Abstract
The aim of this study was to investigate the efficacy of dietary endotoxin binders [bentonite (BEN) and Saccharomyces cerevisiae cell wall (SCW)] on acute-phase protein (APP) response and liver function in cows during the transition period. Twenty-four multiparous Holstein cows were randomly assigned to one of four treatment groups. The experimental groups consisted of (1) the basal diet (BD) + SCW, (2) BD + SCW + BEN, (3) BD + BEN, and (4) BD (control). Blood samples were taken at 1, 3 and 4 weeks before and 1 and 3 weeks after parturition and serum concentrations of non-esterified fatty acids (NEFA), beta-hydroxybutyrate (BHBA), glucose, haptoglobin (Hp), serum amyloid A(SAA), albumin, g-glutamyl transferase (GGT), aspartate aminotransferase (AST), cholesterol, iron, and lipopolysaccharide (LPS) were measured. The concentrations of LPS, SAA, albumin, and Hp in the blood were within reference range at all times. The level of blood LPS was not high enough to initiate an APP response. Mean BHBA concentration was highest at 1 week after calving. For NEFA, the pattern was similar, with a peak at 1 week after calving. Cholesterol concentration was lower in the SCW group, probably due to a lower lipoprotein concentration. Mean AST concentration was highest at 1 week after calving, especially in the SCW + BEN group. The results of a current study showed that, if the carbohydrate level is not high in the diet to cause rumen acidosis, it is not profitable to supplement BEN and SCW for adsorbing endotoxins in the diet, in transition cows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transition period is defined as the last 3 weeks before parturition to 3 weeks after parturition and is described by a number of challenges for the dairy cows due to critical physiologic changes that occur during that period (Grummer 1995). These changes include a greater demand for nutrients due to the rise of performance required for milk production as well as decreased dry matter intake leading to negative energy balance (Ametaj 2005; Mulligan and Doherty 2008; Hammon et al. 2009; Mullins et al. 2012). Ketosis and hypocalcemia are key players in the transition period negative energy balance (Weaver et al. 2017). One important change is based on the fast mobilization of energy sources from tissue depots in the form of non-esterified fatty acids (Veenhuizen et al. 1991; Ingvartsen 2006; Geelen and Wensing 2006). Excessive liver uptake of non-esterified fatty acids from adipose tissues can lead to hepatic lipidosis, which makes high-producing dairy cows being susceptible, in particular (Grummer 1993; Bobe et al. 2004). Fatty liver syndrome results in impaired health, reduced immunity, and decreased reproductive performance (Zerbe et al. 2000; Grummer et al. 2004; Burton et al. 2005).
Furthermore, immediately after parturition, most cows have markedly increased plasma NEFA (non-esterified fatty acid) concentrations. A recent evidence shows that there is a poor correlation between circulating NEFA and BHBA (McCarthy et al. 2015), suggesting that ketosis is not simply the result of excessive fat mobilization. In this regard, detailed pathophysiology of ketosis is still unclear, and thus, it is of particular interest to determine why some cows are more predisposed to ketosis than others.
Some authors suggest that inflammation resulting from endotoxin circulation may play a role in the pathogenesis of metabolic diseases (Bradford et al. 2009) and that inflammatory response might be the missing link in the pathology of metabolic disorders in transition cows (Drackley 1999).
Endotoxin release during the transition period likely results from changes in rumen pH due to reduced forage to concentrate ratio in the diet of cows fed a high non-structural carbohydrate diet. This release may lead to ruminal acidosis that can either facilitate growth of Gram-negative bacteria in the rumen or their death and cell lysis (Dougherty et al. 1975; Eckel and Ametaj 2016) and consequently translocation of endotoxins from the rumen wall into systemic circulation (Emmanuel et al. 2008;Khafipour et al. 2009b). Gram-negative bacteria such as Escherichia coli can result in a greater activation of the immune system and therefore a more comprehensive transfer of blood components including IgG (Wall et al. 2016), and the study by Hernández-Castellano et al. 2016) also suggests that the immune status can influence the transfer of immune components from blood to colostrum. The liver plays an important role in absorption and detoxification of these endotoxins (Nolan 1975; Munford 1978), and decreased liver function due to hepatic lipidosis is associated with a decreased capacity for clearance of endotoxins (Andersen et al. 1996).
Acute-phase proteins (APPs) play a contributory role in this process by binding and neutralizing the endotoxins that enter into the blood circulation and bind with serum amyloid A (SAA), which is associated with lipoproteins, especially with high-density lipoproteins (Levels et al. 2001). Lipopolysaccharide (LPS) as cell wall component of Gram-negative bacteria elicits an immune response when entering the blood circulation (Berczi et al. 1966) by upregulating acute-phase proteins such as Hp (haptoglobin), SAA (serum amyloid A), and LPS-binding protein (Khafipour et al. 2009a; Plessers et al. 2015), which can be measured as markers of bovine systemic inflammation (Ceciliani et al. 2012). Furthermore, a grain-based diet has been shown to induce sub-acute ruminal acidosis leading to an increase in substances like lactate (including the d-isomer), ethanol, histamine, tyramine, tryptamine, and bacterial endotoxin (LPS) in the rumen (Plaizier et al. 2009).
Clay minerals such as bentonite are naturally derived substances that can bind toxins by trapping them between the layers of a multilayered structure, which creates a large surface area. A net positive charge between the layers in the sheet of bentonite can attract negatively charged ions and organic elements, such as LPS (Murray 2000; Spieker 2010), and bind them.
Bentonite and yeast cell wall (YCW) have both been used as inorganic and organic adsorbent additives in animal diets. Lei et al. (2013) showed that dietary supplementation of bentonite and yeast cell wall can decrease the free LPS concentration in plasma feces and digesta. Other studies have shown that yeast cells are able to bind different molecules such as killer toxins and metal ions on complex binding structures on the cell wall surface (Brady et al. 1994; Orrihage et al. 1994; Bolognani et al. 1997; Santos et al. 2000; Breierova et al. 2002). However, whether YCW can effectively bind LPS still remains unclear.
A growing body of evidence suggests that circulating LPS might play a role in disease around calving like fatty liver. Accordingly, it would be beneficial for the health of bovines to bind and eliminate these toxins. Therefore, the objective of this study was to determine the beneficial effect of dietary supplementation of endotoxin binders (bentonite and Saccharomyces cerevisiae cell wall) on APPs and liver function in high-producing dairy cows during the transition period.
Material and methods
Animals and experimental design
All animals were treated in accordance with the regulations on the guidelines of the Iranian Council of Animal Care (1995), and the experiment was approved by the Institutional Animal Care Committee for Animals Used in Research and we further followed the recommendations of the European Council Directive (86/609/EC) of November 24, 1986, regarding the standards of protecting animals used for experimental purposes. In this work, 24 high-producing Holstein dairy cows (3rd to 5th lactation) with an initial body weight (BW) of 567.5 ± 40.3 kg (mean ± SD), body condition score of 3.5 ± 0.26 out of 5 (mean ± SD), and average milk production of previous lactation of 35.8 ± 1.6 kg/day (mean ± SD) were randomly allocated to one of four experimental treatments (n = 6) in a completely randomized design during the transition period (− 28 days before the expected calving date until 21 days after calving date). The criteria for selecting them as high milk-producing (Dobson et al. 2007) was according to average milk yield of their previous lactation. The cows were selected from a 1000-cow industry dairy herd in Khorasan Razavi province in the northeast of Iran (36° 17′ 52.8″ N, 59° 36′ 20.52″ E 36.298, 59.6057, 1810 m above sea level) in summer 2016. All animals were randomly divided into four equal experimental groups containing a control (n = 6) and three treatment (n = 6) groups. Control group received a balanced ration that met all the NRC nutrient requirements based on the cow condition (National Research Council (NRC) 2001) since 4 weeks before the predicted parturition time until 3 weeks after the parturition. The treatment groups received the same ration at similar intervals; furthermore, those groups were supplemented with a toxin binder based on bentonite and Saccharomyces cerevisiae cell wall. The experimental groups consisted of (1) BD + SCW, (2) BD + BEN + SCW, (3) BD + BEN, and (4) BD (control). Experimental cows were housed in a pen with four stalls and six cows kept in each stall. After calving, experimental animals were moved to another pen near the milking parlor, which consisted of four stalls and their diet changed from close-up diet to fresh cow diet. Treatment animals had free access to water and were fed twice a day at 1000 h and 1700 h and milked twice at 0700 h and 1400 h. Treatment animals’ diet was made separately for each group and endotoxin binder was mixed every day to their diet. The cows’ places were bedded with clean wood shavings and dry manure, and the bedding was refreshed daily if necessary.
Diet
The bentonite with a particle size less than 37 μm according to manufacturer’s introduction was manufactured by Zarin Binder (Mashhad, Iran), and the Saccharomyces cerevisiae cell wall was purchased from Active MOS, Biorigin, Sao Paulo, Brazil (dry matter ≥ 94.0%, chitin ≥ 2.0, β-glucan ≥ 30.0%, and mannoprotein ≥ 20.0%). Dietary doses of endotoxins binder were based on the manufacturer’s recommendation for dairy cows (12 gr/day/cow for SCW and 150 gr/day/cow for bentonite). The total mixed ration (TMR) was formulated to meet or exceed the requirements of metabolizable protein (MP), net energy of lactation (NEL), minerals, and vitamins for close-up and fresh cows separately (National Research Council (NRC) 2001). The ingredients and chemical composition of the basal diet are detailed in Table 1.
Sampling procedure
Blood samples were obtained in the milking parlor, five times during the trial period, before the morning feeding on all cows through the tail vein in 10-mL plain tubes. Samples were taken 1 week before adding the toxin binders to the diet, 3 and 1 weeks before parturition, and 1 and 3 weeks after parturition (according to the predicted date of calving). Samples were cooled down at room temperature and serum was separated by centrifugation (Sigma 1-15p-Sigma-Laborzentrifugen, Munich, Germany) at 3000×g for 10 min and stored at − 22 °C until being assayed. In this study, serum BHBA measured by commercially available kits according to the manufacturers’ instructions by enzymatic (UV) method (Biorex Fars, Shiraz, Iran) (assay range, 0.01–0.32 mmol/L; sensitivity, 0.01 mmol/L; intra-assay, CV < 6.6%; and inter-assay, CV < 10%). NEFA was evaluated with the Biorex Fars kit (Shiraz, Iran) by calorimetric method (product code: BXC0473) (assay range, 0.01–0.4 mmol/L; sensitivity, 0.1 mmol/L; intra-assay, CV < 3.25%, and inter-assay CV < 3.47%). Glucose was evaluated with enzymatic colorimetric method (GOD-PAP) using the Biorex Fars (Shiraz, Iran) kit (assay range, 60–6500 mg/L; sensitivity 60 mg/L; intra-assay, CV < 1.75%; and inter-assay, CV< 1.86%). Albumin was measured with bromocresolgreen (BCG) method by a commercially available kit (Biorex Fars, Shiraz, Iran) (assay range, 5–60 g/L; sensitivity, 5 g/L; intra-assay, CV < 1.13%; and inter-assay, CV < 2.48%). GGT was determined with enzymatic (SZAS) method with a commercially kit (Biorex Fars, Shiraz, Iran) (assay range, 4–410 U/L; sensitivity, 4 U/L; intra-assay, CV < 2.3%; and inter-assay, CV < 2.85%). AST concentrations were determined with International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method without pyridoxal phosphate using a commercially available kit (Biorex Fars, Shiraz, Iran) (assay range, 5–300 U/L; sensitivity, 5 U/L; intra-assay, CV < 1.23%; and inter-assay CV < 1.16%). SAA was evaluated in serum by Hangzhou Eastbiopharm Co., Ltd., (Hangzhou, USA) kit (cat. no.: CK-E90546). The kit uses a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) to assay the level of bovine serum amyloid A (assay range, 0.1–40 μg/mL; sensitivity, 0.054 μg/mL; intra-assay, CV < 10%; and inter-assay, CV < 12%). Hp was determined in serum by a double-antibody sandwich ELISA with a commercially available kit (Hangzhou Eastbiopharm Co., Ltd., Hangzhou, USA) (cat. no: CK-E90126) (with assay range, 3–900 μg/ml; sensitivity, 1.36 μg/ml; intra-assay, CV < 10%; and inter-assay, CV < 12%). LPS concentration in serum was detected via limulus amebocyte lysate (LAL) test using chromogenic assay with Thermo Scientific Pierce LAL chromogenic endotoxin quantitation kit (Rockford, USA). The correlation between absorbance and LPS concentration is linear in the 0.1–1.0 EU/mL range. In this method, bacterial endotoxin catalyzes the activation of a proenzyme in the modified limulus amebocyte lysate (LAL); then, the activated proenzyme catalyzes the splitting of p-nitroalanine (pNA) from the colorless substrate. The activation rate is proportional to the sample endotoxin concentration. After stopping the reaction, the released pNA was photometrically measured at 404–410 nm. The developed color intensity is proportional to the amount of LPS present in the sample and can be calculated using a standard curve. Cholesterol concentrations were analyzed by colorimetric reactions using specific enzymatic kits according to the manufacturers’ instructions (Biorex Fars, Shiraz, Iran). Iron was determined by the photometric method with Ferene using a commercial laboratory kit (ParsAzmun, Karaj, Iran).
Statistical analyses
A repeated measures linear mixed model was used to compare the mean concentrations of different serological factors within similar weeks between four different experimental groups. The model had the serological measure as the dependent variable with the group as the independent variable and time as a repeated measure alongside the interaction between group and time. Statistical analysis was performed using SPSS software (SPSS for Windows, version 16, SPSS Inc., Chicago, IL). The level of statistical significance was set at p < (0.05).
Results
Effect of the group, time, and group × time on blood parameters
The p values from the linear mixed models of the effect of the treatment group, time, and their interaction on the mean concentrations of the measured analyzed are shown in Table 2.
LPS concentration in serum
The effect of treatment group and time on the concentration of LPS is shown in Fig. 1. Mean LPS concentrations were highest at 1 week before calving (0.48 [(95% confidence interval (CL) 0.4–0.57] endotoxin units (EU)/mL).
APP concentration in the blood
The effect of treatment group on albumin and SAA concentrations in serum are shown in Figs. 2 and 3 respectively. Mean albumin concentrations were highest at 1 week before calving (31 [(95% CL) 27–34] g/L). The mean concentration of SAA was lowest at 3 weeks after calving (6.6 [(95% CL) 6–7.1] μg/mL). No effect of the treatment group was found for Hp at any time point even though with a significant interaction.
Energy status
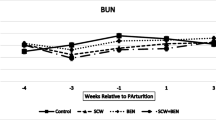
The effects of the treatment group on BHBA and NEFA concentrations are summarized in Figs. 4 and 5 respectively. Mean BHBA concentrations were highest at 1 week after calving (1.08 [(95% CL) 0.5–1.5] mmol/L), although the overall interaction between time and the treatment group was not significant at 5%. At 1 week after calving, cattle treated with SCW + BEN (2.3 [(95% CL) 2.1–2.7 mmol/L) had higher mean BHBA concentrations than control cows (0.6 [(95% CL) 0.4–0.8 mmol/L) (p = 0.056). The pattern was similar for mean NEFA concentration, with a peak at 1 week after calving but only because SCW + BEN cows had significantly elevated concentrations at that time point. The effect of the treatment group was significant only at 3 weeks before calving (p < 0.05). No effect of the treatment group was found for glucose at any time point even though with a significant interaction.
Liver enzyme activity
The effect of the treatment group on AST and GGT activity in serum are summarized in Figs. 6 and 7 respectively. There was an effect of the group only at 1 week after calving; at that time point, the mean AST activity in the group SCW + BEN was higher than the control group (p < 0.05). The overall effect of the treatment group on GGT activity was significant at the 5% (p = 0.054), with which treatment groups (22 [(95% CL) 19–26] U/L, 22 [(95% CL) 18–26] U/L and 20 [(95% CL) 17–24] U/L for SCW, SCW + BEN, and BEN groups respectively) being different from the control (26 [(95% CL) 22–30] U/L) group.
Cholesterol and iron
The effect of the treatment group and time on concentrations of cholesterol and iron are summarized in Figs. 8 and 9 respectively. Mean cholesterol concentrations in the SCW group was lowest (1194 ± [1092–1296(CL)] mg/L) in that group mean cholesterol concentrations was lower than the BEN group (p < 0.05). Mean cholesterol concentrations was lowest at 1 week after parturition (1140 ± [996–1283(CL)] mg/L). Mean Fe concentrations at 3 weeks after calving was lowest (87.6 ± [72.1–103.1(CL)] μg/dL). At that time point, the mean Fe concentration was lower than 4, 3, and 1 week before calving (p < 0.05).
Correlation between blood factors
The correlation between BHBA, NEFA, glucose, AST, and GGT are shown in Table 3. There was a statistically significant positive correlation between BHBA serum concentrations in response to NEFA serum concentrations (τ = 0.168, p < 0.05). An increase in NEFA serum concentrations was associated with an increase in AST serum concentrations (τ = 0.303, p < 0.05). Analysis of the obtained data revealed that an increase in NEFA serum concentrations was associated with a decrease in glucose serum concentrations (τ = − 0.220, p < 0.05). There was a significant negative correlation between serum glucose concentrations and AST serum concentrations (τ = − 0.286, p < 0.05) and an increase in the AST serum concentrations was associated with an increase in the GGT serum concentrations (τ = 0.283, p < 0.05).
Discussion
Emmanuel et al. (2008) reported that dairy cows on a diet containing barley grain at concentrations of 0%, 15%, 30%, and 45% of DM had LPS blood concentrations of 654, 790, 5021 and 8870 ng/mL respectively. The barley content of the diet fed in the present study was between 12 and 14.2%. In another study, dairy cows were fed a control diet containing 70% of forage and 30% mixed concentrates (DM basis), a high grain diet (38% wheat-barley pellets, 32% mixed concentrates, and 30% of forages), or a diet containing alfalfa pellets (45% of mixed concentrates, 32% of alfalfa pellets, and 23% of other forages). The author showed that the ruminal LPS concentrations were 8333; 124,566; and 18,425 EU/mL, respectively (Li et al. 2010). The experimental diet of our study contains 48.71% mixed concentrates (DM basis) for close-up cows and 60.02% mixed concentrates (DM basis) for fresh cows. Although LPS concentrations in the rumen were not measured, we expected that this diet could increase the LPS concentration in the rumen.
Magata et al. (2015) investigated the concentrations of bacterial LPS in the blood and uterine fluid of a clinical case of bovine metritis and the LPS concentrations in plasma and uterine fluid were 0.94 and 6.34 EU/mL, respectively. Gozho et al. (2007) induced sub-acute ruminal acidosis in dairy cows and measured LPS concentrations in the serum. They showed that, serum LPS concentrations in both control and SARA cows were less than the detection limit of < 0.01 EU/mL for the assay. In the present study, the mean LPS concentration in serum was 0.29 ± 0.01 EU/mL. According to our results, differences between the LPS concentration in blood in the control group and groups that received endotoxin binders was not statistically significant, probably because translocation of free ruminal LPS into the blood circulation depends on the integrity of the rumen wall. Damaged rumen epithelium can lead to a pathogen infiltration like LPS (Nordlund et al. 1995). We did not induce sub-acute ruminal acidosis; probably the rumen epithelium was not damaged to translocate LPS into the blood circulation or liver succeeded in detoxification of free ruminal LPS. Andersen (2003) reported that the liver can detoxify free ruminal LPS that translocates into the hepatic portal circulation before the LPS reaches the blood circulation. Studies by Andersen and Jarlov (1990) and Andersen et al. (1994) did not detect LPS in the peripheral blood circulation when acute acidosis was induced. LPS concentration in blood was higher in the BEN group at 1 week before calving. According to a similar level of diet concentration which treatment animals received, the elevation of the LPS concentration in the BEN group might be due to another source of endotoxin. Although there was not any sign of infection in experimental animals, they might have had a subclinical infection.
It is widely accepted that acute-phase proteins are increased in the blood when free LPS translocate from the digestive tract into circulation (lipopolysaccharide binding protein, C-reactive protein, SAA, and Hp) in dairy cows and beef cattle (Emmanuel et al. 2008; Khafipour et al. 2009c; Zebeli and Ametaj 2009).
The APP concentrations are related to the severity of the disorder and the extent of tissue damage in the affected animal; quantification of their concentration can provide diagnostic and prognostic information (Murata et al. 2004).
Major APPs in cattle that increase markedly in the acute-phase response are haptoglobin (Hp) and serum amyloid A (SAA) (Conner et al. 1986; Alsemgeest et al. 1994). Nazifi et al. (2008) showed that the concentration of Hp in clinically healthy cattle is 0.20 ± 0.03 mg/mL. Takahashi et al. (2007) reported that SAA concentrations range in healthy cows from 3–135 μg/ml with a median of 14 μg/ml. SAA plays a role in detoxification of endotoxin and it may be involved in the local defense mechanism of the gut to endotoxin challenge (McDonald et al. 2001). However, the rise in SAA concentration can be found in cows at parturition (Alsemgeest et al. 1993) or subjected to physical stress (Alsemgeest et al. 1995),suggesting that any APP response is not indicative of inflammation. Hp reduces the oxidative damage associated with hemolysis by binding to hemoglobin which also attributes to the variety of immunomodulatory effects (El-Ghmati et al. 1996; Yang et al. 2003).
Results of this study show that SAA and Hp and albumin were within the reference range during the whole study period. We suggest that an increase in APP depends on the severity of the inflammation which is in accordance with the finding by Alsemgeest et al. (1994) and Horadagoda et al. (1999). The results suggest that the levels of blood LPS through feeding the diet used in this work were low and not able to initiate an APP response.
Declined concentrations of albumin and cholesterol may be a consequence of a reduced liver synthesis of usual proteins. Accordingly, lower lipoprotein concentrations result in lower serum cholesterol. Reduced serum concentrations of albumin and cholesterol can occur during the acute-phase response when the activity of the liver starts to produce other proteins such as SAA and Hp (Bertoni and Trevisi 2013). Low plasma cholesterol is associated with disturbances of plasma amino acids and severity of the acute-phase response (Chiarla et al. 2004). In the study of Chiarla et al. (2004), cholesterol measurement for the whole study during sepsis was 3.1 ± 1.1 mmol/L which is equal to 558 mg/L. In the current study, cholesterol measurement for the whole study was 1290 mg/L which is equal to 7.1 mmol/L. The results of this study showed that cholesterol concentration was higher in the experimental cows of our study compared to Chiarla’s study during sepsis. We suggest that the acute-phase response was not stimulated in the experimental cows of this study. In addition, acute-phase proteins (albumin, SAA, and Hp) were in reference range. Cholesterol concentration was lower in the SCW group, probably due to a lower lipoprotein concentration. Cholesterol reference values in cattle are 650–2200 (mg/L) (Constable et al. 2017).
Baydar and Dabak (2014) reported a declined Fe concentration during the inflammation. Zebeli et al. (2010) showed a strong inverse relationship between rumen endotoxin and plasma concentration of Fe. Fe reference value in cattle is 570–1620(μg/L) (Constable et al. 2017). The result of our study showed that the Fe concentration decreased since 1 week before parturition. Although it might be due to inflammation, we selected healthy animals with no sign of infection. Moreover, APPs were in reference range; however, the decrease in the Fe concentration might be due to subclinical infection.
Despite the advantages of measuring of serum activities of hepatic enzymes, it also involves some limitations (Bogin et al. 1988; Sevinc et al. 1998, 1999). AST activity in serum is fairly well correlated to hepatic lipidosis, but this enzyme is nonspecific to hepatic tissue (Reid and Roberts 1983, Roussel et al. 1997). Moreover, GGT is more specific to liver tissue, but the correlation of these serum activities with hepatic lipidosis is not as high (Body et al. 1964).
Sevinc et al. (2001) showed that GGT, AST, and albumin concentrations seem to be helpful parameters for measuring liver function in cows with fatty liver. However, because of considerable individual variations of these results, they should be interpreted with caution. The AST reference range in cattle is 78–132 (units/L), for GGT is 6.1–17.4 (units/L), and for albumin is 21–36 (g/L) (Constable et al. 2017).
In this study, AST concentrations increased from 1 week before calving until 1 week after calving except in the control group and it was higher especially in the SCW + BEN group; these results might be attributed to the liver damage that was present in cows of this group. GGT concentrations increased from 1 week before calving in all four groups especially in the SCW + BEN group. Elevation of liver enzymes especially in the SCW + BEN group might be due to liver damage.
The concentration of NEFAs in blood reflects the degree of adipose tissue mobilization (Pullen et al. 1989). The BHB reference range in cattle is 0.35–0.47 mmol/L (Constable et al. 2017). High concentrations of BHBA and NEFA reflect the high level of fat mobilization. A study by Drackley (2000) suggests that normal NEFA blood concentrations are less than 0.2 mmol/L. Glucose concentrations decreased from 3 weeks before parturition in each group. Lower blood glucose concentrations should be correlated with lower insulin concentrations, leading to an increase in lipolysis and glycogenosis (Herdt 2000).
Some studies support the possible role of endotoxins released by gut flora in diseases around parturition such as fatty liver (Andersen 2003; Ametaj 2005; Ametaj et al. 2010). Ametaj et al. (2010) hypothesized that fatty liver develops as a result of a rapid removal of endotoxin particles by liver hepatocytes. SAA in association with lipoproteins especially high-density lipoprotein bounds and neutralizes endotoxins, which enter into the blood circulation. Besides, the clearance of the endotoxin-SAA-lipoprotein complex by hepatocytes results in the accumulation of triglyceride-rich lipoprotein and development of fatty liver (Ametaj et al. 2010).
In this study, BHBA and NEFA of the blood in the SCW + BEN group increased 1 week after calving even though the treatment animals received endotoxin binders. It is likely because average milk yield of the previous lactation in SCW + BEN group was higher than that of other groups. The average milk production of the previous lactation for SCW, SCW + BEN, BEN, and control groups were 34.2, 38, 35.3, and 35.7 respectively. High-producing dairy cows are more prone to production disorders around parturients (Dobson et al. 2007). Obviously, the negative energy balance was more pronounced in the SCW + BEN group at 1 week after calving, probably due to their higher milk yield and consequently higher energy demand for milk production. The LPS concentration in blood was low, and also, APPs were in the reference range. Accordingly, we suggest that if there was evidence of liver damage, it could not be due to the clearance of the endotoxin-SAA-lipoprotein complex by hepatocytes and accumulation of triglyceride-rich lipoprotein in this study.
We observed a correlation between the increase in NEFAs and BHBA and a decrease in glucose with blood GGT and AST levels. We assume that an increase in NEFAs and BHBA following an increase in energy demand results in the accumulation of triglycerides in the liver and damage to the liver can result in increased levels of liver enzymes (GGT, AST). It supports the theory that around parturition and during negative energy balance, the liver might be prone to damage due to the accumulation of excessive triglyceride in hepatocytes (Bobe et al. 2004). Further research, however, is needed to verify this theory and the effect of endotoxin binders on the performance of dairy cows.
Due to an increase of carbohydrates in diet relative to forage, the concentrations of LPS in the rumen should have increased. The pH of the rumen fluid was not measured so it is not known if the diet resulted in acute or sub-acute ruminal acidosis. Our results show that even in the control group that did not receive endotoxins binders, LPS concentrations were not elevated and the difference in LPS concentrations between groups was not significant.
In conclusion, although we did not measure the LPS concentration in feces or rumen, according to the level of concentration that we used in this study, the LPS concentration in blood was not high. So, it did not stimulate the acute-phase response and, therefore, increased the APP concentration in the blood. We think that at the level of concentration that we used in this study, it is not profitable to use endotoxin binders for adsorbing endotoxins in the diet because the LPS concentration in blood was not high to stimulate the immune system and has an impact on animals’ health even in the control group that did not receive endotoxin binders. Due to the increased cost of nutrition that we use in dairy cow diets, it is essential to evaluate benefits of ration additives. Evidence of liver damage was present in one of the experimental groups (SCW + BEN) expressed by an increase in the concentration of liver enzymes (GGT and AST). Moreover, due to the pronounced negative energy balance in that group, we suggest that they might be in some stage of fatty liver. However, according to low blood LPS, we think that, it is not due to the clearance of the endotoxin-SAA-lipoprotein complex by hepatocytes and accumulation of triglyceride-rich lipoprotein. Results of this study showed that there is no evidence of a benefit in the combined or separate feeding of bentonite and Saccharomyces cerevisiae cell wall for adsorbing endotoxins in the diet in transition cows. We suggest further research for evaluating the efficacy of endotoxin binders on cow’s health in the transition period and the role of endotoxin in development of disease around parturition like fatty liver.
References
Alsemgeest, S.P., Taverne, M.A., Boosman, R., Van Der Weyden, B.C., Gruys, E., 1993. Peripartum acute-phase protein serum amyloid-A concentration in plasma of cows and fetuses. American Journal of Veterinary Research 54, 164–167.
Alsemgeest, S.P.M., Kalsbeek, H.C., Wensing, T.H., Koeman J.P., Van Ederen, A.M., Gruys E., 1994.Concentrations of serum amyloid A (SAA) and haptoglobin (Hp) as parameters of inflammatory diseases in cattle. Veterinary Quarterly,16, 21–23.
Alsemgeest, S.P., Lambooy, I.E., Wierenga, H.K., Dieleman, S.J., Meerkerk, B., VanEderen, A.M., Niewold, T.A., 1995. Influence of physical stress on the plasma concentration of serum amyloid-A (SAA) and haptoglobin (Hp) in calves. Veterinary Quarterly 17, 9–12.
Ametaj, B.N., 2005. A new understanding of the causes of fatty liver in dairy cows. Advanced Dairy Science and Technology, 17, 97–112.
Ametaj, B. N., Q. Zebeli, and S. Iqbal. 2010. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Revista Brasileira de Zootecnia, 39:433–444. https://doi.org/10.1590/S1516-35982010001300048
Andersen, P. H. 2003. Bovine endotoxicosis—some aspects of relevance to production diseases. A review. Acta Veterinaria Scandinavica, 98:141–155. DOI: https://doi.org/10.1186/1751-0147-44-S1-P57
Andersen, P. H., Jarlov, N., 1990. Investigation of the possible role of endotoxin, TXA2, PG12 and PGE2 in experimentally induced rumen acidosis in cattle. Acta Veterinaria Scandinavica. 31:27–38.
Andersen, P. H., Bergelin, B., Christensen, K. A., 1994. Effect of feeding regimen on concentration of free endotoxin in ruminal fluid of cattle. Journal of Animal Science, 72:487–491.
Andersen, P.H., Jarløv N., Hesselholt, M., Bæk, L., 1996. Studies on in vivo endotoxin plasma disappearance times in cattle. Journal of Veterinary Medicine, A. 43, 93-101.
Baydar, E. & Dabak, M., 2014, Serum iron as an indicator of acute inflammation in cattle. Journal of Dairy Science, 97, 222-228. DOI: https://doi.org/10.3168/jds.2013-6939
Berczi, I., Bertók, L., Bereznai, T., 1966. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Canadian Journal of Microbiology, 12, 1070–1071.
Bertoni, G., and E. Trevisi. 2013. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Veterinary Clinics of North America: Food Animal Practice, 29:413–431. DOI: https://doi.org/10.1016/j.cvfa.2013.04.004.
Bobe, G., Young, J.W., Beitz, D.C., 2004. Invited review: pathology, etiology, prevention, and treatment of fatty liver in dairy cows. Journal of Dairy Science, 87, 3105–3124. DOI: https://doi.org/10.3168/jds.S0022-0302(04)73446-3.
Body, J.W., Douglas, T.A., Gould, C.M. and Grimes, F.C., 1964. The interpretation of serum enzyme assay in cattle. Veterinary Record, 76, 567-574.
Bogin, E., Avidan, Y., Meron, M., Soback, S., Brenner, G., 1988. Biochemical changes associated with the fatty liver syndrome in cows. Journal of Comparative Pathology, 98, 337-347.
Bolognani, F., Rumney, C. J., Rowland, I. R., 1997. Influence of carcinogen binding by lactic acid-producing bacteria on tissue distribution and in vitro mutagenicity of dietary carcinogens. Food and Chemical Toxicology, 35, 535–545.
Bradford, B.J., Mamedova, L.K., Minton, J.E., Drouillard, J.S., Johnson, B.J., 2009. Daily injection of tumor necrosis factor-{alpha} increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. Journal of Nutrition, 139, 1454–1456. DOI: https://doi.org/10.3945/jn.109.108233.
Brady, D., Stoll, A. D., Strake, L., Dunkan, J. R., 1994. Chemical and enzymatic extraction of heavy metal binding polymera from isolated cell walls of Saccharomyces cerevisiae. Biotechnology and Bioengineering, 44, 297–302.
Breierova, E., Vajczikova, I., Sasinkova, V., Stratilova, E., Fiserac, M., Gregor, T., et al., 2002. Biosorption of cadmium ions by different yeast species. Journal of Biosciences, 57(7-8), 634-639.
Burton, J.L., Madsen, S.A., Chang, L.C., Weber, P.S.D., Buckham, K.R., Van Dorp, R., Hickey, M.C., Earley, B., 2005. Gene expression signatures in neutrophils exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Veterinary Immunopathology, 105, 197–219.
Ceciliani, F., Ceron, J., Eckersley, P.D., Sauerwein, H., 2012. Acute phase proteins in ruminants. Journal of Proteomics, 75, 4207–4231. DOI: https://doi.org/10.1016/j.jprot.2012.04.004.
Chiarla, C., Giovannini, I., Siegel, J.H., 2004.The relationship between plasma cholesterol, amino acids and acute phase proteins in sepsis. Amino Acids, 27:97–100. DOI: https://doi.org/10.1007/s00726-004-0064-x.
Conner, J.G., Eckersall, P.D., Doherty, M., Douglas, T.A., 1986. Acute phase response and mastitis in the cow. . Research in Veterinary Science, 41, 126–128.
Constable P. D., Hinchcliff K. W., Done S. H., Grünberg, W., 2017. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goats, 11th edn., Elsevier ltd. Veterinary Medicine, 2, 2217–2219.
Dobson, H., Smith, R.F., Royal, M.D., Knight, C.H., Sheldon, I.M., 2007. The high-producing dairy cow and its reproductive performance. Reproduction in Domestic Animals, 42 (Suppl.2), 17–23.
Dougherty, R.W., Coburn, K.S., Cook, H.M., Allison, M.J., 1975. Preliminary study of appearance of endotoxin in circulatory system of sheep and cattle after induced grain engorgement. American Journal of Veterinary Research, 36(6), 831-832.
Drackley, J.K., 1999. Biology of dairy cows during the transition period: the final frontier. Journal of Dairy Science 82, 2259–2273.
Drackley, J.K., 2000. Use of NEFA as a Tool to monitor energy balance in transition dairy cows, pp. 1–3. http://www.Livestocktrail.uiuc.Cdu.uploods/dairynet/pp.
Eckel, E.F., Ametaj, B.N., 2016. Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. Journal of Dairy Science 99, 5967–5990. DOI: https://doi.org/10.3168/jds.2015-10727.
El-Ghmati, S.M., Van Hoeyveld, E.M., Van Strijp, J.G., Ceuppens, J.L., Stevens, E.A., 1996. Identification of haptoglobin as an alternative ligand for CD11b/CD18. Journal of Immunology 156, 2542–2552.
Emmanuel, D.G., Dunn, S.M., Ametaj, B.N., 2008. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. Journal of Dairy Science 91, 606–614. DOI: https://doi.org/10.3168/jds.2007-0256.
Geelen, M.J.H., Wensing, T., 2006. Studies on hepatic lipidosis and coinciding health and fertility problems of high-producing dairy cows using the “Utrecht fatty liver model of dairy cows”. A review. Veterinary Quarterly, 28, 90–104.
Gozho, G.N., Krause, D.O., Plaizier, J.C., 2007, Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced sub-acute ruminal acidosis in dairy cows, Journal of Dairy Science 90,856–866. DOI: https://doi.org/10.3168/jds.S0022-0302(07)71569-2.
Grummer, R.R., 1993. Etiology of lipid-related metabolic disorders in periparturient dairy cows. Journal of Dairy Science 76, 3882–3896.
Grummer, R.R., 1995. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. Journal of Animal Science, 73, 2820–2833.
Grummer, R.R., Mashek, D.G., Hayirli, A., 2004. Dry matter intake and energy balance in the transition period. Veterinary Clinics of North America: Food Animal Practice, 20,447–470. DOI: https://doi.org/10.1016/j.cvfa.2004.06.013.
Hammon, H.M., Stürmer, G., Schneider, F., Tuchscherer, A., Blum, H., Engelhard, T., Genzel, A., Staufenbiel, R., Kanitz, W., 2009. Performance and metabolic and endocrine changes with emphasis on glucose metabolism in high-yielding dairy cows with high and low fat content in liver after calving. Journal of Dairy Science 92, 1554–1566.
Herdt, T.H., 2000. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Veterinary Clinics of North America: Food Animal Practice, 16, 215–230. DOI: https://doi.org/10.1016/S0749-0720(15)30102-X.
Hernández-Castellano, L.E., Hernandez, L.L., Weaver, S., Bruckmaier, R.M., 2016. Increased serum serotonin improves parturient calcium homeostasis in dairy cows. Journal of Dairy Science. 100:1–8 https://doi.org/10.3168/jds.2016-11638
Horadagoda, N.U., Knox, K.M., Gibbs, H.A., Reid, S.W., Horadagoda, A., Edwards, S.E., Eckersall, P.D., 1999. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Veterinary Record, 144, 437–441.
Ingvartsen, 2006. Feeding- and management-related diseases in the transition cow. Animal Feed Science and Technology, 126,175–213. DOI: https://doi.org/10.1016/j.anifeedsci.2005.08.003.
Iranian Council of Animal Care, 1995. Guide to the Care and Use of Experimental Animals, vol. 1 Isfahan University of Technology, Isfahan, Iran.
Khafipour, E., Krause, D.O., Plaizier, J.C., 2009a. A grain-based sub-acute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. Journal of Dairy Science 92, 1060–1070. DOI: https://doi.org/10.3168/jds.2008-1389.
Khafipour, E., Krause, D.O., Plaizier, J.C., 2009b. Alfalfa pellet-induced sub-acute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation. Journal of Dairy Science 94, 1712–1724. DOI: https://doi.org/10.3168/jds.2008-1656.
Khafipour, E., Li, S., Plaizier, J. C., Krause, D. O., 2009c. Rumen microbiome composition determined using two nutritional models of sub-acute ruminal acidosis. Applied and Environmental Microbiology, 75, 7115–7124. DOI: https://doi.org/10.1128/AEM.00739-09.
Lei, C.L., Dong, G.Z., Jin, L., Zhang, S., Zhou, J., 2013. Effects of dietary supplementation of montmorillonite and yeast cell wall on lipopolysaccharide adsorption, nutrient digestibility and growth performance in beef cattle. Livestock Science Journal, 57–63. DOI:https://doi.org/10.1016/j.livsci.2013.08.019.
Levels, J.H., Abraham, P.R., Van Den Ende, A., Van Deventer, S.J., 2001. Distribution and kinetics of lipoprotein-bound endotoxin. Infection and Immunity. 69, 2821-2828.
Li, S., Kroeker, A., Khafipour, E., Rodriguez, J.C., Krause, D.O., Plaizier, J.C., 2010. Effects of sub-acute ruminal acidosis challenges on lipopolysaccharide endotoxin (LPS) in the rumen, cecum, and feces of dairy cows [abstract]. Journal of Animal Science, 88(E-Suppl 2), 433-434. DOI: https://doi.org/10.3168/jds.2011-4447.
Magata, F., Ishida, Y., Miyamoto, A., Furouka, H., Inokuma, H., Shimizu, T., 2015, Comparison of bacterial endotoxin lipopolysaccharide concentrations in the blood, ovarian follicular fluid and uterine fluid: a clinical case of bovine metritis, Veterinary Medicine and Science, 77(1), 81–84. DOI: https://doi.org/10.1292/jvms.14-0333.
McCarthy, M., Mann, M.S., Nydam, D.V., Overton, T.R., McArt, J.A.A., 2015. Short communication: concentrations of non-esterified fatty acids and β-hydroxybutyrate in dairy cows are not well correlated during the transition period. Journal of Dairy Science, 98, 6284–6290. DOI: https://doi.org/10.3168/jds.2015-9446.
McDonald, T.L., Larson, M.A., Mack, D.R., Weber, A., 2001. Elevated extra-hepatic expression and secretion of mammary associated serum amyloid A 3 (M-SAA3) into colostrum. Veterinary Immunology and Immunopathology 83, 203–211.
Mulligan, F.J., Doherty, M.L., 2008. Production diseases of the transition cow. Veterinary Journal, 176, 3–9.
Mullins, C.R., Mamedova, L.K., Brouk, M.J., Moore, C.E., Green, H.B., Perfield, K.L., Smith, J.F., Harner, J.P., Bradford, B.J., 2012. Effects of monensin on metabolic parameters, feeding behavior, and productivity of transition dairy cows. Journal of Dairy Science 95, 1323–1336.
Munford, R.S., Endotoxin(s) and the liver. Gastroenterology, 1978, 75, 532-535.
Murata H., N. Shimada, M. Yoshioka, 2004. Current research on acute phase proteins in veterinary diagnosis: an overview, Vet. J., 168, 28–40.
Murray H.H., 2000.Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Applied Clay Science, 17, 207–221.
National Research Council (NRC), 2001. Nutrient requirements of dairy cattle, 7th revised ed. National Academic Press, Washington, DC.
Nazifi, S., Rezakhani, A., Koohimoghadam, M., Ansari-lari, M., Esmailnezhad, Z., 2008. Evaluation of serum haptoglobin in clinically healthy cattle and cattle with inflammatory diseases in Shiraz, a tropical area in southern Iran. Bulgarian Journal of Veterinary Medicine, 11, NO 2, 95−101.
Nolan J.P., 1975. The role of endotoxin in liver injury. Gastroenterology, 69, 1346-1356.
Nordlund, K. V., Garrett, E. F., Oetzel, G. R., 1995. Herd-based rumenocentesis: a clinical approach to the diagnosis of sub-acute rumen acidosis. Compendium Contin Education Practice Veterinary, 17:s48–s56.
Orrihage, K. E., Sillerstrom, E., Gustafsson, J.A., Nord, C. E., Rafter, J., 1994. Binding of mutagenic heterocyclic amines by intestinal lactic acid bacteria. Mutation Research, 311, 239–248.
Plaizier, J.C., Krause, D.O., Gozho, G.N., McBride, B.W., 2009. Sub-acute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Veterinary Journal, 176, 21-31. DOI:https://doi.org/10.1016/j.tvjl.2007.12.016
Plessers, E., Wyns, H., Watteyn, A., Pardon, B., De Backer, P., Croubels, S., 2015. Characterization of an intravenous lipopolysaccharide inflammation model in calves with respect to the acute-phase response. Veterinary Immunology and Immunopathology, 163, 46–56. DOI: https://doi.org/10.1016/j.vetimm.2016.01.007.
Pullen, D.L, Palmquist, D.L, Emery, R.S., 1989. Effect on days of lactation and methionine hydroxyl analog on incorporation of plasma fatty acids into plasma triglycerides. Journal of Dairy Science, 72, 49–58.
Reid, I.M., Roberts, C.J., 1983. Subclinical fatty liver in dairy cows. Irish Veterinary Journal, 37, 104-110.
Roussel, J.A., Whitney, S.M., Jole, J.D., 1997. Interpreting a bovine serum chemistry profile; part II. Veterinary Medicine,6, 559- 566.
Santos, A., Marquina, D., Leal, J. A., Peinado, J. M., 2000. (166)- B-D-glucan as cell wall receptor for Pichia membranifaciens killer toxin. Applied and Environmental Microbiology, 66, 1809–1813.
Sevinc, M., Basoglu, A., Oztok, I., Sandikci, M., Birdane, F.M., 1998. The clinical-chemical parameters, serum lipoproteins and fatty infiltration of the liver in ketotic cows. Turkish Journal OF Veterinary and Animal Sciences, 22, 443-447.
Sevinc, M., Basoglu, A., Birdane, F.M., Gokçe, M., Kucukfindik, M., 1999. The changes of metabolic profile in dairy cows during dairy period and after. Turkish Journal OF Veterinary and Animal Sciences, 23, 475-478.
Sevinc, M., Basoglu, A., Birdane, F.M., Boydak, M., 2001, Liver function in dairy cows with fatty liver, Turkish Journal OF Veterinary and Animal Sciences, 152, 4, 297-300.
Spieker, H., 2010. Efficacy of clay minerals and activated charcoal to bind endotoxins in rumen fluid. University of Utrecht, Utrecht, the Nether-lands (Ph.D. thesis). https://dspace.library.uu.nl/handle/1874/44548
Takahashi, E., Yuzuka Y., Tanabe, S.Satoh, M.Furouka, H., 2007. Serum amyloid and haptoglobin levels in bovine amyloidosis. Journal of Veterinary Medicine Science, 69(3)-321.323
Veenhuizen, J.J., Drackley, J.K., Richard, M.J., Sanderson, T.P., Miller, L.D., Young, J.W., 1991. Metabolic changes in blood and liver during development and early treatment of experimental fatty liver and ketosis in cows. Journal of Dairy Science, 74, 4238–4253.
Wall, S.K., Wellnitz, O., Hernández-Castellano, L.E., Ahmadpour, A., Bruckmaier, R.M., 2016. Supraphysiological oxytocin increases the transfer of immunoglobulins and other blood components to milk during lipopolysaccharide and lipoteichoic acid–induced mastitis in dairy cows. Journal of Dairy Science. 99:1–9, https://doi.org/10.3168/jds.2016-11548
Weaver, S.R., Prichard, A.S., Maerz, N.L., Prichard, A.P., Endres, E.L., Hernández-Castellano, L.E., Akins, M.S., Rupert M. Bruckmaier, R.M., Hernandez, L.L., 2017. Elevating serotonin pre-partum alters the Holstein dairy cow hepatic adaptation to lactation. PLoS One, 12(9):e0184939. Doi: https://doi.org/10.1371/journal.pone.0184939
Yang, F., Haile, D.J., Berger, F.G., Herbert, D.C., Van Beveren, E., Ghio, A.J., 2003. Haptoglobin reduces lung injury associated with exposure to blood. American Journal of Physiology – Lung and Cell Molecular Physiology 284, L402–409.
Zebeli, Q., Ametaj, B.N., 2009. Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. Journal of Dairy Science, 92, 3800-3809. https://doi.org/10.2527/jas.2009-2203.
Zebeli, Q., Dunn, S.M. and Ametaj, B.N., 2010. Strong associations among rumen endotoxin and acute phase proteins with plasma minerals in lactating cows fed graded amounts of concentrate. Journal of Animal Science, 88, 1545–1553.
Zerbe, H., Schneider, N., Leibold, W., Wensing, T., Kruip, T. A. M. and Schuberth, H. J., 2000. Altered functional and immunophenotypical properties of neutrophilic granulocytes in postpartum cows associated with fatty liver. Theriogenology, 54, 771–786. DOI: https://doi.org/10.1016/S0093-691X(00)00389-7.
Acknowledgments
This study was financed by Ph.D student project grant by School of Veterinary Medicine, Shiraz University, Shiraz, Iran. The authors would like to thank the Moghufat Malek industry for provision of cows especially Mr.Miri, Mr.Naghavi and Mr.Ershadi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of Animal Rights
All animals were treated in accordance with the regulations on the guidelines of the Iranian Council of Animal Care (1995), and the experiment was approved by the Institutional Animal Care Committee for Animals Used in Research and We further followed the recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the standards of protecting animals used for experimental purposes.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razavi, S.A., Pourjafar, M., Hajimohammadi, A. et al. Effects of dietary supplementation of bentonite and Saccharomyces cerevisiae cell wall on acute-phase protein and liver function in high-producing dairy cows during transition period. Trop Anim Health Prod 51, 1225–1237 (2019). https://doi.org/10.1007/s11250-019-01815-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-01815-3