \( \bar {\mathrm{y}} \):
-
the mean of the values of trait y
Simple and multiple regression with groups was used to predict body weight of WAD goats from linear body measurements based on the models 1 and 2 respectively, using GenStat (Discovery Edition 12):
where:
- BW:
-
body weight
- β/β0:
-
the intercept of the regression equation
- X:
-
a given linear body trait
- βi:
-
the ith regression coefficient of the ith linear body trait retained in the model (Xi)
- βn:
-
the nth regression coefficient of the nth linear body trait retained in the model (Xn)
- ε:
-
the random error
The data for the animals were grouped into four age categories (years 1, 2, 3, and 4) and then subdivided into males and females. A preliminary simple regression analysis was carried out to identify six (6) most important linear body variables (chest girth [CG], body length [BL], withers height [WH], rump length [RL], rump width [RW], and shoulder point width [SPW]) for predicting body weight and were the only traits retained for the multiple regression analysis to ascertain their pooled predictive ability.
Multivariate (discriminant) analysis
The canonical discriminant and stepwise discriminant analyses of the PROC CANDISC and PROC STEPDISC of SAS (SAS 2011) University Edition Software program respectively were used to differentiate and classify the four WAD goat populations from the four corresponding agro-ecological zones, using the Mahalanobis distance (based on covariance matrix Rao 1973) according to the formula:
where:
- D2:
-
the Mahalanobis squared distance
- x:
-
the vector of observation of morphometric trait (row in a dataset)
- m:
-
the vector of mean values of independent morphometric variables (mean of each column)
- T:
-
transposed matrix (a new matrix whose rows are the columns of the original)
- C−1:
-
the inverse covariance matrix of independent morphometric variables
Results and discussion
Effect of agro-ecological zone, sex, and age on morphometric traits of WAD goats
The descriptive statistics of quantitative (morphometric) traits are presented in Table 2. Generally, agro-ecological zone significantly (p < 0.05) influenced most traits (body weight [BW], body length [BL], withers height [WH], chest girth [CG], chest depth [CD], shoulder point width [SPW], rump length [RL], rump width [RW], head length [HL], head width [HW], shin circumference [SC], ear length [EL], and tail length [TL]) with the exception of horn length (HoL) and hair length (HaL). However, WAD goat population in the transitional zone showed considerably higher values for these traits than other populations except for shin circumference (SC), ear length (EL), and tail length (TL). This trend was followed closely by the goats in the semi-deciduous forest which shares boundary with the transitional zone. Meanwhile, rainforest and coastal savannah goats were found to be similar in some traits (body weight [BW], body length [BL], withers height [WH], chest depth [CD], head length [HL], horn length [HoL], and hair length [HaL]) whereas the former recorded higher values for traits such as shoulder point width (SPW), rump length (RL), rump width (RW), head width (HW), ear length (EL), and tail length (TL) than the latter.
The significant (p < 0.05) effect of agro-ecological zones on morphometric traits observed in this investigation (Table 2) is an indication of the differences in availability of feed resource base (in terms of quantity and quality), availability of natural and established grazing field, and the management conditions which the animals were subjected to in these different locations. Also, the relatively higher superiority of WAD goats in the transitional zone than the other populations could be attributed to the fact that WAD goat population in the transitional zone may have been improved through selection and good management practices as this has been the focus of the National Goat Breeding Station at Kintampo in the transitional zone through its out-grower and Open Nucleus Group Breeding Scheme (ONGBS) as nearly half of the animals (45 out of 96) sampled from 15 households in the transitional zone had owners who were members of the out-grower and Open Nucleus Group Breeding Scheme.
Sex, however, did not influence most of the traits except shin/canon circumference (SC) and hair length (HaL) which exhibited sexual dimorphism in that bucks were found to have larger shin and longer hair than does. According to Coyne et al. (2008), the genetic basis of sexual dimorphisms is an intriguing problem of evolutionary genetics because dimorphic traits are limited to one sex. Such traits can arise genetically in two ways. First, the alleles that cause dimorphisms could be limited in expression to only one sex at their first appearance. Alternatively, dimorphism alleles could initially be expressed in both sexes, but subsequently be repressed or promoted in only one sex by the evolution of modifier genes or regulatory elements. The latter may however explain the reason for the observed sexual dimorphism in shin circumference (SC) and hair length (HaL) of the WAD goat.
Results of the current work showed that age influenced all the morphometric traits studied such that as the goat ages, the values of these traits also increase until 4–5 years old (Table 2). This pattern is expected since growth increases with age but ceases (plateaus) and possibly declines at a particular point in time depending on the genotype (population, breed, species, etc.) and the environment (nutrition, medication/health, and housing). These results agree with the trends and values of earlier works (Birteeb et al. 2015; Ebegbulem et al. 2011; Idrissou et al. 2017; Ibnelbachyr et al. 2015) on WAD goats in different African countries.
Pearson’s correlation among quantitative variables (body weight and linear body traits)
The phenotypic correlations among quantitative variables (body weight and linear body traits) were generally and significantly (p < 0.05) low to high which were both negative and positive (Table 3) and comparable to the findings of other works (Birteeb and Lomo 2015; Chacón et al. 2011). The results obtained showed low (0.06–0.39) phenotypic correlations between body weight and linear body traits such as chest depth, head length, head width, shin/canon circumference, horn length, ear length, hair length, and tail length, indicating that these linear body traits make little contribution to total body weight and hence may not be good predictors of body weight. It further suggests that selection for these traits may not necessarily improve body weight. Moderate (0.44–0.59) correlations were however noticed between body weight and body length, withers height, chest/heart girth, shoulder point width, rump length, and rump width; these are indicators of the body weight and carcass yield and therefore improvement in these linear traits may improve body weight and carcass yield reasonably (Morales-delaNuez et al. 2012). However, a high correlation of 0.67 was observed between rump length and rump width which implies that selection for one will improve the other if this high correlation is caused by genetic factors rather than the environment. The rump length and width define the size of the pelvic region and relates to the ease of giving birth by female animals, and therefore, selection for improved rump length and width may improve the ease of parturition when selecting for increased birth weight (Frandson et al. 2009).
The present findings agree in part with the outcome of Birteeb and Lomo (2015) who detected that most phenotypic correlations among body weight and linear body traits of the WAD goats reared in the transitional zone of Ghana were low to moderate (0.19–0.58) with the highest between body weight and heart/chest girth (0.67) and body weight and body length (0.71). Admittedly, the correlations observed in the current study are generally lower than those reported in earlier studies (Ahmed et al. 2016; Dorantes-Coronado et al. 2015) but may confirm other works including Chacón et al. (2011) who noted that correlation coefficients between linear body traits of Cuban Creole goats and their crossbreds were generally medium as well as Sam et al. (2016) who reported that the correlation coefficients between body weight and linear body measurements in WAD goats of Obio Akpa town of the Akwa Ibom State of Nigeria were significantly (p < 0.001) medium to high with values ranging from 0.57 to 0.68 and additionally supporting the results of Idrissou et al. (2017) who reported medium to high correlation of 0.58–0.83 for the dwarf goats of Benin.
Regression equations to predict body weight of WAD goats from linear body traits
The regression analyses for prediction of live body weight from linear body measurements of WAD goats are presented in Table 4. The linear body dimensions that could predict the body weight of WAD goats are CG, BL, WH, RL, RW, and SPW because they contain the main musculoskeletal system (made up of the bones of the skeleton, muscles, body fluids, cartilages, tendons, ligaments, joints, and other connective tissue that supports and binds tissues and organs together) which essentially make up the animal’s body weight. When live weight was regressed on each of these six linear body measurements in multiple linear regressions, all the regression models were highly significant (p < 0.001) with low to high prediction accuracies as the resultant percentage variances accounted for or adjusted R2 (adjusted coefficients of determination) were generally low to high which could have been the consequence of the low-to-moderate/high correlations between body weight and each of these linear body traits considered. The percentage variance accounted for (when expressed as a proportion instead of a percentage is called the adjusted R2) is a summary of how much of the variability of a given set of response measurements can be explained by the fitted model. The results showed that largely, a single trait which may be used to predict the body weight of male WAD goats of ages 1–4 years is chest/heart girth (CG) followed by body length (BL). Specifically, the prediction equations with the adjusted R2 for the various ages are as follows: age 1 (these are goats between 1 and 2 years old), BW = − 22.96 + 0.71CG (adj. R2 = 0.74); age 2 (these are goats between 2 and 3 years old), BW = − 5.86 + 0.40CG (adj. R2 = 0.36); age 3 (these are goats between 3 and 4 years old), BW = − 1.96 + 0.43BL (adj. R2 = 0.36); and age 4 (these are goats between 4 and 5 years old), BW = − 10.15 + 0.50CG (adj. R2 = 0.49). On the other hand, body weight of female counterparts of the various age groups may be predicted from mainly withers height (WH) and then body length (BL) as follows: age 1 (BW = − 14.63 + 0.70WH; adj. R2 = 0.38), age 2 (BW = −9.25 + 0.55BL; adj. R2 = 0.41), age 3 (BW = −12.38 + 0.69WH; adj. R2 = 0.39), and age 4 (BW = 9.44 + 0.26WH; adj. R2 = 0.22). Consequently, a single variable weight estimator measuring tape based only on either the chest girth, withers height, or body length prediction equations may be developed for WAD goat keepers to use in the absence of weighing scale as suggested (FAO 2012). Such measuring tapes are user-friendly because for every centimetre (cm) on the tape, there is a corresponding indicative weight (kg) on the same tape which makes it easier and convenient for farmers to use in their households where weighing scales are virtually non-existent. The results of the current study support the findings of Birteeb and Lomo (2015) who noticed that most of the regression models were highly significant (p < 0.001) in predicting the body weight of WAD goats within the transitional zone of Ghana but with low prediction accuracies as the adjusted R2 were low/moderate (the range of 25.3% to 63.4%) when similar variables (CG, BL, WH, and RH) were used and additionally stated that body length (X) could predict body weight (Y) with the equation, y = − 23.7 + X, with coefficient of determination (R2 = 49.8%) and further agree with the work of Hassen et al. (2012) who detected that the use of chest/heart girth yielded 80.7% and 9.46% while the use of body length generated 53.2% and 29.0% for two goat populations respectively in Ethiopia.
Furthermore, the results of this contemporary study aligns with that of Sam et al. (2016) who showed that the body weight of some Nigerian WAD goats of 1-year-, 2-year-, and 3-year-old could be predicted with chest/heart girth with prediction accuracies (R2) of 0.48, 0.62, and 0.46 respectively.
Moreover, when the six major explanatory or independent variables (CG, BL, WH, RL, RW, and SPW) for predicting body weight were combined in a multiple regression analysis to ascertain their pooled predictive ability, the outcome was positive as the percentage variance accounted for or adjusted R2 values increased significantly, implying that combination of two or more of these variables in a model improves the prediction of the live weight. These equations may be useful in predicting body weight of WAD goats in on-farm research where weighing scales are not available. These findings conform to other works (Birteeb and Lomo 2015; Sam et al. 2016) which confirmed that body weight of goats could be estimated more accurately by combination of two or more variables with heart girth in multiple regressions.
Differentiation and classification of four WAD goat populations in Ghana
Multivariate analyses of morphometric traits are used to evaluate total/combined variation within and between populations when all morphometric variables are considered simultaneously, with the objective of differentiating and classifying populations or breeds or species (FAO 2012). In the current investigation, the canonical discriminant and stepwise discriminant analyses using the PROC CANDISC and PROC STEPDISC of SAS respectively were able to differentiate and classify the four WAD goat populations from the corresponding agro-ecological zones.
Differentiation of populations
The degree of differentiation was indicated by the Mahalanobis distance as shown (Table 5 and Fig. 2).
From Table 5, all the populations were significantly (p < 0.0001) distant (differentiated/separated) from each other. However, the largest (4.62) estimated Mahalanobis distance (D2) was between WAD goat populations in the transitional zone (P1) and the rainforest (P3) while the least (1.61) was observed between transitional zone (P1) and semi-deciduous forest zone (P2); the former could be due to the fact that there has been minimal interbreeding between the populations as a result of large geographical separation whereas the latter might be due to inbreeding or high interbreeding or genetic exchange that has taken place overtime facilitated by geographical proximity. The large distance between WAD goat populations 1 and 3 may also be attributable to natural and artificial selection as well as adaptation to environmental conditions. Thus, geographical isolation with ecological variation can create differences in livestock populations. It is however important to maintain the diversity found between goats in the transitional zone and the rainforest in order to take advantage of a well-designed interbreeding programme to improve the economic traits (growth rate, weight gain, matured weight) of these goats. Advisedly, the National Goat Breeding Station in the transitional zone which is earmarked to be restocked with new goats under the ‘Rearing for Food and Jobs Initiative’ may consider screening and obtaining goats from different districts of the rainforest. On the other hand, the short Mahalanobis distance between populations 1 and 2 suggest intermingling between these goat populations and hence may be homogenous and share similar genetic identities (genes). This could nevertheless be checked by discouraging the seemingly unrestricted and indiscriminate mating of goats from these two neighbouring populations since further reduction in the distance might result in high inbreeding levels and a decrease in diversity. One way to curb the uncontrolled movement of goats between P1 and P2 is to setup goat market within the middle belt as part of the goat value chain where sellers and buyers can trade; and by using animal identification, the Ghana Post GPS digital address and digital photograph of the animal (taken at the point of sale) as requirements for selling or buying or moving (veterinary movement permit) goat, we may be able to check the migration of identified animals. Again, since ecological closeness has been found to aid indiscriminate mating of animals, in the absence of animal migration and molecular genetic characterization data (on inbreeding among populations), the rule of thumb should be that animals to be used for breeding must be selected from populations that are separated by wide geographical area. The findings of the present study agree in part with that of Radhika et al. (2018) who noted greatest Mahalanobis distance (D2) of 8.92 and shortest (0.28) for goat genetic groups of India as well as the outcome of the investigation by Yakubu and Ibrahim (2011) who reported that all between-breeds (pair-wise) Mahalanobis distances were significant (p < 0.0001) and ranged from largest distance (4.83) to smallest distance (1.79) in sheep breeds of Nigeria. The outcomes of the current work also align with that of Goitom et al. (2019) who noted a Mahalanobis distance of 1.93 between Highland and Western lowland cattle, 5.10 between Highland and East Coast cattle, and 4.72 between Western lowland and East Coast cattle populations of Eritrea.
Classification of populations
The level of similarity between individuals within populations which is used to classify the former into the latter is shown in Table 6.
In the contemporary work, the discriminant analysis was able to classify most of the individual goats into their respective populations (except those in the transitional zone P1) by assigning an observation the class/population that it is closest to (Table 6), based on the Mahalanobis distance (Table 5). This implies that the canonical components (linear combinations) captured most of the between-class/population variation of the data and therefore the highlighted (italicized) values on the main diagonal (P2 versus P2, P3 versus P3, and P4 versus P4) are large with the exception of P1 versus P1, compared to the off-diagonal values (misclassified). The highest similarity between individuals within population was found in the rainforest (79.76%) and the lowest in the transitional zone (47.13%). The results further indicate that 32.18% of goats in the transitional zone were misclassified as semi-deciduous forest goats whereas 16.51% of the latter were wrongly assigned to the former, providing additional evidence of intermixing of the two populations. In effect, it is possible to use canonical discriminant analysis to discriminate (differentiate) between these populations of WAD goats by using three canonical components that are linear combinations of physical measurements or morphometric traits; the first three canonical components are good discriminators for the populations. Attempting to discriminate by using only two canonical components leads to classification errors, because the projection onto the span (the span of a set of vectors is the vector space consisting of all linear combinations of the vectors) of the first two canonical components does not separate the populations completely, especially P1 and P2 as illustrated in the graph in Fig. 2. These findings agree somehow with that of Yakubu and Ibrahim (2011) who detected that most Balami sheep (61.45%) were classified into their source genetic group, while 41.22% of Uda sheep were misclassified as Yankasa sheep while 35.35% of Yankasa were wrongly assigned as Uda sheep, showing the level of genetic exchange that had taken place between the two breeds in the past. The outcome further conforms to that of Sanni et al. (2018) who reported that 70% of Kalahari Red, 68.9% of Red Sokoto, and 79.5% of Sahel goats were classified into their genetic groups. The results of the current research are however lower than that of Batubara et al. (2011) who recorded a classification rate of 82.50–100%, but nonetheless higher than the values obtained by Yakubu et al. (2010) who stated that only 24.38% of rainforest, 22.86% of Guinea savannah, and 45.95% Sudan savannah WAD goats in Nigeria were classified into their source population. The variations in the findings of the present work and that of others might be due to the differences in species, breeds, populations, and agro-ecological zones.
Partitioning of between-population variances
Table 7 displays the total canonical structure (original morphometric traits and their canonical coefficients used to derive the canonical components) whereas Table 8 summarizes the total canonical structure into the three canonical functions/components/variables (Can1, Can2, and Can3) and their highest possible multiple correlations (r) with the populations, eigenvalues (dimension-reduction or transformational factor of the covariance matrix), and proportion/percentage of between-population variation explained by each eigenvalue and its corresponding canonical component. The relative importance of the canonical components in partitioning the variance between populations depends on the eigenvalue; the higher the eigenvalue, the greater the proportion of variance accounted for and therefore the first few canonical components with high eigenvalues account for much of the variation in the dataset.
Linear combinations of canonical (Can) variables/functions/components of total canonical structure (based on Tables 7 and 8)
Can1 = 0.08895BW + 0.27370BL + ……0.62946TL (r = 0.62)Eigenvalue (0.63); percentage variance explained is 49.56%
Can2 = − 0.00081BW − 0.42330BL + …… − 0.104191TL (r = 0.55)Eigenvalue (0.44); percentage variance explained is 34.79%
In the current investigation, the three canonical components (Can1, Can2, and Can3) with corresponding eigenvalues of 0.63, 0.44, and 0.20 explained the total (100%) between-population variation in morphometric traits. However, in partitioning of the variance, the first canonical component (Can1) with the highest eigenvalue explained relatively higher proportion (49.56%) whereas the second (Can2) with the next highest eigenvalue explained 34.79%, thus both (with cumulative eigenvalue of 1.07) accounting for cumulative proportion of 84.35% (a high representation) of between-population variance in morphometric traits of WAD goats from different agro-ecological zones. These results are parallel to the findings of the study by Jimcy et al. (2011) who documented that the two first canonical variables represented a cumulative total of 89.9% of total variation and further agrees with other works including Dauda et al. (2018) and Vargas et al. (2019).
Most discriminating traits for the populations
The stepwise selection summary (Table 9) shows traits that have higher discriminating power (contributing significantly to the aggregate differentiation/variation) depending on the partial R2 and F value.
The comparative importance of the morphometric variables in discriminating between the WAD goat populations was evaluated using the level of significance (p < 0.0001), partial R2 and F statistic/value (the higher these values, the greater the discrimination) in a stepwise discriminant function procedure which showed that chronologically, tail length (TL), head length (HL), ear length (EL), shin circumference (SC), head width (HW), and shoulder point width (SPW) were the most differentiating variables responsible for the variations/diversities among the four goat populations. These six morphometric variables have also been found by other works (Dauda et al. 2018; Yakubu and Ibrahim 2011; Pundir et al. 2015) to be powerful discriminators in differentiating among species, breeds, and populations of livestock in other countries and therefore support the findings of the current study. Consequently, these traits might be priority traits when discriminating and classifying WAD goat populations based on morphometric variables.
Conclusions
The study revealed the influence of agro-ecological zone and age on the performance of most morphometric traits while there was no sexual dimorphism regarding the traits measured. The regression analysis also revealed that a single trait which may be used to predict the body weight of male WAD goats of ages 1–4 years is chest/heart girth (CG) and that of female counterparts is withers height (WH). Again, the differences in performance of the traits across the various agro-ecological zones were as a result of differences in genetics and environmental conditions and as such the superior performance of goats in the transitional zone could be due to the fact that these goats may have been improved through selection and good management practices. The high differentiation (low similarity) between WAD goats in the transitional zone and the rainforest could be due to the fact that there has been minimal interbreeding between the populations as a result of large geographical separation and the low differentiation (high similarity) between the transitional zone and semi-deciduous forest populations might be due to inbreeding or high interbreeding or genetic exchange that has occurred overtime facilitated by geographical proximity. However, since ecological proximity has been found to aid indiscriminate mating of animals, in the absence of animal migration and molecular genetic characterization data (on inbreeding among populations), it is important that animals to be used for breeding are selected or obtained from populations that are separated by a wide geographical area. In summary, morphometric variations exist among West African Dwarf goat populations of Ghana. However, further studies on molecular genetic diversity of the populations are suggested to support a sustainable breed improvement strategy. Also, equations have been developed that can be used to reliably predict the body weight of WAD goats and future research should also focus on developing single variable weight estimator measuring tapes based only on either the chest girth, withers height, or body length prediction equations for farmers to use in the absence of weighing scale.
References
Ahmed, S., Kefelegn, K. & Kefena, E. (2016). Morphological characterization of indigenous goats in Western Ethiopia: implication for community based breeding programmes. In FAO, Animal Genetic Resources. Pp. 1-10
Batubara, A., Noora, R. R., Farajallah, A., Tiesnamurti, B. & Doloksaribu, M. (2011) Morphometric and Phylogenic Analysis of Six Population Indonesian Local Goats. Media Peternakan 165-174.
Birteeb, P. T. & Lomo, R. (2015). Phenotypic characterization and weight estimation from linear body traits of West African Dwarf goats reared in the transitional zone of Ghana. Livestock Research for Rural Development. Volume 27, Article #175. Retrieved from http://www.lrrd.org/lrrd27/9/birt27175.htm
Birteeb, P.T., Peters, S.O., Yakubu, A., Adeleke, M.A. and Ozoje, M.O. (2012). Multivariate characterisation of the phenotypic traits of Djallonke and Sahel sheep in Northern Ghana. Trop. Anim. Health Prod. 45: 267-274.
Birteeb, P. T., Danquah, B. A. & Abdul-Rahaman S. S. (2015). Growth performance of West African Dwarf Goats Reared in the Transitional Zone of Ghana. Asian J. Anim. Sci., 9 (6), 370-378
Chacón, E., Macedo, F. Velázquez, F., Paiva, S. R., Pineda, E. & McManus, C. (2011). Morphological measurements and body indices for Cuban Creole goats and their crossbreds. Revista Brasileira de Zootecnia, 40(8), 1691-1679
Coyne, J. A., Kay, E. H. & Pruett-Jones, S. (2008). The genetic basis of sexual dimorphism in birds, Evolution 62(1), 214–219. Retrieved https://doi.org/10.1111/j.1558-5646.2007.00254.x
Dauda, A., Abbaya, H. Y. & Ebegbulem, V. N. (2018) Application of Multifactorial Discriminant Analysis of morphostructural differentiation of Sheep. Journal of Genetics and Genetic Engineering, 2(2), 11-16
Dorantes-Coronado, E. J., Torres-Hernández G. O., Hernández-Mendo, O & Rojo-Rubio, R. (2015). Zoometric measures and their utilization in prediction of live weight of local goats in southern México. Springer Plus 4:695
Ebegbulem, V. N., Ibe, S. N., Ozung, P. O. & Ubua, J. A. (2011). Morphometric trait characteristics of West African Dwarf Goats in Abia State, South East Nigeria. Continental J. Agricultural Science, 5(2), 1 - 6
FAO 2012 Phenotypic characterization of animal genetic resources. FAO Animal Production and Health Guidelines No. 11. Rome.
FAO (2014). "FAOSTAT." Retrieved from http://faostat.fao.org/
Frandson, R. D., Wilke, W. L. & Fails, A. D. (2009). Anatomy and Physiology of Farm Animals (7th edition). Wiley-Blackwell, Hoboken
Goitom, S., Gicheha, M. G., Ngeno, K. & Njonge, F. K. (2019) Morphological characterisation of indigenous cattle breeds in Eritrea. Adv. Anim. Vet. Sci. 7(10), 848-857
Hassen, H., Baum, M., Rischkowsky, B. & Tibbo, M. (2012). Phenotypic characterization of Ethiopian indigenous goat populations. African Journal of Biotechnology 11(73), 13838-13846.
Huson, H. J., Sonstegard, T. S., Silverstein, J., Woodward-Greene, M. J., Masiga, C., Muchadeyi, F., Rees, J., Sayre, B., Elbetagy, A., Rothschild, M., Mujibi, F. D., Mwai, O., Kemp, S., Colli, L., Ajmone Marsan, P., Crepaldi, P., Abegaz, S., Soelkner, J., Van Tassell, C.P., & African Goat Improvement Network (AGIN) (2014). Genetic and phenotypic characterization of African goat populations to prioritize conservation and production efforts for small-holder farmers in Sub Saharan Africa. In: Proceedings of the 10th World Congress of Genetics Applied to Livestock Production (pp.1-4). Vancouver, BC. Canada.
IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.
Ibnelbachyr, M., Boujenane, I. & Chikhi, A. (2015). Morphometric differentiation of Moroccan indigenous Draa goat based on multivariate analysis. Animal Genetic Resources 57, 81-87. https://doi.org/10.1017/S2078633615000296
Idrissou, N. D., Ahounou, S. G., Tougan, U., Tamimou, M. I., Hounmanou, Y. M. G., Mensah, G. A., Karim, I. Y. A. (2017). Morphometric and zootechnical characterization of dwarf goats in Northeastern Benin. Int. J. Agron. Agri. R., 11(3), 26-42
Islam, M. R., Saadullah, A. R., Howlider, M.A.R. & Huq, M. A. (1991). Estimation of live weight and dressed carcass weight from different body measurements in goats. J. Anim. Sci. 61(4): 460-461.
Jimcy J., Raghavan, K. C. & Sujatha, K. S. (2011) Diversity of local goats in Kerala, India, based on morphobiometric traits. Livestock Research for Rural Development, 23(5), 1-9. Retrieved May 28, 2019, from http://www.lrrd.org/lrrd23/5/jimc23119.htm
Ministry of Food and Agriculture [MoFA] 2016 Ghana Livestock Development Policy and Strategy. Accra: MoFA
Morales-delaNuez, A., Rodríguez, C., Santana, M., Hernandez-Castellano, L.E., Niño, T., Reyes-Chacon, R.J., Moreno-Indias, I. Castro, N. & Capote, J. (2012). Morphological measurements and indexes as a tool to support molecular genetic studies: an example in Canary Islands. Journal of Applied Animal Research, 40:3, 215-221, DOI: https://doi.org/10.1080/09712119.2012.658062
Ndao, S., Moulin, C., Traoré, E.H. et al. Contextualized re-calculation of enteric methane emission factors for small ruminants in sub-humid Western Africa is far lower than previous estimates. Trop Anim Health Prod 51, 919–928 (2019). https://doi.org/10.1007/s11250-018-1775-4
Ofori, S. A. & Hagan, J. K. (2020). Genetic and non-genetic factors influencing the performance of the West African Dwarf (WAD) goat kept at the Kintampo Goat Breeding Station of Ghana. Trop Anim Health Prod. http://www.springer.com/home?SGWID=0-0-1003-0-0&aqId=4016277&download=1&checkval=ba6323830f57df580c6c355423cfdfba
Oldenbroek, K., & Waaij, Liesbeth van der (2015). Textbook Animal Breeding: Animal Breeding and Genetics for BSc Students. Wageningen, the Netherlands: Centre for Genetic Resources and Animal Breeding and Genomics Group, Wageningen University and Research Centre – 311
Pundir, R. K., Singh, P. K. & Sadana, D. K. (2015) Multivariate analysis of morphometric traits of three different indigenous cattle populations from North East States of India. Indonesian Journal of Animal and Veterinary Sciences (JITV), 20(2), 79-86
Radhika, G., Raghavan, K. C. Mercey, C. K. A. Sunanda, & Rojan, P. M. (2018). Assessment of genetic diversity in goat genetic groups of Kerala, (India) using morphobiometric markers. Indian J. Anim. Res., 52 (3), 331-336
Rao, C. R. (1973) Linear Statistical Inference and its Applications, 2nd Wiley, Chichester
Sam, I., Ekpo, J., Ukpanah, U., Eyoh, G. & Warrie, M. (2016). Relationship between linear body measurement and live body weight in West African Dwarf Goats in Obio Akpa. Journal of Biology, Agriculture and Healthcare, 6(16), 118-124
Sanni, M. T., Okpeku, M., Onasanya, G. O., Adeleke, M. A., Wheto, M., Adenaike, A. S., Oluwatosin, B. O., Adebambo, O. A. & Ikeobi, C. O. N. (2018) Genetic morphometry in Nigerian and South African Kalahari Red goat breeds. Agricultura Tropica Et Subtropica, 51(2), 51–61
SAS Institute Inc. (2011) SAS/STAT® Software, Version 9.3 (University Edition) & User’s Guide. Cary, NC, USA
Sowande, O.S. and Sobola, O.S. (2008). Body measurements of West African dwarf sheep as parameters for estimation of live weight. Trop. Anim. Hlth. Prod. 40(6): 433-9.
Tadesse, A. and Gebremariam, T. (2010). Application of linear body measurements for live body weight estimation of Highland Sheep in Tigray Region, North-Ethiopia. J. The Dry lands. 3(2): 203-207
Vargas, P. A. T., González, F. J. N., Landi, V., Jurado, J. M. L. & Bermejo, J. V. D. (2019) Sexual Dimorphism and Breed Characterization of Creole Hens through Biometric Canonical Discriminant Analysis across Ecuadorian Agroecological Areas. Animals, 10 (32), 1-24
Verma, S.K., Dahiya, S.P., Malik, Z.S., Patil, C.S. and Patil, H.R. (2016). Biometrical characterization of Harnali sheep: A new synthetic strain. Indian J. Vet. Res. 25(1): 16-21.
VSN International (2012) GenStat for Windows 12th Edition. VSN International, Hemel Hempstead, UK. Web page: GenStat.co.uk
Yakubu, A. and Ibrahim, A. I. (2011) Multivariate analysis of morphostructural characteristics in Nigerian indigenous sheep. Italian Journal of Animal Science, 10(17), 83-86
Yakubu, A. Salako, A. E., Imumorin, I. G., Ige, A. O. & Akinyemi, M. O. (2010) Discriminant analysis of morphometric differentiation in the West African Dwarf and Red Sokoto goats. South African Journal of Animal Science, 40(4), 381-387
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics declaration
The authors would like to declare unequivocally that this paper has not been submitted to another journal and that the paper is original and has never been published elsewhere in any form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ofori, S.A., Hagan, J.K. & Kyei, F. Morphometric characterization and differentiation of West African Dwarf goat populations in Ghana. Trop Anim Health Prod 53, 69 (2021). https://doi.org/10.1007/s11250-020-02498-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-020-02498-x