Abstract
Five avian infectious bronchitis virus (IBV) isolates were isolated from broiler chickens showing respiratory and renal lesions. The isolated strains were characterized by reverse transcriptase polymerase chain reaction and sequence analysis of the hypervariable region 3 of the S1 spike glycoprotein gene. Three out of five isolates formed a distinct phylogenetic group with the Egypt/Beni-Suef/01 variant (Var 1). Two of the five isolates showed 89 and 84 % amino acid sequence identity and 89 and 88 % nucleotide sequence identity to the Egyptian variant 1 and the IS/885 strains, respectively. The Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 strains showed 15 and 20 and 12 and 18 amino acid substitutions relative to Egypt/Beni-Suef/01 and IS/885, respectively. The results indicate that Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 can be considered a new IBV variant. This study demonstrates a constant evolution of IBV in Egypt that necessitates continuous monitoring to control the spread of infections, and the development and use of vaccines based on indigenous viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Avian infectious bronchitis virus (IBV) is a positive-sense, single-stranded RNA virus belonging to the order Nidovirales, family Coronaviridae, genus Gammacoronavirus [5]. To date, dozens of different genotypes of IBV have been identified all over the world [9]. The IBV genome encodes four main structural proteins: phosphorylated nucleocapsid protein (N), membrane glycoprotein (M), spike glycoprotein (S) and small membrane protein (E) [12]. The S glycoprotein is proteolitically cleaved into two fragments, S1 and S2 [21]. Three hypervariable regions (HVRs) have been identified in the S1 subunit [8, 17, 18]. The S1 subunit induces neutralizing, serotype-specific, and haemagglutination-inhibiting antibodies [12, 21].
In spite of routine IBV vaccination, outbreaks of IB frequently occur in the field due to the presence of different serotypes as well as the emergence of multiple subtypes, generated by point mutations, insertions, deletions, or RNA recombination of the S1 genes [6, 13]. Accordingly, genotyping of IBV field strains is very important for screening the emergence of new variants as well as evaluating the existing vaccination programs. IBV strains related to the Massachusetts D3128, D274, D-08880 and 4/91 genotypes have been detected at different poultry farms in Egypt [1, 10, 20, 22]. The Egyptian variant, Egypt/Beni-Suef/01, was isolated from different poultry farms in 2001 [2] and is closely related to the Israeli variant strain.
In the current study, we present an analysis of the partial S1 gene sequences of five IBV isolates from broiler chickens in Egypt (GenBank accession numbers JX174184 to JX174188) and compare them with the Egyptian variant Egypt/Beni-Suef/01 (JX174183).
IBV was isolated from five different Governorates (Beni-Suef, Al-Fayoum, Giza, Qalubia, and Alexandria Governorates), which represent the northern and middle parts of Egypt. The chicken flocks showed signs of respiratory distresses. Mortality rates ranged from 15 to 30 %. Postmortem findings included petechial haemorrhages in the larynx and trachea, and severe congestion of the kidney. Pooled tracheal scrapings and renal homogenates were routinely processed and isolated in specific-pathogen-free 10-day-old embryonated hen eggs. Viral RNA was extracted from infected allantoic fluid of the third egg passage using a DNA/RNA extraction kit (Koma Biotech, Korea) as recommended by the supplier. One-step reverse transcriptase polymerase chain reaction (RT-PCR) (Koma Biotech, Korea) for the S1 gene of IBV was conducted on the five isolates as well as the Egypt/Beni-Suef/01 strain as described previously [4]. The five isolates as well as the Egypt/Beni-Suef/01 strain reacted positively in RT-PCR, and the isolated strains were designated Ck/Eg/BSU-1/2011 to Ck/Eg/BSU-5/2011. RT-PCR amplicons for the IBV S1 gene were sequenced directly using an ABI Prism BigDye Terminator Cycle Sequencing Kit (Applied Biosystems). A BLAST analysis was initially performed to exclude sequence redundancy with the existing GenBank entries. ClustalW analysis of selected nucleotide and amino acid sequences was conducted (http://www.genome.jp/tools/clustalw), while deduced amino acid and nucleotide sequences were used for phylogenetic analysis using MEGA 4.1.
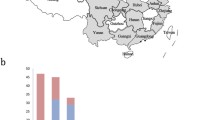
IBV has been detected in poultry in Egypt since 1954 [3]. Despite the current immunization with live attenuated vaccines, IB outbreaks have occurred frequently in chicken flocks. BLAST analysis revealed that three out of five isolates were found to be closely related to the ancestor Egypt/Beni-Suef/01 variant, while two of the five strains were found to be different form all known genotypes. Phylogenetic analysis revealed that the sequences of the recent Egyptian stains formed two main groups (Fig. 1). The first group included Egypt/Beni-Suef/01, IS/1494/06 and Ck/Eg/BSU-1/2011, Ck/Eg/BSU-4/2011 and Ck/Eg/BSU-5/2011 strains. The second group was subdivided into two subgroups: one subgroup including the Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 sequences while the second subgroup included IS/885, Sul/01/09 and IR-Razi-HKM3-2010 (Fig. 1).
Phylogenetic tree based on a partial sequence of the S1 gene, showing the relationship between different IBV isolates. The robustness of individual nodes of the tree was assessed using 1000 replications of bootstrap re-sampling of the originally aligned nucleotide sequences. Viruses isolated in the current study are in shown in blue
The Ck/Eg/BSU-4/2011 and Ck/Eg/BSU-5/2011 strains were isolated from broiler chicken flocks from Giza and Qalubia Governorates and found to be identical to each other (Table 1). Strains Ck/Eg/BSU-1/2011, Ck/Eg/BSU-4/2011 and Ck/Eg/BSU5/2011 showed high nucleotide sequence identity to each other and to the ancestor Egypt/Beni-Suef/01 variant as well as the Israeli variant IS/1494/06 (Table 1). Ck/Eg/BSU-1/2011, Ck/Eg/BSU-4/2011 and Ck/Eg/BSU-5/2011 possessed high nucleotide and amino acid sequence identity to an Egyptian isolate that was isolated in 2009 (Eg/101-ck: 99 % nucleotide identity and 100 % amino acid identity). Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 isolates showed a high degree of nucleotide sequence identity (97 %) and 94 % amino acid sequence identity to each other. These strains were isolated form Alexandria and Fayoum Governorates, which are located in the northern and in the middle part of Egypt, respectively. The sequences of these two strains were distant from all available IBV sequences in the GenBank database. The new Egyptian variants Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 differ from IS/885 by 35 and 46 nucleotide point mutations and 12 and 18 amino acid substitutions, while they differ from Egypt/Beni-Suef/01 by 43 and 51 nucleotide point mutations and 15 and 20 amino acid substitutions, respectively (Fig. 2). Interestingly, some serotypes differ in S1 by as few as 10 amino acids [7], suggesting that only a few epitopes may induce most of the VN antibody [11]. The two new isolates (Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011) clustered alone (Fig. 1) and had the genetic signature of a new variant, as they had unique substitutions compared to the remaining sequences (Fig. 2). Accordingly, they represent a new genotype variant (variant 2) that circulates in Egypt together with Egypt/Beni-Suef/01 (variant 1), which was isolated in 2001 in the Alexandria and Beni-Suef Governorates [2].
Deduced amino acid and nucleotide sequences of different Egyptian IBV variant strains compared to the ancestor strains Egypt/Beni-Suef /01 and Israeli variants. The horizontal line represents the sequence limits of HVR-3. Underlined triplet letters indicate NXT/S glycosylation motifs of the S1 glycoprotein. Dots indicate identical sequences
The genetic diversity of IBV strains arises primarily by mutations, which are induced both by the high error rate and limited proofreading capability of the viral RNA-dependent RNA-polymerase and by recombination [13]. The S1 protein commonly differs by 20 to 25 % of the amino acids among different IBV serotypes [15, 16]; however, some IBV serotypes show as little as 2 % differences in S1 [7]. The antigenic sites in the amino acid sequence of the spike sequence were determined [14]. Three HVRs are located within residues of 38-67, 91-141 and 274-387 [8, 17, 18]. The new Egyptian variant 2 isolates (Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011), possess 7 and 11 amino acid substitutions, respectively, relative to IS/885 in the HVR3 (274-387). Interestingly, Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011 also possess 7 and 11 amino acid substitutions that differ from Egypt/Beni-Suef/01 in the HVR3 (Fig. 2). Ck/Eg/BSU-3/2011 differs from the Ck/Eg/BSU-2/2011 at amino acid position 238, resulting in an S-to-C substitution and the loss of one of the N-glycosylation sites in this region. The spread of the Egyptian variants in distant Governorates in Egypt may be due to the live-poultry trade in Egypt and/or the role of wild birds in the dissemination of the IBV strains. Previous studies on IBV in wild birds have indicated a potential role of wild birds in IBV dissemination [19].
Ck/Eg/BSU-1/2011, Ck/Eg/BSU-4/2011 and Ck/Eg/BSU-5/2011 showed a W-to-R substitution at amino acid 243 (H120 numbering) from the ancestor strain Egypt/Beni-Suef/01. Interestingly, amino acid R at 243 is present in both virulent and attenuated strains of the Israeli isolate IS/1494/06. Ck/Eg/BSU-1/2011 showed a different amino acid substitution, namely, R to M at amino acid no. 355.
Our results are in accordance with the notion that IBV mutates commonly and that endemic variants 1, 2 are co-circulating in Egypt. We confirmed the existence of two Egyptian variants: variant 1, represented by Egypt/Beni-Suef/01, Ck/Eg/BSU-1/2011, Ck/Eg/BSU-4/2011, and Ck/Eg/BSU-5/2011, and variant 2, represented by Ck/Eg/BSU-2/2011 and Ck/Eg/BSU-3/2011. We have presented, for the first time, sequence data for Egyptian variant 2 (Ck/Eg/BSU-2/2011/ Ck/Eg/BSU-3/2011 isolates), revealing this to be of a previously undescribed genotype. Launching of nationwide genotyping of IBV strains is needed to determine the IBV virus genotype map and to develop and adopt suitable vaccination regimes. Further epidemiological surveillance studies are needed in order to explain the mechanism of emergence of variants and their biological properties, including pathogenicity, along with developing suitable vaccines from endemic virus strains. Continuous surveillance of new IBV strains is important for understanding the molecular evolution of different genotypes and for selecting candidate virus strains for vaccination regimes.
References
Abdel-Moneim AS, El-Kady MF, Ladman BS, Gelb J Jr (2006) S1 gene sequence analysis of a nephro-pathogenic strain of avian infectious bronchitis virus in Egypt. Virology J 3:78
Abdel-Moneim AS, Madbouly HM, Gelb J Jr, Ladman BS (2002) Isolation and identification of Egypt/Beni-Suef/01 a novel genotype of infectious bronchitis virus. Vet Med J Giza 50:1065–1078
Ahmed HN (1954) Incidence and treatment of some infectious viral respiratory diseases of poultry in Egypt. PhD thesis Fac Vet Med Cairo Univ Egypt
Capua I, Minta Z, Karpinska E, Mawditt K, Britton P, Cavanagh D, Gough RE (1999) Co-circulation of four types of infectious bronchitis virus (793/B, 624/I, B1648 and Massachusetts). Avian Pathol 28:587–592
Carstens E (2009) Report from the 40th meeting of the Executive Committee of the International Committee of Taxonomy of Viruses pp 1571–1574
Cavanagh D, Davis PJ, Cook J (1992) Infectious bronchitis virus: evidence for recombination within the Massachusetts serotype. Avian Pathol 21:401–408
Cavanagh D, Davis PJ, Cook JKA, Li D, Kant A, Koch G (1992) Location of the amino-acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol 21:33–43
Cavanagh D, Davis PJ, Mockett APA (1988) Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res 11:141–150
Cavanagh D, Naqi S (2003) Infectious bronchitis. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE (eds) Diseases of Poultry, 11th edn. Iowa State University Press, Ames, pp 101–119
El-Kady MF (1989) Studies on the epidemiology and means of control of infectious bronchitis disease in chickens in Egypt, PhD thesis. Faculty of Veterinary Medicine, Cairo University, Egypt
Hodgson T, Casai R, Dove B, Britton P, Cavanagh D (2004) Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J Virol 78:13804–13811
Holmes KV, Lai MM (2001) Coronaviridae: the virus and their replication. In: Fields BN, Knipe DM, Howley PM (eds) Fundamental virology, 4th edn. Lippincott-Raven, Philadelphia, pp 1163–1203
Jackwood MW, Hall D, Handel A (2012) Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol 12(6):1305–1311
Kant A, Koch G, van Roozelaar DJ, Kusters JG, Poelwik FA, van der Zeijst BA (1992) Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J Gen Virol 73:591–596
Keeler CL, Reed KL, Nix WA, Gelb J Jr (1998) Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S-1) gene. Avian Dis 42:275–284
Kingham BF, Keeler CL, Nix WA, Ladman BS, Gelb J Jr (2000) Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Dis 44:325–335
Koch G, Hartog L, Kant A, van Roozelaar DJ (1990) Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J Gen Virol 71:1929–1935
Moore KM, Jackwood MW, Hilt DA (1997) Identification of amino acids involved in a serotype and neutralization specific epitope within the S1 subunit of avian infectious bronchitis virus. Arch Virol 142:2249–2256
Muradrasoli S, Bálint A, Wahlgren J, Waldenström J, Belák S, Blomberg J, Olsen B (2010) Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS One 5(10):e13640
Sheble A, Sabry MZ, Davelaar FG, Burger AG, Khafagy AK, Moustafa F, Moustafa MM, Henna M (1986) Present status of infectious bronchitis in Egypt. J Egyp Vet Med Assoc 4:393–411
Stern DF, Sefton BM (1982) Coronavirus proteins: structure and function of the oligosaccharides of the avian infectious bronchitis virus glycoproteins. J. Virol 44:804–812
Sultan HA, Tantawi L, Youseif AI, Ahmed AAS (2004) Urolithiathis in white commercial egg laying chickens associated with an infectious bronchitis virus. 6th Sci Conf Egyp Vet Poult Assoc pp 155–169
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Moneim, A.S., Afifi, M.A. & El-Kady, M.F. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch Virol 157, 2453–2457 (2012). https://doi.org/10.1007/s00705-012-1445-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1445-1