Abstract
The aim of this study was to evaluate the effect of antioxidant agents and freezing methods on the ability of ram sperm to preserve its post-thaw quality characteristics. Six Chios rams were subjected to 52 weekly semen collections. Each ram was used as semen donor for freezing experiments once every 2 weeks. Equal number of good quality spermatozoa from each ejaculate (concentration ≥1 × 109 spermatozoa/ml, motility ≥70%, motility score ≥3.5) were pooled. Three equal aliquots of the pooled sample were diluted using three different fractions of a milk-based and glycerol extender (control, quercetin-enriched, α-tocopherol-enriched). Three freezing methods were applied (slow and fast freezing rate in a programmable freezer, vapors of liquid nitrogen) in every aliquot. Sperm aliquots were tested before freezing, immediately after thawing and after 3 h of incubation at 37 °C. Sperm motility (%) was evaluated microscopically. The percentage of membrane and acrosome-intact spermatozoa (IL%) as well as the percentage of membrane-intact and acrosome-reacted spermatozoa (ARL%) were determined by eosin-nigrosin stain. Furthermore, the percentage of hypo-osmotic swelling (HOS) test-positive spermatozoa was estimated. The results revealed no beneficial effect of the antioxidant treatment on the parameters of post-thaw semen (P > 0.05). However, the slow freezing rate method was more beneficial regarding motility, IL, ARL and HOS-positive spermatozoa compared to the other methods. In conclusion, the antioxidant agents used in this study failed to protect sperm against cryopreservation stress; however, the choice of the appropriate freezing method could contribute to the improvement of post-thaw ram sperm quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous researchers and practitioners are involved in ram semen cryopreservation and sheep artificial insemination (AI). A high variability of field fertility results have been reported, depending on the quality of the frozen-thawed ram semen and the method of AI (Windsor 1997). Differences have also been attributed to the breed or to the quality of the ram ejaculate (Fair et al. 2005). Spermatozoa’s membranes are susceptible to lipid peroxidation since they are rich in polyunsaturated fatty acids (Peris et al. 2004). It is well known that sperm freezing negatively affects the frozen-thawed sperm quality. Specifically, the freezing process induces osmotic stress, high production of reactive oxygen species (ROS), sperm DNA damage, destabilization of sperm membrane and dysfunction of sperm mitochondria (Pena et al. 2011).
In order to avoid the detrimental effects of oxidative stress during semen freezing, previous studies have used antioxidants or modified freezing protocols (Curry et al. 1994; Kumar et al. 2003; Sicherle et al. 2011; Silva et al. 2013). The supplementation of semen extenders with antioxidants has been shown to be beneficial for the maintenance of semen quality after freezing (Uysal and Bucak 2007). Among them, vitamin E provides greater structural integrity and kinematics for ram spermatozoa after cryopreservation (Silva et al. 2013), while crocin protects sperm DNA (Mata-Campuzano et al. 2015). However, controversial results have been reported concerning the effect of antioxidant agents specifically on ram frozen-thawed semen quality. The addition of resveratrol or quercetin to the Tris-egg yolk-glycerol extender reduced the potential of sperm mitochondrial membrane (Silva et al. 2012), while combined cysteine and glutathione in soy lecithin-based semen extender had a detrimental effect on the quality of post-thawed ram semen (Zhandi and Sharafi 2015).
During the freezing procedure, the temperature area between −10 and −25 °C is crucial for ram spermatozoa (Salamon and Maxwell 1995). In addition, rapid freezing rates induce sperm death, while ram spermatozoa are particularly susceptible to cold shock because they have low cholesterol levels and a high ratio (>2.5) of unsaturated to saturated fatty acids (Bailey et al. 2000). For the above mentioned reasons, previous studies have tested various protocols to measure the effect of the freezing rate on ram sperm post-thaw quality, reporting various results (Byrne et al. 2000; Kumar et al. 2003).
The aim of the present study was to investigate the effect of the antioxidant agents α-tocopherol and quercetin and the effect of different freezing methods on the post thawed quality of Chios ram semen.
Materials and methods
Chemicals, semen extenders
All reagents used in this study were purchased from Sigma Aldrich, Seelze, Germany, unless otherwise indicated.
Two basic extenders were used. Extender A, a milk-based extender, was prepared by the addition of skimmed milk powder in distilled water (11% w/v), heated to 95 °C for 10 min, and cooled to room temperature before the addition of 5.5% egg yolk (v/v). Finally, 120.000 IU penicillin and 0.04 gr streptomycin were added per 100 ml of the solution (Kulaksiz et al. 2012).
Extender B was prepared by the addition of glycerol (17.5% v/v) and fructose (5% w/v) to extender A.
Two additional types of extenders were prepared for the study’s purposes.
Extender Q, was prepared by the addition of the antioxidant agent quercetin to extender B to a final concentration of 0.5 mM.
Extender T, was prepared by the addition of antioxidant agent α-tocopherol to extender B to a final concentration of 0.5 mM.
The concentration of both antioxidants was selected based on preliminary trials, where concentrations of 0.5 mM, 1 mM, 2 mM and 4 mM were compared regarding motility and viability of thawed semen. The concentration of 0.5 mM demonstrated better results after 3 h of incubation at 37 °C (data not shown).
Animals, semen collection
Six trained adult rams (3–6 years old) of Chios breed were used in this study. The animals were housed in the Institute of Reproduction and Artificial Insemination of Thessaloniki, Greece (40°40′52.6″N 22°51′57.4″E). The rams were selected out of 19 available rams based on the freezability of their semen. During a 3 months preliminary period, the weekly ejaculates of the 19 rams were evaluated concerning total and progressive motility and percentage of morphological abnormalities after freezing-thawing. Thawed semen having total motility ≥45%, vigor progressive motility ≥3.5 (scale 0–5) and morphological abnormalities ≤5% was considered good freezable. During the experimental period, the animals were under invariable controlled feeding and housing conditions. A clinical examination of the rams was performed weekly to confirm their health status.
The main experimental period lasted over 1 year. A total of 156 ejaculates, 26 from each ram were used. All the selected rams were weekly semen collected by an artificial vagina. The ejaculates of three rams were used for the experimental purposes once a week, while the remaining ejaculates were used for the routine duties of the Institute. As a result each ram was used as semen donor for freezing experiments once every 2 weeks.
Semen processing
The collected semen samples were immediately extended 1:1 v/v by the extender A (37 °C) and transferred to the laboratory. After that, they were evaluated for motility parameters and concentration by a phase contrast microscope (200×) equipped with a heated plate (37 °C) and by means of a Neubauer haemocytometer, respectively. If the samples fulfilled the quality criteria of total motility ≥70%, vigor progressive motility ≥3.5 and a minimum concentration of 1 × 109 spermatozoa/ml they were further processed. From each sample, a volume with the same number of spermatozoa was removed and was used to prepare the final pooled semen sample. Thus, the pooled sample contained the same number of spermatozoa per used ram, to avoid any individual effect. Finally, the sample was diluted by extender A (25 °C) to a final concentration of 1.6 × 109 spermatozoa/ml and was transferred to the freezer at 5 °C. The pooled sample was cooled to 5 °C within 1 h and was separated in three equal aliquots. A second dilution was performed for each aliquot at 5 °C. The first aliquot was diluted by extender B, the second by extender Q and the third by extender T, creating the groups of control frozen semen (group C), frozen semen plus quercetin (group Q) and frozen semen plus α-tocopherol (group T), respectively. The addition of the glycerol-extenders was performed slowly (20–30 min), to a final concentration of 7% glycerol in semen. After the second dilution, the samples were equilibrated at 5 °C for 120 min. Straws of 0.25 ml volume (Minitub, Germany) were filled and were further processed for freezing using three different methods.
Semen freezing methods
All semen groups were frozen using three major methods; slow freezing rate, fast freezing rate and liquid nitrogen vapor straws’ plunging. The first two methods were performed in a programmable freezer (Digicool 5300; IMV, France). In the latter case, the straws were placed 4.5 cm above the level of the liquid nitrogen for 15 min and then they were plunged into a liquid nitrogen tank for storage (Bucak et al. 2007). In the programmable freezer, a slow (−3 °C/min from +5 to −8 °C; −25 °C/min from −8 to −130 °C) and a fast (−6 °C/min from +5 to −8 °C; −50 °C/min from −8 to −130 °C) freezing rate were applied. Thereafter, the straws were also plunged directly into liquid nitrogen (−196 °C).
Frozen semen thawing: incubation processing
Two straws from each group were thawed at 50 °C for 9 s in a water bath. Semen quality evaluation was performed immediately after thawing using the first straw, as well as, 3 h later, after incubation of the second straw in a water bath at 37 °C.
Semen evaluation
All semen samples were evaluated for the same parameters at three time points: (a) time 1, before the cooling and freezing process, as pooled semen diluted with extender A (25 °C) to a final concentration of 1.6 × 109 spermatozoa/ml, (b) time 2, immediately after thawing and (c) time 3, incubated semen in a water bath at 37 °C, 3 h after thawing.
Evaluation of motility
Sperm motility was subjectively evaluated by a phase contrast microscope (200×) equipped with a heated plate (37 °C). A 20 μl drop of semen was placed on a pre-warmed slide tempered, was covered with a coverslip and spermatozoa with fast straight movement were evaluated on a scale from 0 to 100%. Ten different microscopic fields in each sample were observed. The mean of the ten estimations was used as the final motility score.
Evaluation of viability and acrosomal status
Viability and acrosome integrity were assessed by eosin-nigrosin double staining method (Ramón et al. 2013). Briefly, a 5 µl drop of semen and a 10 µl drop of eosin-nigrosin solution were mixed in a tube of 1 ml volume. The tube was placed in a water bath at 37 °C for 3 min and a smear was prepared and allowed to air dry. Viability was assessed by counting 400 cells under phase-contrast at (1000×). Spermatozoa that displayed purple staining were considered dead. Depending on acrosome status, spermatozoa were classified as live with intact acrosome (IL) and live with reacted acrosome (ARL). All the aforementioned parameters were expressed as percentages.
Hypo-osmotic swelling test (HOST)
The hypo-osmotic swelling test (HOST) was used to evaluate the functional integrity of spermatozoa membranes. According to Paulenz et al. (2002), a hypo-osmotic solution of 100 m Osm (9 g fructose + 4.9 g sodium citrate per liter of distilled water) was prepared and used. A volume of 10 μl of each semen sample was incubated with 100 μl of the hypo-osmotic solution at 37 °C for 45 min. After incubation, 20 μl of semen was spread on a slide. Two hundred spermatozoa per slide were microscopically evaluated (1000×) and were classified as HOST positive (spermatozoa with swollen or coiled tails) or HOST negative (spermatozoa with non-swollen coiled tails). The results were expressed as percentages.
Statistical analysis
The Statistical Analysis Systems version 9.3 (SAS Institute Inc., 1996, Cary, N.C., U.S.A.) was used to conduct the analysis. For the purposes of this study, the relative changes (%) of motility, IL, ARL and HOST positive spermatozoa between time 1 and 2 and between time 2 and 3 were analyzed. Normality of the data was tested using the Shapiro–Wilk Test (PROC UNIVARIATE) and the parameters that did not follow a normal distribution were normalized by square root transformation. The least square means were analyzed with a General Linear Mixed Model (PROC MIXED). The model included the antioxidant treatment, the freezing method and their interaction as fixed effects. Pairwise comparisons were performed with the PDIFF command incorporating the Tukey–Kramer adjustment. Statistically significant difference was defined as P < 0.05.
Results
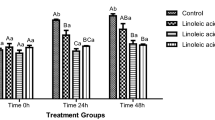
The changes of the evaluated parameters between time 1 (before cooling/freezing) and 2 (immediately after thawing) are shown in Fig. 1. A significantly higher reduction of motility was noticed in the fast freezing rate compared to the slow rate (P = 0.0008) and the LN vapors method (P = 0.03). Moreover, the relative reduction of HOST positive spermatozoa was significantly lower in the fast freezing rate compared to the slow rate (P = 0.04) and the LN vapors method (P = 0.003). There was a lower reduction of intact live spermatozoa in the slow freezing rate compared to the remaining methods (both P < 0.001). Additionally, a higher increase of acrosome reacted live spermatozoa was found for the slow freezing rate method compared to the LN vapors (P < 0.05).
Changes of the a motility, b intact membrane live spermatozoa (IL), c acrosome reacted live spermatozoa (ARL) and d positive spermatozoa in Hypo-osmotic swelling test (HOST) between time 1 (before cooling/freezing) and 2 (immediately after thawing), after the performance of different freezing methods (FM) and the addition of antioxidant agents (AT). Slow rate: −3 °C/min from +5 to −8 °C; −25 °C/min from −8 to −130 °C; Fast rate: −6 °C/min from +5 to −8 °C; −50 °C/min from −8 to −130 °C; LN liquid nitrogen; Data are presented as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.0001)
The changes of the evaluated parameters between time 2 (immediately after thawing) and 3 (3 h after thawing at 37 °C) are shown in Fig. 2. The reduction in sperm motility was as a tendency lower in the liquid nitrogen vapors compared to the other methods (both 0.10 < P < 0.05). The relative reduction of HOST positive spermatozoa was significantly higher in the fast freezing rate compared to the other methods (both P = 0.04). No statistical differences were noticed between the freezing methods regarding the relative reduction of intact live spermatozoa and the relative increase of acrosome reacted live spermatozoa (P = 0.32 and P = 0.19, respectively).
Changes of the a motility, b intact membrane live spermatozoa (IL), c acrosome reacted live spermatozoa (ARL) and d positive spermatozoa in Hypo-osmotic swelling test (HOST) between time 2 (immediately after thawing) and 3 (3 h after thawing at 37 °C), after the performance of different freezing methods (FM) and the addition of antioxidant agents (AT). Slow rate: −3 °C/min from +5 to −8 °C; −25 °C/min from −8 to −130 °C; Fast rate: −6 °C/min from +5 to −8 °C; −50 °C/min from −8 to −130 °C; LN liquid nitrogen; Data are presented as the mean ± SEM (*P < 0.05)
The addition of the antioxidants had no significant effect on any of the evaluated parameters. Moreover, no significant interactions between the freezing methods and the antioxidants addition were apparent.
Discussion
Two antioxidant agents and three freezing processes for ram semen cryopreservation were tested. The results demonstrate that the freezing method affects ram semen quality immediately after thawing, as well as, after incubation of thawed semen at 37 °C for 3 h. Fiser et al. (1991) reported that spermatozoa that maintain their motility during incubation have higher potential to keep their functionality into the female genital tract, thus support fertilization. In our study, ram semen freezing in liquid nitrogen vapors and in an automated freezer with the application of a slow rate protocol, displayed lower reduction of motility after thawing compared to a fast freezing rate protocol. However, after incubation at 37 °C for 3 h, a beneficial effect was apparent only for the semen produced using the nitrogen vapors procedure, although it was weak. These results indicate a clear disadvantage of the fast freezing rate method in the automated freezer. The motility of post thaw sperm reflects the influence of the cryopreservation process on spermatozoa’s membranes structure, metabolism and mitochondrial functionality. Semen motility is an essential and frequently used parameter for the evaluation of ram sperm’s fertilizing potential (Yániz et al. 2015). Our results agree with a previous study that reported a beneficial effect of a freezing rate of 5 °C/min from +5 to −25 °C compared to a slower one (0.5 °C/min) (Byrne et al. 2000). This beneficial effect was reflected also in vivo, since the aforementioned freezing rate resulted in a significantly higher pregnancy rate (Byrne et al. 2000). Moreover, Kumar et al. (2003) performed ram semen freezing from −5 to −50 °C in rates of −1, −30 or −50 °C/min. They found an improvement of post thaw motility after a freezing rate of −50 °C/min. However, the highest acrosome integrity was observed at −30 °C/min rate. These results demonstrate that a careful control of the cooling/freezing rate is essential for the recovery of ram spermatozoa.
The assessment of sperm viability and plasma membrane can be useful for the prediction of sperm fertilizing ability (Rodríguez-Martínez 2003). The freezing process induces damage of membranes and acrosomes affecting the fertilization capacity of spermatozoa. Resistance of spermatozoa at freezing is related with membrane properties, such as the phospholipid and cholesterol composition, the permeability and the osmotic behavior of the membrane (Sieme et al. 2015). Ram spermatozoa acrosomes are susceptible to cryopreservation process and their integrity is affected by the freezing-thawing practices (Alcay et al. 2016). In the present study, the relative reduction of intact live spermatozoa after thawing was significantly lower in the slow freezing rate compared to the other two methods. This indicates a protective effect of this protocol in the fertilizing ability of ram spermatozoa. However, live spermatozoa with altered acrosomes were also higher in the slow freezing rate method (although significantly only compared to LN vapors method), probably because of the overall higher population of live spermatozoa in this group. Similar results have been reported by Gadea et al. (2013) in goat frozen-thawed semen, who found that the semen group with the higher percentage of viable spermatozoa with intact acrosome showed the higher percentage of viable spermatozoa with altered acrosome. It is noteworthy that after 3 h of thermal incubation no further differences were noticed between the freezing methods regarding intact and reacted spermatozoa. This is indicative that in the slow rate method the live spermatozoa with altered acrosomes did not further increase, in contrary to what happened to the remaining groups. Thus, the spermatozoa in the fast freezing and in the liquid nitrogen method showed greater acrosomal instability during incubation.
Concerning HOST positive spermatozoa our results were not homogeneous across the two study time points. After thawing, the fast freezing rate method revealed a lower reduction of HOST positive spermatozoa. However, after incubation, the slow freezing rate and the liquid nitrogen methods performed better. Cryopreservation exposes spermatozoa to severe osmotic stress (Sieme et al. 2015). An optimal cooling rate is related to the membrane permeability, which in term is influenced by the properties of the used cryoprotective agent and the contents and status of the membrane (Akhoondi et al. 2011). According to Pena et al. (2004), the responsiveness of spermatozoa to osmotic challenge is strongly related to cryo-preservability. Moreover, the damaged ram spermatozoa are not functional under HOST performance, so that this test can be utilized to evaluate the quality of post thaw ram sperm (Fukui et al. 2004). Considering that the freezing process degrades semen characteristics and that fertilization is a complicated and time-consuming process which requires high quality semen, the slow freezing rate protocol applied in this study could enhance the fertilization potential.
It is well known that cryopreservation negatively affects sperm by lipid peroxidation induction (Peris et al. 2007). For this reason numerous studies have tried to preserve semen characteristics by the addition of antioxidants to the freezing extenders with divergent results. In our study, the addition of α-tocopherol and quercetin failed to supply greater protection to spermatozoa, as no significant differences between groups were observed regarding the evaluated parameters. This is in agreement with the results of Silva et al. (2012), who did not find any beneficial effect on ram frozen semen after the addition of either resveratrol or quercetin to a Tris egg yolk-glycerol extender. This study reported no effect on progressive motility and on plasma membrane integrity and found a reduced mitochondrial membrane potential after the antioxidant treatment. In contrast, Silva et al. (2013) reported that the addition of vitamin E had a positive effect on sperm plasma membrane integrity and on ram semen progressive motility, although no effect was found on acrosome integrity. However, in this study much lower concentrations of the antioxidant substance and a different freezing rate (−15 °C/min decrease from 5° to −120 °C, then storage in liquid nitrogen −196 °C) were applied. A similar to our study approach was used by Câmara et al. (2011), where the supplementation of the Tris-egg yolk extender with three different antioxidants (glutathione, superoxide dismutase or catalase) did not increase the total antioxidant capacity of semen, nor enhanced the quality of the post-thaw ram semen. Controversial results from the use of antioxidants are not uncommon, although a beneficial effect is generally expected based on their physiological properties. These results have been attributed to the studied animal species, the concentration of the antioxidants or even to the fact that the effect of antioxidants on sperm function may be reversible (Desroches et al. 2005; Gadea et al. 2013; Sikka 2004).
In conclusion, based on our results a slow freezing rate (−3 °C/min from +5 to −8 °C; −25 °C/min from −8 to −130 °C) provides higher motility and integrity of the plasma membrane of Chios ram sperm post-cryopreservation as well as after incubation. The used antioxidant agents failed to sufficiently protect sperm against cryopreservation stress; however, the choice of the appropriate freezing method could contribute to the improvement of post-thaw ram sperm quality.
References
Akhoondi M, Oldenhof H, Stoll C, Sieme H, Wolkers WF (2011) Membrane hydraulic permeability changes during cooling of mammalian cells. Biochim Biophys Acta 1808:642–648
Alcay S, Ustuner B, Nur Z (2016) Effects of low molecular weight cryoprotectants on the post-thaw ram sperm quality and freezing ability. Small Rumin Res 136:59–64
Bailey JL, Bilodeau JF, Cormier N (2000) Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon. J Androl 21:1–7
Bucak MN, Ateşşahin A, Varışlı Ö, Yüce A, Tekin N, Akçay A (2007) The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology 67:1060–1067
Byrne GP, Lonergan P, Wade M, Duffy P, Donovan A, Hanrahan JP, Boland MP (2000) Effect of freezing rate of ram spermatozoa on subsequent fertility in vivo and in vitro. Anim Reprod Sci 62:265–275
Câmara DR, Silva SV, Almeida FC, Nunes JF, Guerra MMP (2011) Effects of antioxidants and duration of pre-freezing equilibration on frozen-thawed ram semen. Theriogenology 76:342–350
Curry MR, Millar JD, Watson PF (1994) Calculated optimal cooling rates for ram and human sperm cryopreservation fail to conform with empirical observations. Biol Reprod 51:1014–1021
Desroches NR, McNiven MA, Foote KD, Richardson GF (2005) The effect of blueberry extracts and quercetin on capacitation status of stored boar sperm. Cell Preserv Technol 3:165–168
Fair S, Hanrahan JP, O’Meara CM et al (2005) Differences between Belclare and Suffolk ewes in fertilization rate, embryo quality and accessory sperm number after cervical or laparoscopic artificial insemination. Theriogenology 63:1995–2005
Fiser PS, Hansen C, Underhill L, Marcus GJ (1991) New thermal stress test to assess the viability of cryopreserved boar sperm. Cryobiology 28:454–459
Fukui Y, Togama M, Abe N et al (2004) Validation of the sperm quality analyzer and the hypo-osmotic swelling test for frozen-thawed ram and minke whale (Balaenoptera bonarensis) spermatozoa. J Reprod Dev 50:147–154
Gadea J, Gumbao D, Gómez-Giménez B, Gardón JC (2013) Supplementation of the thawing medium with reduced glutathione improves function of frozen-thawed goat spermatozoa. Reprod Biol 13:24–33
Kulaksiz R, Çebi Ç, Akcay E (2012) The effect of different extenders on the motility and morphology of ram sperm frozen or stored at 4 C. Turk J Vet Anim Sci 36:177–182
Kumar S, Millar JD, Watson PF (2003) The effect of cooling rate on the survival of cryopreserved bull, ram, and boar spermatozoa: a comparison of two controlled-rate cooling machines. Cryobiology 46:246–253
Mata-Campuzano M, Álvarez-Rodríguez M, Álvarez M, Tamayo-Canul J, Anel L, de Paz P, Martínez-Pastor F (2015) Post-thawing quality and incubation resilience of cryopreserved ram spermatozoa are affected by antioxidant supplementation and choice of extender. Theriogenology 83:520–528
Paulenz H, Söderquist L, Pérez-Pé R, Berg KA (2002) Effect of different extenders and storage temperatures on sperm viability of liquid ram semen. Theriogenology 57:823–836
Pena FJ, Johannisson A, Wallgren M, Rodriguez Martinez H (2004) Antioxidant supplementation of boar spermatozoa from different fractions of the ejaculate improves cryopreservation: changes in sperm membrane lipid architecture. Zygote 12:117–124
Pena FJ, Macías García B, Samper JC, Aparicio IM, Tapia JA, Ortega Ferrusola C (2011) Dissecting the molecular damage to stallion spermatozoa: the way to improve current cryopreservation protocols? Theriogenology 76:1177–1186
Peris SI, Morrier A, Dufour M, Bailey JL (2004) Cryopreservation of ram semen facilitates sperm DNA damage: relationship between sperm andrological parameters and the sperm chromatin structure assay. J Androl 25:224–233
Peris SI, Bilodeau JF, Dufour M, Bailey JL (2007) Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol Reprod Dev 74:878–892
Ramón M, Pérez-Guzmán MD, Jiménez-Rabadán P et al (2013) Sperm cell population dynamics in ram semen during the cryopreservation process. PLoS ONE 8:e59189
Rodríguez-Martínez H (2003) Laboratory semen assessment and prediction of fertility: still utopia? Reprod Domest Anim 38:312–318
Salamon S, Maxwell WMC (1995) Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination. Anim Reprod Sci 37:185–249
Sicherle CC, Maia MS, Bicudo SD, Rodello L, Gallego IC (2011) Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen supplemented with catalase or Trolox. Small Rumin Res 95:144–149
Sieme H, Oldenhof H, Wolkers WF (2015) Sperm membrane behaviour during cooling and cryopreservation. Reprod Domes Anim 50:20–26
Sikka SC (2004) Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl 25:5–18
Silva ECB, Cajueiro JFP, Silva SV, Soares PC, Guerra MMP (2012) Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology 77:1722–1726
Silva SV, Soares AT, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MMP (2013) Vitamin E (Trolox) addition to Tris-egg yolk extender preserves ram spermatozoon structure and kinematics after cryopreservation. Anim Reprod Sci 137:37–44
Uysal O, Bucak MN (2007) Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet Brno 76:383–390
Windsor DP (1997) Variation between ejaculates in the fertility of frozen ram semen used for cervical insemination of merino ewes. Anim Repod Sci 47:21–29
Yániz JL, Palacín I, Vicente-Fiel S, Sánchez-Nadal JA, Santolaria P (2015) Sperm population structure in high and low field fertility rams. Anim Reprod Sci 156:128–134
Zhandi M, Sharafi M (2015) Negative effect of combined cysteine and glutathione in soy lecithin-based extender on post-thawed ram spermatozoa. Cell Tissue Bank 16:443–448
Acknowledgements
This article is dedicated to the memory of our beloved colleague and friend Leonidas Vichas (PhD student, first author), who performed all the experiments and suddenly left us before the writing process. The authors would like to thank the scientific and technical staff of the Institute of Reproduction and Artificial Insemination of Thessaloniki, for their assistance to the completion of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Leonidas Vichas—deceased.
Rights and permissions
About this article
Cite this article
Vichas, L., Tsakmakidis, I.A., Vafiadis, D. et al. The effect of antioxidant agents’ addition and freezing method on quality parameters of frozen thawed ram semen. Cell Tissue Bank 19, 113–121 (2018). https://doi.org/10.1007/s10561-017-9633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9633-6