Abstract
Poultry spermatozoa are prone to oxidative damage and adversely affect their fertility. Bioactive constituents of citrus fruit confer antioxidant enrichment on its juice and are candidates to combat oxidative load in poultry semen. Computer-assisted semen analyser and oxidative status were used to evaluate the potency of two tropical citrus varieties (sweet orange and tangerine) as natural diluents for rooster semen. Fresh and ripe sweet orange and tangerine fruits were obtained and processed into juices using a standard protocol and included in dextrose saline at 0%, 10%, 20%, 30%, 40%, 50% and 60% as semen diluent. Semen pool from 30 breeder roosters of 35–40 weeks of age was allotted randomly in triplicates to the different fruit juice-dextrose at 1:2 dilution rate and evaluated for 5 h at room temperature. Diluted semen, according to treatments, was evaluated for sperm kinetics using a computer-assisted sperm analyser, and seminal plasma was assayed for lipid peroxidation and total antioxidant activity. The result obtained shows that tangerine and sweet orange juice inclusion significantly (p < 0.05) enhance progressive spermatozoa motility and semen kinetics compared favourably with undiluted semen. The diluents had a proportionate increase in antioxidant activity with juice inclusion at 0 h and the antioxidant activity of 40%, 50% and 60% fruit juice-based diluents was higher than undiluted semen at 5 h. There was a reduced lipid peroxidation in juice inclusive diluents and increase lipid peroxidation rate in undiluted semen and 0% juice inclusion. In conclusion, tangerine and sweet orange juice inhibit lipid peroxidation in rooster semen and enhance progressive spermatozoa motility and maintain rooster semen kinetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chicken spermatozoa are particularly susceptible to lipid peroxidation due to its high proportion of polyunsaturated fatty acids (PUFAs) and account for a decrease in motility and viability during handling, processing and preservation (Balogun et al. 2017). Under physiological condition, spermatozoa produce a small amount of free radical (FR) and reactive oxygen species (ROS) which has a significant role in capacitation and the acrosomal reaction (Abd El-baseta et al. 2010). However, elevated concentrations of ROS induce sperm immotility via depletion of intracellular ATP and subsequent decrease in the phosphorylation of axonemal and can result in sperm cell death (Bansal and Bilaspuri 2011). The balance between the production of ROS and antioxidants defence is considered to be an essential determinant of semen quality, particularly its fertilizing ability (Khan 2011). Endogenously, semen ejaculates contain appreciable amounts of antioxidants that balance lipid peroxidation (Partyka et al. 2012). However, its capacity may be insufficient during storage or dilution (Balogun and Jimoh 2017; Balogun et al. 2016). Thus, it is imperative for antioxidant supplementation during handling (Jimoh et al. 2020). Many natural plants and their seed, leaf or root extracts which are rich in polyphenols, flavonoids, carotenes, gallic acid, tannins and essential oil as antioxidants have been recognized to be better than synthetic antioxidants due to lower cytotoxicity and residue (Zhong and Zhou 2013). Antioxidant capacities of different juices varied markedly; orange juice is believed to be a superior antioxidant candidate (Al-Daraji 2012). Citrus sinensis (sweet orange) and Citrus reticulata (tangerine) are the most abundant citrus variety farmed and consumed in Western Africa and their bioactive compounds make it a potent antioxidant candidate (Zhuo et al. 2016; Chen et al. 2020). The inclusion of citrus to rooster semen has been reported previously by Al-Daraji (2012). Most works on citrus have been centred or limited to sweet orange, at the expense of other readily available varieties which are also promising antioxidant candidates in semen processing. Thus, it is important to establish varieties with similar potentials as orange, to reduce the competition and overdependence on orange juice alone within the industry. Their comparative potential to maintain oxidative stability in rooster semen extension is worthy of investigating. Interestingly, the antioxidant system in the semen of mammals has been well characterized, and only a few studies are available about the antioxidant system in avian semen (Partyka et al. 2012). Thus, the strength of poultry antioxidant capacity needs to be investigated through dilution and assesses the extent of protection in ambient room temperature. The potential of fruit juices to mitigate oxidative load can be best evaluated at room temperature (handling temperature for artificial insemination) (Jimoh et al. 2020). At such a stage, spermatozoa are metabolically and physiologically actively to generate its high oxidative load for potential scavenge, unlike at semi-hibernated handling such as freezing and cooled storage. Most authors are unable to distinguish between the antioxidant contribution of the seminal fluid and exogenous antioxidants in extenders during handling and storage. This study aims to assess the role of sweet orange and tangerine inclusion in rooster semen diluents on spermatozoa kinetics and oxidative stability.
Materials and methods
This research was undertaken with approval from the institutional ethics committee of the Department of Agricultural Technology, Federal Polytechnic, Ado-Ekiti. The institutional and national standards for the care and use of animals for research were followed, and appropriate measures were taken to minimize pain or discomfort on the animals.
Thirty ISA brown breeder roosters of 35–40 weeks of age were obtained from a reputable breeder farm, housed in a battery cage containing one (1) rooster per cell and were used for this study. The birds were managed based on breeder’s recommendations and fed a commercial diet with the NRC requirement for poultry 1994. The 30 roosters were trained using the double abdominal massage for 2 weeks, after which semen was be harvested twice a week and ejaculate taken to the laboratory for in vitro analysis. All roosters were assessed for fertility, and only roosters of high fertility (spermatozoa kinetics and viability of 90% grade and above,) were used; care was taken to avoid any contamination of semen with faeces.
Diluent preparation
Fresh and ripe sweet orange and tangerine fruit were purchased, washed, peeled and the fruit pulp blended separately. The fruit juices were clarified with a juice extractor (Mikachi model No 1706). The juice obtained was designated as sweet orange juice (SWJ) and tangerine juice (TGJ) were of pH 4.3 and were kept frozen at − 4 °C in disposable 5-ml sterile sample bottles until it is required for use. Dextrose saline (5% dextrose in 0.9% normal saline; Unique Pharmaceuticals, Nigeria) was procured for the study.
The experimental design was completely randomized, consisting of seven treatments and three replicate in three repeated trials. For tangerine; 7 treatments consist of different diluent formulated: undiluted semen (G1) and others were semen diluted in dextrose saline + 10% TGJ (G2), dextrose saline + 20% TGJ (G3), dextrose saline + 30% TGJ (G4), dextrose saline + 40% TGJ (G5), dextrose saline + 50% TGJ (G6) and dextrose saline + 0% TGJ (G7).
For sweet orange; 7 treatments consist of different diluent formulated: undiluted semen (W1) and others were semen diluted in dextrose saline + 10% SWJ (W2), dextrose saline + 20% SWJ (W3), dextrose saline + 30% SWJ (W4), dextrose saline + 40% SWJ (W5), dextrose saline + 50% SWJ (W6) and dextrose saline + 0% SWJ (W7).
Semen collection, dilution and evaluation
The pooled semen were allotted randomly to treatments in replicates and diluted at 1:2, (semen:diluents), respectively. Diluted samples were mixed gently to allow equilibration in line with standard protocol for handling semen and semen assessment took place immediately. Semen qualitative and oxidative assay were assessed for each treatment at three repeated trials. Diluted semen, according to treatments, was evaluated for sperm kinetics using computer-assisted sperm analyser (SpermAnalyzeWin7 Xuzhon city, China, setting of CASA as in 5th WHO manual, 51 sperm tracks, evaluated magnification × 10, image acquisition rate: number frames/s 60): percentage motility, progressive motility, non-progressive motility, curvilinear velocity (um/s), average path velocity (um/s), straight line velocity (um/s), linearity, straightness, amplitude of lateral head (um), beat cross frequency (Hz), wobble, while sperm concentration and liveability using convention procedures. Sperm concentrations (duplicates per sample) were determined using Neubauer haemocytometer (TH-100; HechtAssistant, Sondheim, Germany) and expressed as spermatozoa × 108/ml. Liveability was done by placing a drop of semen on a glass slide, one drop of eosin-nigrosin stain added and mixed gently, then smeared on a slide, air-dried and viewed under the microscope at a magnification of × 400.
For the seminal oxidative assay, the respective diluted semen was prepared according to their treatments. The various treatment samples were constituted and stored at room temperature for 5 h. Triplicate samples were taken at 0 h and 5 h, centrifuged at 2000g for 15 min to separate seminal plasma. The seminal plasma was assayed for lipid peroxidation and total antioxidant activity as outlined in Jimoh and Ewuola (2018).
Statistical analysis
The data obtained were subjected to descriptive statistics, t test for comparison between the two citrus variety (W/G) inclusions in semen diluent for parameters such as seminal lipid peroxidation, total antioxidant activity at 0 h and 5 h and one-way analysis of variance for comparison among treatments G1–G7 and W1–W7 for parameters such as semen kinetics at p < 0.05. Differences in mean value were separated using Duncan’s new multiple range test, using IBM SPSS 20.
The statistical model is as follows:
where Yijk represents the value of spermatozoa kinetics and oxidative stability measured in the ith diluted semen; μ is the overall mean for each character; Bi is the fixed effect of ith rooster semen diluted citrus juice (i = undiluted semen (positive control), the citrus juice was incorporated into dextrose saline at 0%, 10%, 20%, 30%, 40%, 50%); and eijk is the random residual effect.
Result
Rooster semen quality in tangerine-dextrose saline diluent is shown in Table 1. The percentage motility of undiluted rooster semen was not significantly different from tangerine-dextrose saline diluted groups. Progressive motility of semen in G3–G6 diluent was significantly (p < 0.05) highest across the treatment and the least values were obtained in G7. The non-progressive motility of G2 and G7 semen groups was higher (p < 0.05) than G4–G6. Undiluted semen groups had statistically (p > 0.05) similar non-progressive motility and average path velocity with all diluted semen groups. Spermatozoa liveability and kinetics were (p > 0.05) similar across the treatments.
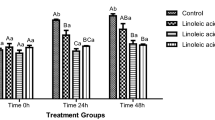
The rate of lipid peroxidation in rooster semen in tangerine-dextrose–based diluent is shown in Fig. 1. The rate of lipid peroxidation declined in tangerine inclusive semen diluents from 0 to 5 h. However, the lipid peroxidation in G1 and G7 semen groups increased from 0 to 5 h. The total antioxidant activity of rooster semen diluted in tangerine-based diluents is shown in Fig. 2. Antioxidant activity of the undiluted semen group compared favourably statistically with G4–G6 semen groups at 0 h. At 5 h, the antioxidant activity of G3–G6 semen groups were significantly (p < 0.05) higher than undiluted (G1), G2 and G7 semen groups. G1 and G7 semen groups dropped sharply from 0 to 5 h compared with other semen groups.
Lipid peroxidation of cock semen in tangerine-dextrose–based diluent. abc: different superscripts at 0 h are significantly (p < 0.05) different. mno: different superscripts at 5 h are significantly (p < 0.05) different. *: indicate a significant difference between 0 and 5 h within a treatment. G1, G2, G3, G4, G5, G6, G7 represent undiluted semen (G1); others were semen diluted in dextrose saline + 10% TGJ (G2), dextrose saline + 20% TGJ (G3), dextrose saline + 30% TGJ (G4), dextrose saline + 40% TGJ (G5), dextrose saline + 50% TGJ (G6), dextrose saline + 0% TGJ (G7)
Antioxidant activity of cock semen in tangerine-dextrose–based diluent. abc: different superscripts at 0 h are significantly (p < 0.05) different. mno: different superscripts at 5 h are significantly (p < 0.05) different. *: indicate a significant difference between 0 and 5 h within a treatment. G1, G2, G3, G4, G5, G6, G7 represent undiluted semen (G1); others were semen diluted in dextrose saline + 10% TGJ (G2), dextrose saline + 20% TGJ (G3), dextrose saline + 30% TGJ (G4), dextrose saline + 40% TGJ (G5), dextrose saline + 50% TGJ (G6), dextrose saline + 0% TGJ (G7)
The result of the semen quality of rooster semen in sweet orange-dextrose is shown in Table 2. The percentage motility of rooster semen was statistically (p > 0.05) similar across all treatment groups. The progressive motility of semen in W2 and W1 is statistically similar but were significantly (p < 0.05) lower than semen in groups W3–W6. Semen non-progressive motility of undiluted semen (W1) is significantly higher than semen in W3. Semen kinetics of undiluted semen was not significantly different from semen in W2–W7.
The rate of lipid peroxidation of rooster semen diluted in sweet orange-dextrose saline is shown in Fig. 3. At 0 h, the rate of lipid peroxidation in W2–W5 diluted semen was significantly (p < 0.05) higher than in W1 and W6 and statistically (p < 0.05), the least value was obtained in W7. At 5 h, there was a sharp increase in seminal lipid peroxidation in W1 and W7 compared to W4–W6, which reduced in comparison with values at 0 h. The total antioxidant activity of rooster semen in sweet orange-dextrose diluent is shown in Fig. 4. The antioxidant activity of undiluted semen and W6 were significantly (p < 0.05) higher than other semen groups at 0 h, while antioxidant activity was enhanced with sweet orange inclusive diluent. However, at 5-h seminal total antioxidant activity of W4–W6 were significantly (p < 0.05) higher than other semen groups. All semen groups had a decline in antioxidant activity at 3 h compared to values at 0 h.
Lipid peroxidation of cock semen in sweet orange-dextrose–based diluent. abc: different superscripts at 0 h are significantly (p < 0.05) different. mno: different superscripts at 5 h are significantly (p < 0.05) different. *: indicate a significant difference between 0 and 5 h within a treatment. W1, W2, W3, W4, W5, W6 and W7 represent undiluted semen (W1); others were semen diluted in dextrose saline + 10% SWJ (W2), dextrose saline + 20% SWJ (W3), dextrose saline + 30% SWJ (W4), dextrose saline + 40% SWJ (W5), dextrose saline + 50% SWJ (W6), dextrose saline + 0% SWJ (W7)
Antioxidant activity of cock semen in sweet orange-dextrose–based diluent. abc: different superscripts at 0 h are significantly (p < 0.05) different. mno: different superscripts at 5 h are significantly (p < 0.05) different. *: indicate a significant difference between 0 and 5 h within a treatment. W1, W2, W3, W4, W5, W6 and W7 represent undiluted semen (W1); others were semen diluted in dextrose saline + 10% SWJ (W2), dextrose saline + 20% SWJ (W3), dextrose saline + 30% SWJ (W4), dextrose saline + 40% SWJ (W5), dextrose saline + 50% SWJ (W6), dextrose saline + 0% SWJ (W7)
Discussion
Citrus fruits are rich in flavonoids, ferulic acid and vitamin C, which confers orange juices as good sources of antioxidants for semen preservation (Adekunle et al. 2018a, b). Zhuo et al. (2016) reported the constituents of citrus that accounts for its antioxidant properties which includes vitamins A, B1, B2, C, E and B3; flavonoids; naringin, hesperidin and naringenin; phenols; coumarins and others. The trend of result shows that tangerine inclusion enhances the progression of diluted semen, and semen kinetics of the diluted semen was similar to undiluted semen. This result is a pointer to the potential of tangerine as a component of semen extension. Similarly, the rate of lipid peroxidation was reduced with the inclusion of tangerine. This trend could have been responsible for the higher progressive motility observed in the tangerine inclusive diluents (G4–G6). This corroborates claims that vitamin C in fruits protects sperm cells from endogenous oxidative DNA and membrane damages (Arabi and Seidaie 2008). The benefit of tangerine inclusion was further established by the increase in the rate of lipid peroxidation in G1 and G7 diluent groups from 0 to 5 h. The trend of total antioxidant activity of rooster semen reveals that the inherent antioxidant present in the seminal fluid was high but was not sufficient to meet the demand over 5 h. This is because the antioxidant activity of undiluted semen dropped drastically at 5 h. The diluents had a proportionate increase in antioxidant activity with tangerine inclusion at 0 h, and the antioxidant activity of G3–G6 diluents was higher than undiluted semen at 5 h. This could be responsible for the lipid peroxide lowering effect in G4–G6 diluent groups and enhance spermatozoa progressive motility.
The role of sweet orange in semen extension was established in this study; the kinetics and percentage motility of spermatozoa of undiluted semen compared favourably with the various diluents. However, the inclusion of sweet orange had an enhancing effect on the progressive motility of spermatozoa, as evident in W3–W6 diluent groups. The suitability of sweet orange as a rooster semen extension constituent is highlighted in this work. Its role in lowering the rate of lipid peroxidation as obtained over 5 h due to its antioxidant enrichment is demonstrated in this study. It is in agreement with claims that improvements in semen parameters when treated with the fruit juices be linked to vitamin C, flavonoids and ferulic acid in the juices which acted synergistically to protect sperm cells from lipid peroxidation (Adekunle et al. 2018a, b). The antioxidant activity of undiluted semen was highest at 0 h but was inferior to W4–W6 at 5 h and does reduced peroxidation over the same period. This trend is common to both citrus variety (tangerine and sweet orange) results. It could indicate the FR and ROS scavenging ability in undiluted semen may be skewed. According to Jimoh and Ewuola (2018), superoxide dismutase, glutathione and catalase have varying activity in seminal fluid and account for different pro-oxidant scavenging. Similarly, Partyka et al. (2012) reported that excessive usage of the antioxidant defence system in chicken semen would lead to its insufficient reserve, necessary for the sperm cells protection against lipid peroxidation. According to Surai et al. (2001), the primary prevention of ROS formation involves SOD, GPx and metal-binding proteins, which are endogenous. The secondary prevention of ROS formation is based on natural antioxidants, such as vitamins A, C, E, uric acid, glutathione and carotenoids (which are the trust of fruit juice), which in conjunction with GPx restrict the production and accumulation of peroxides. This analogy could account for the limitation of oxidative stability exhibited in undiluted semen and the superiority of inclusive citrus semen.
Higher lipid peroxidation in undiluted semen despite high antioxidant activity may be due to lower activity of specific scavengers leading to the accumulation of its oxidants and leading to lipid peroxidation. Contrariwise, fruits juices have been reported to possess a cascade of antioxidants with a broad spectrum of pro-oxidant scavenging activity such as phenolics present in fruits which play a prominent antioxidant role in addition to other hydroxycinnamic derivatives such as dicaffeoylquinic and chlorogenic acids in the fruits (Zhang and Hamauzu 2004). The deteriorating result in semen diluted T7 and G7 (dextrose saline + 0% fruits juice) further confirm the importance of seminal antioxidant during handling. The dilution of semen with dextrose saline would reduce the seminal antioxidant activity and semen handling at an active condition as room temperature would increase its oxidation load. The inclusion of fruit juices in groups 2–6 will provide defence against the oxidation surge during handling and maintain the spermatozoa integrity.
These components could have accounted for the potency of the two fruit juices to lower lipid peroxidation better than groups without juice inclusion. This is similar to the report of Adekunle et al. (2018a, b) that the account of flavonoids and ferulic acid in fruits lowers the level of lipid peroxidation in fruit juice. The wide array of phytoconstituents in these fruit juices with antioxidant activities played a significant role in lowering lipid peroxidation and enhancing spermatozoa progressive motility in rooster semen (Adekunle et al. 2018a, b). This is in line with Zheng and Zhang (1997) that observed vitamin C, flavonoids and ferulic acid in orange suppressed a damaging effect of lipid peroxidation during the storage of rooster’s semen. Similarly, Okiyele et al. (2019) reported that orange juice enhanced sufficient CAT and SOD levels in the cryopreserved semen and efficiently removed reactive oxygen species ROS (Krzyzosiak et al. 2000).
Conclusion
This study establishes the potential of antioxidant enrichment of tangerine and sweet orange to inhibit lipid peroxidation in rooster semen and enhance progressive spermatozoa motility and maintain rooster semen kinetics. These profess natural antioxidants of tropical fruits as economically readily available, rich antioxidant supplements in extenders and diluents of rooster semen handling for artificial insemination.
Abbreviations
- PUFAs:
-
polyunsaturated fatty acids
- FR:
-
free radical
- ROS:
-
reactive oxygen species
- SWJ:
-
sweet orange juice
- TGJ:
-
tangerine juice
- SOD:
-
superoxide dismutase
- GPx:
-
glutathione peroxidase
- CAT:
-
catalase
References
Abd El-baseta S. A. and S. M. Abd El-Wahaba, A. M.A. Mansour, E. A.H. Mohamed 2010 Light and electron microscopic study of the effect of L-carnitine on the sperm morphology among sub fertile men. Middle East Fertility Society Journal 15, 95–10.
Adekunle E. O, J O. Daramola, O S Sowande, J A. Abiona and M O. Abioja 2018 Effects of apple and orange juices on Quality of refrigerated Goat semen. Journal of Agricultural Sciences Vol. 63, No. 1, 2018a Pages 53-65
Adekunle E.O, J O. Daramola, O S Sowande, J A. Abiona and M O. Abioja 2018 Effects of apple and orange juices on Quality of refrigerated Goat semen. Journal of Agricultural Sciences Vol. 63, No. 1, 2018b Pages 53-65
Al-Daraji HJ, 2012. Effect of diluent supplementation with different levels of orange juice on semen quality during liquid storage of roosters’ semen. Inter J Vet Sci, 1(1): 5-9.
Arabi, M., and Seidaie, S.R. 2008 Assessment of motility and membrane peroxidation of bull spermatozoa in the presence of a different concentration of vitamin C. Veterinary Medicine Journal Shahrekord, 2, 39-46.
Balogun, A.S. and Jimoh, O.A. (2017) Efficacy of egg-yolk citrate extender fortified with aqueous garlic extract on rooster semen for artificial insemination. Nigerian Journal of Animal Science 19(1):62 – 70.
Balogun A.S., O. A. Jimoh, Mustapha AED Abdulrahman, Akinosun Akin Akinbobola and Ojo, J.A. (2016) Assessment of different garlic extracts on fertilizing potentials of extended rooster semen used for artificial insemination. Nigeria Poultry Science Journal 12 pp. 84-91.
Balogun A.S., O. A. Jimoh, Olayiwola T.A. and Abubakar Z.Y (2017). Semen quality and fertilizing ability of roosters semen diluted with quail egg-yolk supplemented with polar and non polar dried garlic extracts. Journal of Advances in Biology and Biotechnology 13(2) Pp. 1-12. DOI: https://doi.org/10.9734/JABB/2017/32395
Bansal A.K. and G. S. Bilaspuri 2011 Impacts of Oxidative Stress and Antioxidants on Semen Functions. Veterinary Medicine International, Article ID 686137, doi:https://doi.org/10.4061/2011/686137.
Chen, Q., Wang, D., Tan, C., Hu, Y., Sundararajan, B. and Zhou, Z. 2020 Profiling of Flavonoid and Antioxidant Activity of Fruit Tissues from 27 Chinese Local Citrus Cultivars. Plants, 9, 196. https://doi.org/10.3390/plants9020196.
Khan R.U. 2011 Antioxidants and poultry semen quality. World’s Poultry Science journal 67 (2) 1-12. https://doi.org/10.1017/S0043933911000316
Jimoh, O.A. and Ewuola E.O. 2018 Semen characteristics, seminal biochemical and oxidative stress markers in rabbits during heat stress’ Journal of Veterinary Andrology 3(2):35-44
Jimoh O. A., Akinola M. O., Ayedun E. S., Ayodele S. O., Omoniyi S. I., Kolawole B. J., Ademola O. A. and Lawal A. G. 2020 Oxidative stability and spermatozoa kinetics of Cock semen in pineapple juice based diluent. Livestock Research for Rural Development. Volume 32, Article #108. Retrieved July 2, 2020, from http://www.lrrd.org/lrrd32/7/abuba32108.html
Krzyzosiak J, Evenson D, Pitt C, Jost L, Molan P, Vishwanath R. 2000 Changes in susceptibility of bovine sperm to in situ DNA denaturation, during prolonged incubation at ambient temperature under conditions of exposure to reactive oxygen species and nuclease inhibitor. Reprod Fertil Dev, 12:251-261
National Research Council NRC 1994. Nutrient requirements for poultry. In. Nutrient requirement of Domestic animals. Ninth Revised Edition, 1994. Washington, DC: The National Academies Press. https://doi.org/10.17226/2114.
Okiyele I. J., Ezike C. O., Agbo A.N. 2019 Motility and Oxidative Stress of Cryopreserved Fish Milt using Juices of Orange, Cucumber and Pineapple as Cryoprotectants. World Journal of Innovative Research, 7(3) pp. 46-51.
Partyka, A., E. Łukaszewicz and W. Nizanski 2012 Lipid peroxidation and antioxidant enzymes activity in avian semen. Animal Reproduction Science 134 184– 190
Surai, P.F., Fujihara, N., Speake, B.K., Brillard, J.P., Wishart, G.J. and Sparks, N.H.C., 2001. Polyunsaturated fatty acids, lipid peroxidation and antioxidant protection in avian semen. Asian-Aust. J. Anim. Sci. 14, 1024–1050.
Zhang, D. and Hamauzu, Y. 2004 Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chemistry, 88: 503-509.
Zheng, R.L., and Zhang, D.L. 1997 Effects of ferulic acid on fertile and asthenozoospermic infertile human sperm motility, viability, lipid peroxidation, and cyclic nueleotides. Free Radical Biology and Medicine, 22 (4), 58.
Zhong R. and Zhou D. 2013 Oxidative Stress and Role of Natural Plant-Derived Antioxidants in Animal Reproduction. Journal of Integrative Agriculture 12(10): 1826-1838.
Zhuo Z.,Wanpeng X., Yan H, Chao N., Zhiqin Z. 2016 Antioxidant activity of Citrus fruits. Food Chemistry 196, Pages 885-896. Doi: https://doi.org/10.1016/j.foodchem.2015.09.072
Funding
The research outcome presented in this article was funded by the TETFUND 2017 institutional-based research intervention of the Nigerian government.
Author information
Authors and Affiliations
Contributions
Jimoh O.A. designed the study, carried out the experimental protocol and wrote the first draft. Ayedun E.S. co-supervised the study and managed the semen collection. Ayodele S.O. and Omoniyi I.S. supervised animal handling, management of animals and data collection of fertility trial. Lawal A.A., Ademola O.A. and Kolawole B.J. carried out the fertility protocol and management of animals. Oladepo A.D. evaluated the experimental protocol, managed the data and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the institutional committee on the care and use of animals for the experiment and in accordance with the NIH guide for the care and use of laboratory animals.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jimoh, O.A., Ayedun, E.S., Ayodele, S.O. et al. Oxidative status and spermatozoa kinetics of rooster semen in citrus juice-based diluent. Trop Anim Health Prod 53, 31 (2021). https://doi.org/10.1007/s11250-020-02482-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-020-02482-5