Abstract
The current study investigated the protective efficacy of a formalin-inactivated infectious bronchitis virus (IBV) vaccine derived from the field strain KP729422, which exhibits low S1 spike protein sequence homology (77.1–79.8%) with the currently used vaccine strains in Egypt. Two-week-old, specific-pathogen-free chickens were subcutaneously inoculated with a single dose of the vaccine containing 106.7 50% embryo infective dose (EID50) of the inactivated virus. At 6 weeks of age, the chickens were challenged with 104 EID50 of the same virus strain via the oculonasal route. In comparison with the unvaccinated challenged group, the vaccinated chickens had significantly higher IBV-neutralizing antibody titers and exhibited efficient protection against challenge on the basis of tracheal ciliary activity. However, the challenge virus was recovered from the kidneys and tracheas of these chickens at rates of 40% and 60%, respectively. These findings suggest that a single application of the vaccine may provide sufficient clinical and respiratory protection, but may not ensure complete protection against infection by the challenge virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious bronchitis virus (IBV), an avian gammacoronavirus, causes an acute, extremely contagious, and economically devastating disease of chickens (Hassan et al. 2016). IBV primarily infects the epithelial cells of the respiratory airways and frequently causes loss of the ciliated tracheal cells, which predispose to secondary bacterial diseases and mortality (Vandekerchove et al. 2004; Cook et al. 2012). IBV also targets the ciliated epithelial cells in the reproductive and urinary tracts (Raj and Jones 1997). IBV infection is characterized by respiratory symptoms including tracheal rales, coughing, sneezing, gasping, and nasal discharge. Nephropathogenic IBV strains cause severe renal damage, urolithiasis, and increased mortality (Brown et al. 1987).
The S1 subunit of the spike (S) glycoprotein of IBV carries serotype-specific determinants and thus is the major inducer of neutralizing monoclonal antibodies (Cavanagh et al. 1992). Mutations or recombinations of the S1 gene are largely responsible for the continual emergence of new IBV variants worldwide (Jackwood et al. 2012). Both live attenuated and killed IBV vaccines are universally applied in the control of the disease. The control of IBV infection is mainly based on the development of vaccines from locally prevailing IBV variants (Umar et al. 2016). The protection gained by IBV vaccines is mainly assessed on the basis of clinical protection, ciliary activity, and tissue recovery of the challenge virus (Darbyshire 1985; Hodgson et al. 2004).
Since 1954, increasing prevalence of IBV infection has been recorded in Egypt (Abdel-Moneim et al. 2012). The disease is mainly controlled by vaccination using the Massachusetts strain (Khataby et al. 2016). However, despite the widespread use of vaccines, frequent outbreaks still occur in vaccinated broiler flocks at various locations (Abd El Rahman et al. 2015; Zanaty et al. 2016; Abdel-Sabour et al. 2017; Magouz et al. 2018). This partial or complete vaccination failure may be due to S1 differences between the prevailing local IBV variants and the vaccine strain used.
Differences in the S1 sequence as small as 2–3% (10–15 amino acids) may be sufficient to alter the serotype, and those as small as 5% (27 amino acids) can contribute to poor cross-protection between vaccines and field strains (Cavanagh et al. 1997). Thus, the level of cross-protection can largely be predicted based on the degree of S1 homology between IBV strains, and the best protection is afforded by vaccine strains that are homologous to the emerging IBV variant (Liu et al. 2009).
In our previous investigation (Abdel-Sabour et al. 2017), the IBV strain KP729422 was recovered from a vaccinated broiler flock and exhibited low S1 homology (77.1–79.8%) with the following vaccine strains: H120, MA5, CR88, 4/91, and M41. Thus, the present study aimed to evaluate the protection offered by a formalin-inactivated IBV vaccine against challenge with the field strain KP729422.
Materials and methods
Virus
The KP729422 IBV strain was recovered in 2013 from a commercial broiler flock raised in the Giza governorate. The flock had a history of renal and respiratory disorders despite having been vaccinated with the H120 vaccine strain. The isolate was identified and genotyped as previously described (Abdel-Sabour et al. 2017).
Killed vaccine preparation
The KP729422 IBV strain was propagated by inoculation into the allantoic cavity of 9-day-old, specific-pathogen-free (SPF) chicken embryonated eggs (Kom Oshim farm, Fayoum, Egypt). The infected allantoic fluids were harvested, and the 50% embryo infective dose (EID50) was calculated as previously described (Reed and Muench 1938). The virus was then inactivated using formalin at a final concentration of 0.1% (v/v). The preparation was then mixed thoroughly and incubated at 25 °C for 20 h. The residual infectivity of the virus was checked in SPF chicken embryos. Two blind passages were conducted to ensure that the virus had been successfully inactivated. A water-in-oil emulsion vaccine was prepared by adding the aqueous antigenic phase (inactivated antigen) to Montanide™ ISA 70 VG (SEPPIC, Paris, France) at a ratio of 3:7 (v/v), according to the manufacturer’s instructions. The sterility of the vaccine was checked by inoculation onto different microbiological media. For safety testing, a double dose of the vaccine was injected subcutaneously into ten 21-day-old SPF chickens. For 21 days after this vaccination, the inoculated chickens were observed for any abnormal reactions, as previously described (Berry 1965).
Vaccination of chickens and virus challenge
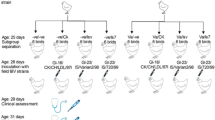
Sixty 14-day-old SPF chickens were used to evaluate the efficacy of the prepared vaccine. The chickens were randomly divided into three groups of 20 birds each: the chickens in group I were injected subcutaneously (into the back of the neck) with approximately 106.7 EID50 of the inactivated virus preparation, while those in groups II and III were kept unvaccinated. Four-week post-vaccination, the chickens in groups I and II were challenged with the same virus strain used in vaccine preparation. A dose of 104 EID50 was administered via the oculonasal route. Meanwhile, chickens in group III were kept as a negative control.
Serological evaluation
Serum samples were collected from five randomly selected chickens in each group on days 0, 7, 14, 21, 28, 35, and 42 post-vaccination. The collected sera were inactivated at 56 °C for 30 min then stored at − 20 °C until use. Protective humoral immunity was determined as previously described (Cowen and Hitchner 1975). Briefly, the inactivated sera were passed through a series of twofold dilutions. Each serum dilution was mixed with the same volume of virus dilution containing 100 EID50/0.1 ml and incubated at 37 °C for 1 h. The virus-serum mixtures were then inoculated into the allantoic cavities of five 9-day-old SPF chicken embryos. Seven-day post-inoculation, the embryos were observed for characteristic IBV lesions, such as curling, stunting, and urates in the kidneys. Neutralizing antibody titers were calculated as previously described (Reed and Muench 1938) and expressed as the reciprocal log2 of the highest reactive dilution.
Evaluation of post-challenge protection
Following challenge, the chickens were monitored daily for any clinical signs of IB infection. Five days post-challenge, five chickens from each group were euthanized by cervical dislocation and necropsied. The kidneys and tracheal swabs were collected and separately processed. IBV re-isolation was attempted through inoculation of the supernatant fluid of each sample into three 9-day-old SPF chicken embryos. Seven-day post-challenge, the ciliary activity in the tracheas of five chickens from each group was assessed as previously described (Cook et al. 1999). Briefly, the tracheas were removed aseptically and immediately immersed in tracheal organ culture medium (Eagle’s serum-free minimum essential medium with glutamine) containing streptomycin (50 mg/ml) and penicillin (50 IU/ml). After washing the tracheas with Eagle’s basal medium, thin rings were prepared using a surgical blade. From each trachea, ten transverse rings (three from the upper and lower parts of the trachea, and four from the middle portion) were examined for ciliary activity using a low-power microscope. The lesions in each ring were scored as follows: 4 (total ciliostasis), 3 (25% of the cilia were beating), 2 (50% of the cilia were beating), 1 (75% of the cilia were beating), and 0 (all cilia were beating). Thus, each chicken was graded on a scale of 0–40 and recorded as protected if the score was less than 20. The percentage protection score was calculated using the following formula: (1 − mean ciliostasis score for vaccinated challenge group/mean ciliostasis score for corresponding unvaccinated challenge group) × 100. Higher scores indicated greater protection conferred by the vaccination trial.
Statistical analysis
One-way analysis of variance was used to determine whether the serum-neutralizing antibody titers differed significantly among the vaccinated chickens. In addition, the chi-square test was used to assess whether virus detection rates and ciliostasis scores differed significantly among the groups. A p value of < 0.05 was considered statistically significant.
Results
Sterility and safety assessment
After inoculation onto nutrient agar, nutrient broth, and Sabouraud’s dextrose agar, the prepared vaccine was found to be sterile and free of bacterial or fungal contamination. The safety of the prepared vaccine was further assessed in ten 21-day-old SPF chickens. None of the inoculated chickens showed any noticeable local or systemic reactions over a period of 3 weeks.
Serological monitoring
The protective serum neutralizing antibodies were recognized 1 week post vaccination at a mean titer of 1.5 log2. The titers continued to rise subsequently, reaching a mean titer of 6.17 log2 at 6 weeks after vaccination (Fig. 1). Non-significant differences (p > 0.05) in antibody titers were detected among the vaccinated chickens. As expected, none of the chickens in group III showed any IBV-specific humoral response.
Post-challenge protection
Following IBV challenge, chickens in group II (unvaccinated challenged) showed mild depression and respiratory symptoms. Necropsy revealed tracheas congested with caseous exudates, turbid air sacs, and urate deposition in the renal tubules. Meanwhile, chickens in groups I (vaccinated challenged) and III (unvaccinated unchallenged) had no detectable clinical signs or gross lesions. No deaths were recorded in any of the three groups during the entire experimental period.
The degree of protection was evaluated on the basis of attempted recovery of the challenge virus from the kidneys and tracheas, as well as assessment of the tracheal ciliary activity (Table 1). The challenge virus was detected in the kidneys and tracheas of chickens in group I (vaccinated challenged) at rates of 40% and 60%, respectively. Meanwhile, the virus was detected in the kidneys and tracheas of all chickens in group II (unvaccinated challenged). As expected, no virus was recovered from chickens in group III (unvaccinated unchallenged). The protection rate was recorded as the percentage of IBV-negative chickens in each group; 40% of the vaccinated challenged chickens, 0% of the unvaccinated challenged chickens, and 100% of the unvaccinated unchallenged chickens were protected.
On the basis of ciliary activity, the vaccinated chickens (group I) had a mean ciliostasis score of less than 20 and a protection score of 68.72%. Meanwhile, the unvaccinated chickens (group II) exhibited extensive ciliostasis when compared with the negative control group (group III). The level of protection against challenge differed significantly between the vaccinated and unvaccinated groups (p < 0.05).
Discussion
IB continues to be a major threat to the developing commercial poultry industry worldwide, because the virus continually evolves and new IBV variants emerge. In addition, the currently used mass vaccines cannot provide adequate protection against most of the newly emerging variant strains (De Wit et al. 2011). Despite widespread vaccination in Egypt, novel IBV variants continue to circulate among vaccinated broiler flocks. The IBV strain employed in the current study, KP729422, was recovered from a vaccinated broiler flock with the H120 vaccine (Nobilis; Intervet, Boxmeer, The Netherland) and exhibited more than 20% S1 amino acid differences with the currently used H120 vaccine strain, GenBank accession number GU393335 (Abdel-Sabour et al. 2017). It has previously been supposed that the S1 amino acid differences of as little as 2–5% (10–27 amino acids) can alter the serotype and contribute to poor cross-protection between vaccines and field strains (Cavanagh et al. 1997). These findings clearly indicate that the KP729422 strain and H120 vaccine strain does not belong to the same protectotype, and necessitate the development of a homologous vaccine for induction of complete protection (Liu et al. 2009). Thus, the present study aimed to evaluate the level of protection induced by a formalin-inactivated IBV vaccine derived from the KP729422 field strain emulsified with Montanide™ ISA 70 VG oil adjuvant.
The protection conferred against homologous challenge was assessed on the basis of absence of clinical signs, presence of normal ciliary activity, and failure to recover the challenge virus from the kidney and trachea (Darbyshire 1985). In comparison to the unvaccinated chickens, a single inoculation of the prepared vaccine induced a significant increase in IBV-neutralizing antibody titers during the experimental period (Fig. 1), also none of the vaccinated challenged chickens displayed any clinical signs or gross IB lesions. In addition, on the basis of ciliary activity, the vaccinated challenged chickens had a protection score of 68.7%, and each individual chicken had a ciliostasis score of less than 20 (Table 1). These findings indicate that the vaccine afforded significant protection against tracheal damage, as suggested by Cook et al. (1999). On the contrary, Martins et al. (1991) concluded that a single dose of inactivated IBV vaccine fails to protect against loss of ciliary activity. Another experiment, conducted by Cavanagh (2003), showed low levels of protection (30–45%) against tracheal damage following a single inoculation of an inactivated M41 vaccine.
In agreement with Cavanagh et al. (1986), 60% of the vaccinated chickens were positive for the challenge virus in the kidneys, trachea, or both (Table 1). This notion corroborates previous data indicating that induced serum antibody titer is not correlated with the level of protection against challenge (Raggi and Lee 1965). Also, indicating that the virus load in the infected tissues was not great enough to induce detectable clinical signs as previously suggested (Sasipreeyajan et al. 2012). Therefore, declaring a bird as unprotected due to the considerably higher rate of virus recovery, even when ciliary activity is normal or only mildly impaired, is too stringent (Cavanagh 2003). By way of comparison, one previous study reported a mortality rate of 10% and severe pathological lesions in a group of chickens that had received an inactivated IBVSX16 vaccine and had 80% protection against infection by the challenge virus (Yan et al. 2013). Moreover, further studies are currently in progress to evaluate the probable enhancement in protection after double applications of the vaccine as previously suggested (Gough et al. 1977).
In conclusion, the present study revealed that a single application of the developed vaccine provides sufficient clinical and respiratory protection, but not complete protection, against infection by the challenge virus. The data from the present study may prompt more effective control strategies against prevailing local IBV variants.
References
Abd El Rahman, S., Hoffmann, M., Lueschow, D., Eladl, A. and Hafez, H. M., 2015. Isolation and characterization of new variant strains of infectious bronchitis virus in Northern Egypt. Advances in Animal and Veterinary, 3, 362–371
Abdel-Moneim, A.S., Afifi, M.A. and El-Kady, M.F., 2012. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Archives of virology, 157, 2453–2457
Abdel-Sabour, M.A., Al-Ebshahy, E.M., Khaliel, S.A., Abdel-Wanis, N.A. and Yanai, T., 2017. Isolation and Molecular Characterization of Novel Infectious Bronchitis Virus Variants from Vaccinated Broiler Flocks in Egypt. Avian diseases, 61, 307–310
Berry, D.M., 1965. Inactivated infectious bronchitis vaccine. Journal of comparative pathology, 75, 409–415
Brown, T.P., Glisson, J.R., Rosales, G., Villegas, P. and Davis, R.B., 1987. Studies of avian urolithiasis associated with an infectious bronchitis virus. Avian diseases, 31, 629–636
Cavanagh, D., 2003. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian pathology, 32, 567–582
Cavanagh, D., Davis, P.J., Darbyshire, J.H. and Peters, R.W., 1986. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. Journal of General Virology, 67, 1435–1442
Cavanagh, D., Davis, P.J., Cook, J.K., Li, D., Kant, A. and Koch, G., 1992. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian pathology, 21, 33–43
Cavanagh, D., Elus, M.M. and Cook, J.K.A., 1997. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathology, 26, 63–74
Cook, J.K., Orbell, S.J., Woods, M.A. and Huggins, M.B., 1999. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477–485
Cook, J.K., Jackwood, M. and Jones, R.C., 2012. The long view: 40 years of infectious bronchitis research. Avian Pathology, 41, 239–250
Cowen, B.S. and Hitchner, S.B., 1975. Serotyping of avian infectious bronchitis viruses by the virus-neutralization test. Avian diseases, 19, 583–595
Darbyshire, J.H., 1985. A clearance test to assess protection in chickens vaccinated against avian infectious bronchitis virus. Avian Pathology, 14, 497–508
De Wit, J.J., Cook, J.K. and Van Der Heijden, H.M., 2011. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathology, 40, 223–235
Gough, R.E., Allan, W.H. and Nedelciu, D., 1977. Immune response to monovalent and bivalent Newcastle disease and infectious bronchitis inactivated vaccines. Avian Pathology, 6, 131–142
Hassan, K.E., Shany, S.A., Ali, A., Dahshan, A.H.M., El-Sawah, A.A. and El-Kady, M.F., 2016. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poultry science, 95, 1271–1280
Hodgson, T., Casais, R., Dove, B., Britton, P. and Cavanagh, D., 2004. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. Journal of virology, 78, 13804–13811
Jackwood, M.W., Hall, D. and Handel, A., 2012. Molecular evolution and emergence of avian gammacoronaviruses. Infection, Genetics and Evolution, 12, 1305–1311
Khataby, K., Fellahi, S., Loutfi, C. and Mustapha, E.M., 2016. Avian infectious bronchitis virus in Africa: a review. Veterinary Quarterly, 36, 71–75
Liu, S., Zhang, X., Wang, Y., Li, C., Liu, Q., Han, Z., Zhang, Q., Kong, X. and Tong, G., 2009. Evaluation of the protection conferred by commercial vaccines and attenuated heterologous isolates in China against the CK/CH/LDL/97I strain of infectious bronchitis coronavirus. The Veterinary Journal, 179, 130–136
Magouz, A., Abdo, W., Abdelsabour, A., Elbestawy, A. and Desouky, A., 2018. Molecular and Histopathological Investigation of Avian Infectious Bronchitis Virus in the Delta of Egypt between 2016 and 2017. Pakistan Veterinary Journal, 38
Martins, N.R., Mockett, A.P.A., Barrett, A.D.T. and Cook, J.K., 1991. IgM responses in chicken serum to live and inactivated infectious bronchitis virus vaccines. Avian diseases, 35, 470–475
Raggi, L.G. and Lee, G.G., 1965. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis vaccine. The Journal of Immunology, 94, 538–543
Raj, G.D. and Jones, R.C., 1997. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathology, 26, 677–706
Reed, L.J. and Muench, H., 1938. A simple method of estimating fifty per cent endpoints. American journal of epidemiology, 27, 493–497
Sasipreeyajan, J., Pohuang, T. and Sirikobkul, N., 2012. Efficacy of different vaccination programs against Thai QX-like infectious bronchitis virus. The Thai Journal of Veterinary Medicine, 42, 73–79
Umar, S., Shah, M.A.A., Munir, M.T., Ahsan, U. and Kaboudi, K., 2016. Infectious bronchitis virus: Evolution and vaccination. World’s Poultry Science Journal, 72, 49–60
Vandekerchove, D., Herdt, P.D., Laevens, H., Butaye, P., Meulemans, G. and Pasmans, F., 2004. Significance of interactions between Escherichia coli and respiratory pathogens in layer hen flocks suffering from colibacillosis-associated mortality. Avian Pathology, 33, 298–302
Yan, F., Zhao, Y., Hu, Y., Qiu, J., Lei, W., Ji, W., Li, X., Wu, Q., Shi, X. and Li, Z., 2013. Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine. Journal of veterinary science, 14, 53–60
Zanaty, A., Arafa, A.S., Hagag, N. and El-Kady, M., 2016. Genotyping and pathotyping of diversified strains of infectious bronchitis viruses circulating in Egypt. World journal of virology, 5, 125–134
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no conflicts of interest.
Ethical statement
The experimental procedures were performed in accordance with the ethical standards applied in Veterinary Serum and Vaccine Research Institute, Abbassia, Egypt.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Ebshahy, E., Abdel-Sabour, M., Abas, O. et al. Protection conferred by a vaccine derived from an inactivated Egyptian variant of infectious bronchitis virus: a challenge experiment. Trop Anim Health Prod 51, 1997–2001 (2019). https://doi.org/10.1007/s11250-019-01898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-01898-y