Abstract
Resistance of one host and three host ticks on cattle to amitraz was studied using samples from five diptanks in the Domboshawa Communal Land Area of Zimbabwe. A random tick profile and a questionnaire survey on the tick control practices of the area were also carried out. Engorged Rhipicephalus (Boophilus) decoloratus, Rhipicephalus appendiculatus and Amblyomma hebraeum females were randomly collected from cattle presented for dipping at the 5 diptanks and were allowed to oviposit separately at T: 28 °C and RH: 85–95%. Larvae obtained were tested for resistance against various amitraz concentrations (1–0.0078125%) using the Larval Packet Test (LPT) and were compared with susceptible reference strains of R. (B.) decoloratus (Makuti strain, 2017), R. appendiculatus (Lake Chivero strain, 2015) and A. hebraeum (Lake Mutirikwi strain, 2017). The most abundant tick species were R. (B.) decoloratus (27.2%), Hyalomma rufipes (20.0%), H. truncatum (16.0%), R. appendiculatus (12.0%) and R. evertsi evertsi (11.9%). Amblyomma hebraeum (8.6%) and A. variegatum (1.8%) were the least common in the collection; this suggests that they were not well established in Domboshawa. Low amitraz resistance (RL = I) was detected only in R. (B.) decoloratus at 2 of the 5 diptanks. In the future, decentralised tick control due to inadequate and inconsistent supply of acaricides could introduce a number of factors which could contribute towards resistance development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are obligate, haematophagous arthropods with direct and indirect effects on livestock health and production in sub-Saharan Africa (Holdsworth et al. 2006; Spickett 2007; Latif 2013; Madder et al. 2013a, b, c). By far, the most important indirect effect of ticks is the transmission of haemopathogens which cause tick-borne diseases (TBDs) that result in high livestock mortalities (De Meneghi et al. 2016). In enzootic areas including Zimbabwe, the control of TBDs is centred upon strategic tick control using acaricides and, when available, vaccination against the TBDs (Marcelino et al. 2012; Abbas et al. 2014).
Tick-borne diseases (TBDs) account for more than 65% of cattle mortalities in Zimbabwe (Sungirai et al. 2015). From November 2017 to May 2018, Zimbabwe lost an estimated 3430 head of cattle due to TBDs, with Mashonaland East Province recording the highest cattle mortalities (Chikwati 2018). The failure of acaricides, including acaricide resistance development by the tick vectors, has been suggested as possible contributing factors to the upsurge in TBDs.
Ticks of domestic animals are grouped into 3 families as follows: Ixodidae, Argasidae and Nuttalliellidae (Walker et al. 2003; Madder et al. 2013a, b, c). The Ixodidae are ixodids (hard ticks) which have a scutum or dorsal shield on their dorsal surface while Argasidae family consists of argasids (soft ticks) which lack a scutum (Walker et al. 2003; Holdsworth et al. 2006). The ixodid ticks of major veterinary significance in Zimbabwe are Rhipicephalus, Rhipicephalus (Boophilus), Hyalomma and Amblyomma species (Spickett 2007; Gono et al. 2014). Domboshawa was selected as the study area because farmers in the area were questioning the effectiveness of the amitraz used, as they claimed that many ticks, particularly blue and brown ear ticks, persisted on their livestock even after dipping. Not many local studies have been carried out to determine the acaricide resistance status of various tick species to amitraz. Ten years after the introduction of amitraz into Zimbabwe, no amitraz resistance was detected at any of the sites sampled (Bruce and Mazhowu 1995). However, Sungirai et al. (2018) detected amitraz resistance in R. (B.) decoloratus from Domboshawa using a polymerase chain reaction (PCR) assay. With the use of PCR, specific nucleotide polymorphisms (SNPs) in the octopamine/tyramine receptor gene sequence linked to amitraz resistance can be detected (Sungirai et al. 2018; Abbas et al. 2014).
Hence, the study initially established the common ixodid tick species in the area at the time of the study and investigated amitraz resistance in Rhipicephalus (Boophilus) decoloratus, Rhipicephalus appendiculatus and Amblyomma hebraeum at selected diptanks. The Larval Packet Test (LPT) was chosen as the method of choice for resistance screening because of its portability, repeatability, simplicity, affordability and potential for international standardisation and comparability (FAO 2004; Jonsson and Hope 2007).
Materials and methods
Study area
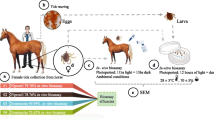
The study was conducted in Domboshawa, a typical communal land area, located in Goromonzi Rural District Council (17.8108° S and 31.3542° E), Mashonaland East Province of Zimbabwe. There are two major seasons: the hot, wet season (October to March) and the cool, dry (April to September). The mean annual rainfall in the district, most of which is received in the hot-wet season, is between 800 and 1000 mm. The district has an altitude of 1300–1550 m. There are two animal health management centres (AHMC), Parirewa and Munyawiri, each with four diptanks (Fig. 1). Each AHMC has an animal health technician and a dip attendant, who supervise animal-related activities such as dipping. According to the Animal Health (Cattle-Cleansing) Regulations, cattle dipping is compulsory in Zimbabwe (Government of Zimbabwe 1993). Dipping and inspection of cattle is done once weekly in the rainy season and once fortnightly or monthly during the dry season. The acaricide most commonly used is amitraz, applied by total immersion in a plunge dip and, occasionally, deltamethrin pour-on (Njagu 2018). The following diptanks were selected as study sites on the basis of easy accessibility, whether plunge dipping was being done or not and presence of engorged female ticks on cattle during the study period: Masikandoro, Mwenda, Runhanga, Cheza, Munyawiri and Pote.
Tick and questionnaire survey
A questionnaire survey was conducted through face to face interviews by veterinarians, dip attendants and animal health inspectors at the selected diptanks to determine the knowledge and perceptions of the farmers in the area of tick control, acaricide usage and tick-borne diseases of cattle. During the visits, adult ticks were also collected from the periocular region, ears, neck and torso including the belly, perineum and tail switch to determine the cattle tick profile for the area at the time of the study.
Reference susceptible tick populations
Susceptible laboratory reference populations are maintained at the Tick Section of the Central Veterinary Laboratory (CVL) in Harare. They were established from engorged female ticks collected from naturally infested cattle and buffalo in the refugia: R. (B.) decoloratus (Makuti strain, 2017), R. appendiculatus (Lake Chivero strain, 2015) and A. hebraeum (Lake Mutirikwi strain, 2017). These ticks are susceptible to the recommended concentrations of amitraz and were used as standard controls in the study.
Collection of suspect ‘acaricide-resistant’ field ticks
At least ten fully engorged and semi-engorged female ticks were collected using a plain thumb forceps between March and November 2018 (FAO 2004), from randomly sampled cattle at each selected diptank before dipping as follows:
-
R. (B.) decoloratus from Munyawiri, Masikandoro, Runhanga, Cheza and Munyawiri
-
R. appendiculatus from Mwenda, Pote and Masikandoro
-
A. hebraeum from Pote, Masikandoro and Munyawiri
Each species was placed separately in aerated, humidified plastic containers to limit movement and provide moisture. The plastic containers were kept away from direct sunlight in a perforated cardboard box and transported to the Tick Section, CVL for confirmation of species, oviposition, egg hatching and acaricide resistance testing.
Laboratory rearing of field tick larvae
Engorged female ticks, > 4 mm in size, were incubated as these are known to lay a sufficiently large number of eggs from which many larvae hatched for use in the acaricide resistance assays. Identified ticks were put into Petri dishes and placed in an incubator (Memmert Incubators Compressor-cooled Perfect (ICP) 400, Germany) at a relative humidity (RH) of 85–95% and a temperature (T°) of 27 °C and examined daily in these conditions until commencement of egg laying. The eggs obtained were separated and allowed to hatch in plastic tubes closed with a lid and organza fabric under the described incubation conditions. Hatched larvae were also maintained for 7–14 days in the same incubation environment to allow them to harden prior to the Larval Packet Test.
Acaricide tested
A widely used, commercially available amitraz was obtained from the manufacturer in its original liquid form, at a concentration of 99.15% (trade name: Amitic Stock Dip 50% M/M; registration number: 2003/80.16.12/9616; manufacturer: Ecomed Manufacturing (Private) Limited).
Acaricide and larval pocket preparation
Pure amitraz (99.15%) was used to make 20 ml of a 1% amitraz stock solution in olive oil/trichloroethylene (trilene) diluent. Twenty millilitres of olive oil/trilene diluent was prepared by mixing olive oil and trilene at a ratio of 1:2, respectively. The volume of the pure acaricide required to prepare the stock amitraz solution (M) was calculated using the formula: \( M=\frac{X\kern0.5em }{\%\mathrm{concentration}\ \mathrm{of}\ \mathrm{original}\ \mathrm{amitraz}}\times \frac{20}{3} \) (Adehan et al. 2016), where X is the desired 1% stock solution, 20 is the required volume (in ml) of stock solution and 3 is a constant. The volume of M was calculated as 67 μl. To prepare the 1% amitraz stock solution, diluent was added to the 67 μl of pure amitraz up to a volume of 20 ml. The stock solution was then serially diluted two-fold using the olive oil/trilene diluent at concentrations ranging from 1 to 0.0078125%.
Each acaricide concentration and the negative control (diluent only) were tested in duplicate. Using a micropipette, a volume of 670 μl of each acaricide concentration and negative control was separately applied onto Whatman filter papers, size 541 (General Electric Healthcare Life Sciences Whatman™, Chicago, IL, USA). Each impregnated filter paper was folded into half to form a pocket. To avoid cross-contamination, filter papers were impregnated and folded starting at the lowest dilution (diluent only) and ending with the highest amitraz concentration. The pockets were then dried under a hood cabinet for 2 h, with gas aspiration to the outside to permit the evaporation of trichloroethylene.
Larval packet assay
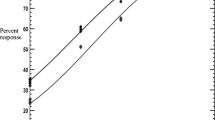
Procedures for the Larval Packet resistance test (LPT) were conducted in accordance with SOP/PAR/007 which complies with the standards recommended by FAO (FAO 2004). Approximately 100 tick larvae were transferred into each of the folded acaricide-impregnated and negative control filter paper pockets using a brush. The pockets were closed with paper clips and placed in a Memmert ICP 400 incubator (T°: 27 °C; RH: 85–95%) for 24 h. Post-incubation, live and dead larval counts were done for each test group. Each pocket was opened separately. Tick larvae were considered to be alive if they moved from one position to another on the filter paper and these were removed by sucking with a vacuum pump. Larvae that did not move or could only move their appendages without changing position were classified as dead. For each test group, dead larvae were counted and recorded for subsequent calculation of average % larval mortality.
Statistical analysis
The data from the tick and questionnaire surveys were recorded and edited in Microsoft Excel®. Acaricide resistance assay data were recorded on acaricide resistance record sheets. Larval percentage mortalities were subjected to probit analysis using IBM Statistical Package for Social Sciences (IBM® SPSS® version 21.0, Chicago, IL, USA) for calculating the lethal concentration (LC) required to kill 50% and 90% of the field and reference ticks, i.e. the LC50 and LC90 values, respectively, with their respective 95% confidence intervals (CI). Differences among the results were considered to be statistically significant when p value was < 0.05.
Calculation and characterisation of the resistance factor
Resistance factor (RF) was obtained by dividing the LC50 of the field ticks by the LC50 of the reference susceptible ticks. The resistance level (RL) in the field population of ticks was classified as follows: susceptible (RF ≤ 1.4), RL I (RF = 1.5–5), RL II (RF = 5.1–25), RL III (RF = 25.1–40) and RL IV (RF > 40) (Kumar et al. 2017).
Results
Ixodid tick species present in Domboshawa
A total of seven ixodid tick species were identified from cattle presenting at the five sampled communal diptanks in the study area. The predominant species were R. (B.) decoloratus followed by H. rufipes and H. truncatum as shown in Table 1. Other tick species found were R. appendiculatus, R. evertsi evertsi, A. hebraeum and A. variegatum. The two hyalommas, R. (B.) decoloratus, R. appendiculatus and R. evertsi evertsi abundancies were similar on cattle at all the sampled sites. Amblyomma hebraeum was found at Mwenda, Masikandoro, Munyawiri and Pote. Runhanga had A. variegatum and no amblyommas were found at Cheza.
Summary of cattle farmers’ perceptions on common ticks, TBDs, tick control methods practised and common acaricides used
According to the dip attendants in the area, the acaricide which was most commonly used during sampling was amitraz but occasionally the pour-on synthetic pyrethroid, deltamethrin, was used due to the unavailability of amitraz. The average dip strength during each dipping session was not known and dipwash strength was not measured. The acaricide classes that have been used in the area to date include organophosphates, formamidines and synthetic pyrethroids. Dip attendants mentioned that farmers were not allowed to treat their animals with acaricides outside of the stipulated dipping schedules.
A total of 151 farmers selected randomly from the study area were interviewed face to face. All farmers interviewed were able to associate high tick infestations on their cattle with the rainy season. Most (82%) of the interviewees knew the pathogenic effects of tick infestations and also were able to mention TBDs such as redwater, January disease and gallsickness as common diseases present in the study area. Approximately half (48%) of the farmers preferred synthetic pyrethroids for tick control. A larger number (72%) of the farmers viewed synthetic pyrethroids and formamidines as effective in controlling ticks. More than half (53%) of the farmers relied on the plunge dip only to control ticks while the remainder (47%) when necessary complemented the plunge dipping with other methods of tick control such as hand spraying with a knapsack and smearing with tick grease. The farmers indicated that quite often if they had to do their own tick control, they would under-dose to allow the treatment of as many animals as possible with the little acaricide they could afford.
In the previous 2 years, especially during the rainy season, 82% of the farmers interviewed claimed to have lost their cattle due to tick-related problems although not many samples were collected from dead animals for confirmatory diagnosis. Diagnosis was mostly presumptive, by local veterinary personnel and/or farmers on the basis of clinical signs shown and post-mortem findings. According to the interviewees, examination of sick cattle and visits by the local veterinary assistants were infrequent. A greater number (57%) of the farmers interviewed wanted to be educated on how to look after livestock and 21% of the interviewees mentioned genetic improvements of their livestock. Most (92%) of the livestock introduced into the area were from within the same province (Mashonaland East) and rarely from other parts of the country. More than half (58%) of the farmers claimed to have sighted wild animals in the area which included mostly hares, baboons, monkeys, duikers and warthogs.
Acaricide resistance
Regarding Rhipicephalus (Boophilus) decoloratus, the LC50 values for Masikandoro, Runhanga and Munyawiri strains were less than that for Makuti reference strain while those for Mwenda and Cheza were higher than that for the reference strain (Table 2). Rhipicephalus (Boophilus) decoloratus larvae in Mwenda and Cheza diptanks were therefore found to be resistant to amitraz (RL: I) while those from Masikandoro, Runhanga and Munyawiri were totally susceptible (RL: S). Rhipicephalus appendiculatus collected from Mwenda, Pote and Masikandoro diptanks produced enough larvae that were tested against the Chivero reference strain. Rhipicephalus appendiculatus larvae from the three diptanks were completely susceptible to amitraz (RL = S) (Table 2). Amblyomma hebraeum collected from Pote, Masikandoro and Munyawiri diptanks produced enough larvae that were tested against the Lake Mutirikwi reference strain. Amblyomma hebraeum larvae from the three diptanks were completely amitraz susceptible (RL = S) (Table 2). Generally, larval mortality increased as the concentration of amitraz increased.
Discussion
The study revealed that the tick species of veterinary significance frequently found on cattle in Domboshawa were Rhipicephalus (Boophilus) decoloratus, Hyalomma rufipes, H. truncatum, R. evertsi evertsi and R. appendiculatus while the occurrence of Amblyomma hebraeum and A. variegatum was sporadic. Rhipicephalus (B.) decoloratus, H. truncatum and H. rufipes were the most numerous, in line with previous findings (Mason and Norval 1980; Norval 1982; Anon. 2014; Sungirai et al. 2015). No Rhipicephalus (B.) microplus was found in the current study despite reports of occasional previous spread into northern inland areas (Norval 1979; Mason and Norval 1980; Katsande et al. 1996; Anon. 2014; Sungirai et al. 2017). Although R. appendiculatus and R. evertsi evertsi were found in the study, they were not as numerous as the Hyalomma ticks and R. (B.) decoloratus. Land degradation common in communal land areas which results in less grass cover significantly reduces the number of R. appendiculatus (Norval 1977; Koch 1990).
The numbers of R. e. evertsi were lower than in the more arid lowveld areas where donkeys are common because equines, including the donkey, the preferred hosts for the tick, are important in determining its abundance (Norval 1981). Indications were that Amblyomma species were still not established in the area (Kakono et al. 2003), with very few A. variegatum found at the most westerly diptank (Runhanga) while three of the other diptanks (to the east) surveyed had A. hebraeum and the two species seemed not to overlap (Norval 1983; Norval et al. 1994).
In this study, resistance level I (RL = I) to amitraz was detected in the 1-host ticks, R. (B). decoloratus populations at two (Cheza and Mwenda) of the 5 diptanks tested while all the 3-host tick populations tested, R. appendiculatus (Mwenda, Pote and Masikandoro) and A. hebraeum (Munyawiri, Pote and Masikandoro) were amitraz susceptible. At the two diptanks with resistance (Cheza and Mwenda), engorged blue ticks were also larger in size in comparison with those of the others. Sungirai et al. (2018) also detected amitraz resistance by R. (B.) decoloratus in the same area using PCR. Acaricide resistance develops faster in 1-host ticks where the larva, nymph and adult spend about 3 weeks on-host and have several generations per year than in 3-host ticks which move on-off the host and, in southern Africa, take a year or more to complete one generation (Walker et al. 2003). In Latin America, Australia and Indian subcontinent, the main cattle tick R. (B.) microplus has been found to be resistant to major classes of acaricides available to date (Rodriguez-Vivas et al. 2018 (review); Rodriguez-Hidalgo et al. 2017; Rodriguez-Vivas et al. 2014 (review)).
Various levels of resistance (including multiple resistance) to chemicals, namely, amitraz, synthetic pyrethroids and organophosphates have been detected in 1-, 2- and 3-host ticks in other parts of Africa including Zimbabwe where acaricide use on animals was less regulated and farmers were responsible for tick control on their animals (Bruce and Mazhowu 1995; Mekonnen et al. 2002; Ntondini et al. 2008; Adehan et al. 2016; Malan 2015; Vudriko et al. 2015). Since the introduction of amitraz into Zimbabwe around 1985 (Choga 2018), it has been the acaricide mostly used in communal land areas where tsetse flies are not a problem. Studies carried out by Bruce and Mazhowu (1995) did not find amitraz resistance in R. (B.) decoloratus, R. appendiculatus and A. hebraeum from both communal and commercial land areas.
The level of amitraz resistance detected was low and appears to be developing slowly. Some have interpreted this slow development of amitraz resistance by the 1-host tick to be due to its use as a single formulation in plunge dips and lower residual effect of 3–5 days (Li et al. 2005; Fernández-Salas et al. 2012). The Zimbabwe DVS still shoulders responsibility of providing farmers with acaricides and manpower to facilitate the dipping of cattle and hence a fairly high quality of acaricide has been maintained. The amitraz used (referred to as the total replacement) has a lower residual effect and is used as single formulation applied by total immersion of animals in plunge dips. Since amitraz degrades very rapidly and is inactivated by dirt, it is applied in a total replacement system to ensure the adequate chemical strength at every dipping (Irvin et al. 1996). The restricted livestock movement, with most livestock exchanges remaining intra-provincial, could also have contributed to the low level of resistance as ticks, including resistant strains, can easily be moved on livestock (Estrada-Peña and Salman 2013; Vudriko et al. 2015).
From approximately the year 2000, there has been a scarcity of acaricides and at times communal land farmers had gone for weeks to months without dipping which has resulted in TBDs outbreaks (Hargreaves et al. 2004; Anon. 2014; Njagu 2018). Farmers then resorted to carrying out their own tick control by hand spraying and applications of tick grease if they noticed high tick infestations. In the questionnaire survey, most of the interviewees mentioned deliberate underdosage to allow treatment of more animals if they had to purchase their own acaricide, concurring with findings from elsewhere where farmers are resource-constrained (Mugabi et al. 2010).
A sub-minimal dose is also not likely to achieve the required knockdown effect; hence, treatment frequencies were likely to be increased. All of the mentioned allude to more selection for resistance in the exposed ticks. When the veterinary service failed to secure amitraz for plunge dips due to shortage of hard currency, it then resorted to the use of the pour-on deltamethrin reserves destined for the less expansive tsetse fly infested area. Use of pour-on products during periods of low tick infestation is also regarded as a contributory factor towards acaricide resistance development because the products have a lengthy sub-lethal decay curve which results in tick exposure to sub-lethal doses of acaricides (FAO 2004).
In India, higher density of R. (B.) microplus ticks resistant to synthetic pyrethroids occurred where intensive cross-bred cattle are reared and deltamethrin and cypermethrin compounds were commonly used (Sharma et al. 2012). Pour-on products have an ease of application and long residual effect but tend to be expensive and are likely to attract under-dosing which contributes towards resistance development. Varying resistance to synthetic pyrethroids (flumethrin, deltamethrin and cypermethrin) was previously found in R. (B.) decoloratus populations in Zimbabwe commercial, small-scale and resettlement areas which did their own tick control but none in communal land areas (Bruce and Mazhowu 1995).
Rhipicephalus (Boophilus) decoloratus tick populations tested at two of the Domboshawa diptanks exhibited a low level resistance to amitraz while the Amblyomma hebraeum and Rhipicephalus appendiculatus showed no amitraz resistance on the Larval Packet Test. A combination of factors including under-dosing and increased frequency of treatment could be the main factors contributing towards observed resistance in the particular area. It is clear that the continued provision of heavily subsidised dipping for livestock by the DVS is unsustainable and at some point these farmers will have to take full responsibility of tick control on their animals. The DVS could play a role in farmer training on sustainable tick control strategies which also mitigate the development of acaricide resistance. There is a need for continual monitoring for the emergence of acaricide resistance which also incorporates molecular-based assays (highly specific and sensitive with low quantities of DNA and assay results out in a couple of days) besides the conventional bioassay techniques (challenges in collection of adequately engorged females and time-consuming (> 35 days)) (George et al. 2004).
Data availability
Data sharing is possible and where necessary it has to be accompanied by appropriate citation(s). New data on the resistance status of selected ticks was obtained from the study area.
References
Abbas, R.Z., Zaman, M.A., Colwell, D.D., Gilleard, J. and Iqbal Z., 2014.Acaricide resistance in cattle ticks and approaches to its management: The state of play. Veterinary Parasitology, 203: 6-20.
Adehan, S.B., Biguezoton, A., Adakal, H., Assogba, M.N., Zounrana, S., Gbaguidi, A.M., Tonouhewa, A., Kande, S., Achi, L., Kagone, H., Adehan, R., Mensah, G.A., De Deken R., Madder, M. and Farougou, S., 2016. Acaricide resistance of Rhipicephalus microplus ticks in Benin. African Journal of Agricultural Research, 11(14):1199-1208.
Anon. 2014. Director Veterinary Services presentation to Commercial Farmers Union (CFU) on Animal Health Situation. Online at: www.cfuzim.org/~cfuzimb/images/disease614.pdf. (accessed on 25 June 2018)
Bruce, D and Mazhowu, W., 1995. Synthetic pyrethroid resistance in Boophilusdecoloratus: a case report. Zimbabwe Veterinary Journal, 26(3/4): 127-131.
Chikwati, E., 2018. ‘Government intensifies control of tick-borne diseases’. The Herald, 10 July, p.3.
Choga, S., 2018. Personal communication.
De Meneghi D, Stachurski F and Adakal H., 2016. Experiences in Tick Control by Acaricide in the Traditional Cattle Sector in Zambia and Burkina Faso: Possible Environmental and Public Health Implications. Frontiers in Public Health 4:239. doi: https://doi.org/10.3389/fpubh.2016.00239.
Estrada-Peña, A. and Salman, M., 2013. Current limitations in the control and spread of ticks that affect livestock:a review. Agriculture:3,221-235;doi:https://doi.org/10.3390/agriculture3020221.
Fernández-Salas, A., Rodriguez-Vivas, R.I. and Alonso-Díaz, M.A., 2012.Resistance of Rhipicephalus microplus to amitraz and cypermethrin in tropical cattle farms in Veracruz, Mexico. The Journal of Parasitology, 98 (5): 1010-1014.
Food and Agriculture Organisation.2004.Resistance management and integrated parasite control in ruminants: guidelines. Module 1. Ticks: Acaricide Resistance: Diagnosis, Management and Prevention. FAO, Rome, Italy, pp. 25-77.
George, J.E., Pond, J.M. and Davey, R.B., 2004. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology, 129: s353-s366.
Gono, R.K., Chireshe F., Muzondiwa, J.V. and Sichewo P., 2014.Acaricide resistance in two tick species of veterinary importance in Zimbabwe. A case study of resistance to amitraz in the Mazowe district. Midlands State University Journal of Science, Agriculture and Technology, 5(1): 111-115.
Government of Zimbabwe 1993.Animal Health (Cattle-Cleansing Regulations, 1993), Statutory Instrument 250 of 1993: 1776-1777.
Hargreaves, S.K., Bruce, D. and Beffa M.L., 2004. Disaster Mitigation Options for Livestock Production in Communal Farming Systems in Zimbabwe. 1. Background information and literature review. ICRISAT, Bulawayo, Zimbabwe and FAO, Rome Italy.
Holdsworth, P.A., Kemp, D., Green, P., Peter, R.J., De Bruin, C., Jonsson, N.N., Letonja, T., Rehbein, S. and Vercruysse, J., 2006.World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of acaricides against ticks (Ixodidae) on ruminants. Veterinary Parasitology, 136:29-43.
Irvin, A.D., McDermott, J.J. and Perry, B.D., 1996. Epidemiology of ticks and tick-borne diseases in eastern, central and southern Africa. Proceedings of a workshop.Publisher: ILRI (aka ILCA and ILRAD). ISBN: 9291460168, 9789291460168, 174 pages.
Jonsson, N.N. and Hope, M., 2007.Progress in the epidemiology and diagnosis of amitraz resistance in the cattle tick Boophilusmicroplus. Veterinary Parasitology, 146 (3):193-198.
Kakono, O., Hove, T., Geysen, D. and Mahan, S., 2003. Detection of antibodies to the Ehrlichia ruminantum MAP1-B antigen in goat sera from three communal land areas of Zimbabwe by an indirect enzyme linked immunosorbent assay. Onderstepoort Journal of Veterinary Research, 70: 243-249.
Katsande, T.S., Mazhowu, B., Turton, J.A., Munodzana, D., 1996. Babesia bovis case reports and the current distribution of Boophilusmicroplus in Zimbabwe. Zimbabwe Veterinary Journal 27: 33-36.
Koch, H.T., 1990. Aspects of the epidemiology of January disease (Theileria parva bovisinfection) in Zimbabwe. PhD thesis in Veterinary Epidemiology.University of Utretcht, 122.
Kumar S.S., Rayulu, V.C., Rao, K.S., Kumar, N.V., 2017. Acaricidal resistance in Rhipicephalus (Boophilus) microplus ticks infesting cattle of Andhra Pradesh. Journal of Entomology and Zoology Studies, 5(6): 580-584.
Latif, A.A., 2013. IIIustrated guide to identification of African tick species: Ticks and tick-borne diseases monograph 2. 1sted, Agri- Connect (Pvt) Ltd, Pretoria.
Li, A.Y., Davey, R.B., Miller, R.J. and George, J.E., 2005. Mode of inheritance of amitraz resistance in a Brazilian strain of the southern cattle tick, Boophilusmicroplus (Acari: Ixodidae). Experimental and Applied Acarology, 37: 183-198.
Madder, M., Horak, I. and Stoltsz, H., 2013a. Ticks- Amblyomma, University of Pretoria. Afrivet, OER Africa and OIE, online at:www.afrivip.org/sites/default/files/Ticks-importance/amblyomma.html.accessed on 6 August 2018.
Madder, M., Horak, I. and Stoltsz, H., 2013b. Ticks- Hyalomma, University of Pretoria. Afrivet, OER Africa and OIE, online at: http://www.afrivip.org/sites/default/files/Ticks_identification/ixod_hyal.html.accessed on 20 April 2020.
Madder, M., Horak, I. and Stoltsz, H., 2013c.Ticks- Rhipicephalus, University of Pretoria. Afrivet, OER Africa and OIE, online at: www.afrivip.org/sites/default/files/Ticks-importance/rhipicephalus.html.accessed on 6 August 2018.
Malan R., 2015. Acaricide Resistance inRhipicephalus (Boophilus)species at a communal dipping system in the Mnisi Community, Mpumalanga Province, University of Pretoria.
Marcelino, I., Martino de Almeida, A., Ventosa, M., Pruneau, L., Meyer, D.F., Martinez, D., Lefrançois, T., Vachiéry, N. and Coelho, A.V., 2012. Tick-borne diseases in cattle: Applications of proteomics to develop new generation vaccines. Journal of proteomics, 75: 4232-4250.
Mason, C.A. and Norval, R.A.I., 1980.The ticks of Zimbabwe. I. The genus Boophilus. Zimbabwe Veterinary Journal, Volume 2 (3-4): 36-43.
Mekonnen, S., Bryson, N.R., Fourie, L.J., Peter, R.J., Spickett, A.M., Taylor, R.J., Strydom, T. and Horak, I.G., 2002.Acaricide resistance profiles of single and multi-host ticks from communal and commercial farming areas in the Eastern Cape and North-west Provinces of South Africa. Onderstepoort Journal of Veterinary Research, 69 (2):99-105.
Mugabi, K.N., Mugisha, A. and Ocaido, M., 2010. Socioeconomic factors influencing the use of acaricides on livestock: a case study of the pastoralist communities of Nakasongola District, Central Uganda. Tropical Animal Health & Production 2010, 42 (1): 131-6.doi: https://doi.org/10.1007/s11250-009-9396-6.
Njagu, S., 2018. Personal communication.
Norval, R. A. I., 1977. Tick problems in relation to land utilization in Rhodesia. Rhodesia Veterinary Journal, 8: 33-38.
Norval, R. A. I., 1979. Tick infestations and tick-borne diseases in Zimbabwe-Rhodesia. Journal of the South African Veterinary Association, 50: 289-292.
Norval, R.A.I., 1981. The ticks of Zimbabwe.Ill. Rhipicephalus evertsi evertsi. Zimbabwe Veterinary Journal, 12:31- 35.
Norval, R.A.I., 1982. The ticks of Zimbabwe. IV. The genus Hyalomma. Zimbabwe Veterinary Journal, 13: 2-10.
Norval, R.A.I., 1983.The ticks of Zimbabwe. VII. The genus Amblyomma. Zimbabwe Veterinary Journal, 14: 3-18.
Norval, R.A.I., Perry, B.D., Meltzer, M.I., Kruska, R.L. and Booth, T.H., 1994. Factors affecting the distributions of the ticks Amblyommahebraeum and A. variegatum in Zimbabwe: implications of reduced acaricide usage. Experimental and Applied Acarology, 18: 383-407.
Ntondini, Z., van Dalen, E. M. S. P. and Horak I. G., 2008. The extent of acaricide resistance in 1-, 2- and 3-host ticks on communally grazed cattle in the eastern region of the Eastern Cape Province, South Africa. Journal of the South African Veterinary Association, 79(3): 130–135.
Rodriguez-Hidalgo, R., Perez-Otanez, X., Garces-Carrera, S., Vanwambeke, S.O., Madder, M. and Benitez-Ortiz, W., 2017.The current status of resistance to alpha-cypermethrin, ivermectin and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PloS ONE, 12(4): 1-15.
Rodriguez-Vivas, R.I., Perez-Cogollo, L.C., Rosado-Aguilar, J.A., Ojeda-Chi, M.M., Trinidad-Martinez, I., Miller, R.J., Li, A.Y., Perez de Leon, A., Guerrero, F. and Klafke, G., 2014.Rhipicephalus (Boophilus) microplus resistant to acaricides and ivermectin in cattle farms of Mexico. Brazilian Journal of Veterinary Parasitology, 23(2): 113-122.
Rodriguez-Vivas, R.I., Jonsson, N.N. and Bhushan, C., 2018.Strategies for the conventional control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitology research, 117:3-29.
Sharma, A.K., Rinesh Kumar, R., Kumar, S., Nagar, G., Kumar Singh, N., Singh Rawat, S., Dhakad, M.L., Rawat, A.K.S. and Ray, D.D. and Ghosh, S. 2012. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Veterinary Parasitology, 188: 337-345.
Spickett, A.M., 2007.Ixodid ticks of major economic importance and their distribution in South Africa. 1sted, Agricultural Research Council-Onderstepoort Veterinary Institute (ARC-OVI), pp 30-39.
Sungirai, M., Madder, M., Moyo, D.Z., De, C.P., Abatih, E.N., 2015. An update on the ecological distribution of the Ixodidae ticks in Zimbabwe. Experimental Applied Acarology, 66:269–280.
Sungirai, M., Abatih, E.N., Moyo, D.Z., Clercq, P.D. and Madder, M., 2017.Shifts in the distribution of ixodid ticks parasitizing cattle in Zimbabwe. Medical andVeterinary Entomology, 31(1):78-87.
Sungirai, M., Baron, S., Moyo, D.Z., De Clercq, P., Maritz-Olivier, C. and Madder, M., 2018. Genotyping acaricide resistance profiles of Rhipicephalus microplus tick populations from communal land areas of Zimbabwe. Ticks and tick-borne diseases, 9(1): 2-9.
Vudriko, P., Okwee-Acai, J., Tayebwa, D.S.,Byaruhanga, J., Kakooza, S., Wampande, E., Omara, R., Muhindo, J.B., Tweyongyere, R., Owiny, D., Hatta, T., Tsuji, N., Umemiya-Shirafuji, R., Xuan, X., Kanameda, M., Fujisaki, K. and Suzuki, H., 2015. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasites and Vectors, 9:4.
Walker, A.T., Bouattour, A., Camicas, J.L., Estrada-Peña A., Horak, I.G., Latif, A.A., Pegram, R.G. and Preston, P.M., 2003. Ticks of Domestic Animals in Africa: a Guide to Identification of Species. Bioscience Reports, Edinburgh, pp 1-27.
Acknowledgements
We would like to thank Professor G.D. Vassilev from University of Zimbabwe, Faculty of Veterinary Science for support during the project. Our deepest gratitude also goes to Mr. N. Sakudawe, Mrs. S. Majonga and Mr. C. Jiti at Central Veterinary Laboratories, Tick Section for technical work assistance. We also want to thank Mr. Muwirimi and Mrs. Jaji from Domboshawa for fieldwork assistance.
Author information
Authors and Affiliations
Contributions
Francis Taenda Makuvadze was the project leader who designed the study, collected data, conducted the experimental work, analysed data and wrote the manuscript. Thokozani Hove was responsible for the design of the project, data analysis and writing and editing the manuscript. Pious Makaya and Emily Waniwa participated in the design of the study and edited the final manuscript. Tinotenda Nemaungwe participated in the study design and assisted during experimental work done. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval to collect ticks from cattle and conduct the acaricide resistance assays was obtained from the Ethical and Higher Degrees committees of the Faculty of Veterinary Science, University of Zimbabwe and the Department of Veterinary Services (DVS), Zimbabwe and the Department of Veterinary Services (DVS), Zimbabwe, Reference number VEHDC 2018/03. The purpose of the study was well explained to the veterinary department employees and farmers during the questionnaire survey and tick collection from cattle was with the farmers’ consent.
Consent for publication
All authors agreed to have the findings of this research published.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makuvadze, F.T., Hove, T., Makaya, P. et al. Resistance of ticks on cattle to amitraz in Zimbabwe. Trop Anim Health Prod 52, 3323–3330 (2020). https://doi.org/10.1007/s11250-020-02364-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02364-w