Abstract
This study was conducted to evaluate the effects of dietary supplementation of endotoxins binders (Saccharomyces cerevisiae cell wall (SCW) and bentonite (BEN)) on blood urea nitrogen (BUN), triglyceride, alkaline phosphates (ALP), and calcium (Ca) in high-producing dairy cows during the transition period. Twenty-four Holstein dairy cows (n = 6, average milk production = 35.8 ± 1.6 kg/day, body weight (BW) = 567.5 ± 40.3 kg, body condition score (BCS) = 3.5 ± 0.26 out of 5) were assigned in a completely randomized design, to the following treatments: (1) basal diet (BD), (2) BD + SCW, (3) BD + BEN, and (4) BD + SCW + BEN. Blood samples were taken 4, 3, and 1 weeks before and 1and 3 weeks after parturition. Serum concentrations of BUN, triglyceride, ALP, and Ca were measured. It was observed that, BUN concentration was lower in SCW + BEN group, probably because of the better utilization of NH3-N and better stimulation for ruminal microbial protein synthesis. Triglyceride plasma concentration was lower in the control group, which might be due to decreased lipolysis and a lower pH. The treatment groups showed no effect for Ca at any time point. Serum ALP concentration was within references range. In conclusion, lower BUN concentration could be a result of better stimulation of ruminal microbial protein synthesis in the treatment groups and also due to lower triglyceride in control group, which might be result of lower pH in the rumen, we suggest that endotoxins binders (especially combined feeding of endotoxin binders) can modulate rumen function by optimizing the rumen pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasing productivity of dairy cows, management of diet for meeting energy required for milk production, becomes more vital for animal health. In many dairy herds after parturition, a diet with high fermentable carbohydrate is often fed to compensate the negative energy balance. Occurrence of sub-acute ruminal acidosis increase when the level of carbohydrate increases in the diet (Owens et al. 1998). Sub-acute ruminal acidosis (SARA) has a negative effect on animal health and profitability of dairy herds (Kleen et al. 2013). Sub-acute ruminal acidosis occurs when ruminal pH declines below 5.6 for a prolonged period for several hours per day (Kleen et al. 2003; Stone 2004; Gozho et al. 2005); but dairy cattle suffered from sub-acute ruminal acidosis has no typical clinical sign of illness (Tajik and Nazifi 2011). Sub-acute ruminal acidosis is associated with inflammations of different organs and results in economic losses in dairy herds (Kleen et al. 2003; Stone 2004; Plaizier et al. 2008; Zebeli and Ametaj 2009). Tajik et al. (2009) did a survey on prevalence of sub-acute ruminal acidosis in Iranian dairy herds and they reported that the prevalence of sub-acute ruminal acidosis was 27% in early lactation and mid-lactation cows. Newly Kleen et al. (2013) reported that incidence of sub-acute ruminal acidosis was 20% in 315 cows in Northern Germany. Sub-acute ruminal acidosis causes pathological changes in the rumen wall (Steele et al. 2011), leading to the increased absorption of lipopolysaccharide (LPS) released from gram negative bacteria in rumen and the lower gut (Gozho et al. 2006; Dong et al. 2011; Plaizier et al. 2008, 2012). Study by Gozho et al. (2006) showed an increase in acute phase protein as a result of sub-acute ruminal acidosis, also liver damage may occur (Ainmalamali et al. 1992). An increase in LPS concentration of blood associated with metabolic disturbances, such as the increase in blood glucose and non-esterified fatty acid which reduce feed intake and affect pattern of hydroxybutyric acid, cholesterol, and minerals, such as Ca, Fe, and Zn (Ametaj et al. 2009; Zebeli et al. 2010). Many studies have addressed different strategies to decrease the incidence of sub-acute ruminal acidosis. A study by Gonzalez et al. (2012) demonstrated that adding feed additives in cows’ diets, such as buffers, natural plant extracts, ionophores, yeasts, and organic acids could reduce the prevalence of sub-acute ruminal acidosis. Bentonites are clays belong to smectite group (Trckova et al. 2004). Smectite minerals have a wide-range absorbent ability, which make it a good absorptive material of heavy metals and bacteria (Hassen et al. 2003; Katsumata et al. 2003) and toxic and anti-nutritive agents (Ditter et al. 1983; Pulsipher et al. 1994; Ibrahim et al. 2000; Phillips et al. 2002). Some studies have reported that many strains of lactic acid bacteria and some strain of Saccharomyces cerevisiae have probiotic activity (Coeuret et al. 2004). For a long time, yeast products have been fed to animals, and nowadays, many commercial yeast products are being specially produced for animal feeding (Celyk et al. 2003). Yeast cell wall also is able to bind sterols from the medium (Adams and Parks 1967), where the binding molecule was identified as the cell wall mannan (Thompson et al. 1973). Study by Lei et al. (2013) in beef cows reported that dietary supplementation of yeast cell wall and montmorillonite (bentonite) can effectively reduce free LPS concentration in plasma, digesta, and the feces. Therefore, the main objectives of this study is to evaluate potential effect of dietary supplementation of endotoxins binders (bentonite and S. cerevisiae cell wall) on serum concentration of BUN, triglyceride, ALP, and Ca in high-producing dairy cows during the transition period.
Material and methods
Animals and experimental design
In this study, 24 multiparous Holstein cows with an initial body weight (BW) of 567.5 ± 40.3 kg (mean ± SD), body condition score of 3.5 ± 0.26 out of 5 (mean ± SD), and average milk production of previous lactation of 35.8 ± 1.6 kg/day (mean ± SD), were assigned in a completely randomized design in four groups (n = 6). Treatment groups received balanced ration in accordance with the NRC nutrient requirements based on the cow condition (NRC 2001). Control group received the same ration at similar intervals; except, it was not supplemented with a toxin binder based on bentonite and S. cerevisiae cell wall. Our experiment conducted from 4 weeks before the expected calving date until 3 weeks after the calving date. Experimental cows were housed in pen with four stalls (n = 6). After calving, experimental animals were moved to another pen near to milking parlor, consisting of four stalls and their diet was changed from close-up diet to fresh cow diet. Pens were bedded with clean wood shavings and dry manure, and bedding was refreshed daily if it was necessary. The cows were selected from a 1000-cow industry dairy herd in Khorasan Razavi province in the northeast of Iran in summer of 2016. The experimental groups consisted of (1) the basal diet (control); (2) the basal diet+ S. cerevisiae cell wall (12 g/day/cow); (3) basal diet + bentonite (150 g/day/cow); and (4) the basal diet + bentonite + S. cerevisiae cell wall on a dry matter basis.
Diet
The bentonite (particle size less than 37 μm according to manufacturer introduction) was purchased from Zarin Binder (Mashhad, Iran). The S. cerevisiae cell wall was supplied from ActiveMOS (dry matter ≥ 94.0%, Chitin ≥ 2.0, β-glucan ≥ 30.0%, and mannoprotein ≥ 20.0%). (Biorigin, Sao Paulo, Brazil). Dietary doses of endotoxins binder were based on the manufacturer recommendation for dairy cows (12 g/day/cow for SCW and 150 g/day/cow for bentonite). The total mixed ration (TMR) was formulated to meet or exceed the requirements for fresh and close-up cows separately (National Research Council (NRC) 2001). Ingredients and chemical formulation of the experimental diets (DM basis) are showed in Table 1. During the trial, cows were fed the experimental diet as a TMR twice daily at 1000 and 1700 h. The experiment animals had free access to water during the trial.
Sampling procedure
Blood samples were taken five times during the trial period, 2 h before the morning feeding on all cows through tail vein in 10-mL plain tubes. One sample was taken 1 week before adding the additives to the diet. The next sample was collected at 3 and 1 weeks before the parturition and 1 and 3 weeks after the parturition. The samples were cooled down quickly and serum was separated by centrifugation (Sigma 1-15p-Sigma-Laborzentrifugen, Munich, Germany) for 10 min at × 3000g and stored at − 22 °C until being assayed. Biochemical analyses and methods of measuring are presented in Table 2.
Statistical analyses
In our study, data were expressed as the mean ± deviation (SD). Comparison of quantitative data between the experimental and control groups was done using linear mixed models to compare mean concentrations of different serological factors within similar weeks between our different experimental groups. Statistical analysis was done using SPSS software (SPSS for Windows, version 16, SPSS Inc. Chicago, Illinois). The statistical significance level was set at P < 0.05.
Results
Blood parameters level
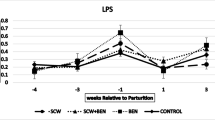
The effect of treatment group and time on concentrations of BUN and triglyceride are summarized in Figs. 1 and 2 respectively. Mean BUN concentration was lowest in group SCW + BEN (136 ± [124–147(CL)] mg/L) and lower than that in the control group and BEN group (p < 0.05). Four outliers were identified and removed prior to analysis as the results were not biologically plausible (BUN concentration > 500 mg/L). All these outliers belong to the group control. Mean triglyceride concentration was lowest in group control (179 ± [114–244(CL)] mg/L). Despite the significant interaction, no effect of treatment group was observed for triglyceride at any time point. Mean triglyceride concentration at 1 week after calving was the lowest (127 ± [107–146(CL)] mg/L). The p values from the linear mixed models of the effect of treatment group, time and their interaction on the mean concentrations of the measured analyzed are shown in Table 3.
Mean (± SEM) serum concentrations of BUN (mg/L) 4, 3, and 1 weeks before and 1 and 3 weeks after parturition (n = 6 six in each group, *p < 0.05) (BUN references value in cattle 60–270(mg/L)) (Constable et al. 2017)
Mean (± SEM) serum concentrations of triglyceride (mg/L) 4, 3, and 1 weeks before and 1 and 3 weeks after parturition (n = 6 six in each group, *p < 0.05) (Triglyceride references value in cattle 10–140 (mg/L)) (Constable et al. 2017)
Serum calcium levels
The effect of treatment group and time on concentrations of Ca is presented in Fig. 3. No effect of treatment group was found for Ca at any time point. The mean Ca concentration was lowest at 1 week before calving (86 [(95% CL) 84–88] mg/L). In that time point, the mean Ca concentration was lower than that at 4 and 3 weeks before calving (p ≤ 0.001).
Mean (± SEM) serum concentrations of Ca (mg/L) 4, 3, and 1 weeks before and 1 and 3 weeks after parturition (n = 6 six in each group, *p < 0.05) (Ca references value in cattle 97–124(mg/L)) (Constable et al. 2017)
Liver enzyme activity
The effect of treatment group and time on concentrations of ALP is shown in Fig. 4. The overall effect of treatment group on ALP activity was statistically significant at 5% level; besides, an interaction between group and time was found. Mean ALP concentration in the control group serum was highest (97 ([95% CL] 84.6–109.3) U/L). Mean ALP concentration in control group was higher than that in group BEN (p < 0.05). Mean ALP concentration in serum was lowest at 1 week before parturition (52.5 ([95% CL] 33.9–71.1) U/L), and it was lower than 4and 30 weeks before parturition (p < 0.05).
Mean (± SEM) serum concentrations of ALP (U/L) 4, 3, and 1 weeks before and 1 and 3 weeks after parturition (n = 6 six in each group, *p < 0.05) (ALP references value in cattle0–500(units/L)) (Constable et al. 2017)
Discussion
A study by Fairfield et al. (2007) on dairy cattle after calving demonstrated that prevalence of sub-acute ruminal acidosis increased when cows received diet with 54% forage DM (34% NDF and 39% NFC, DM basis). A study by Penner et al. (2007) also showed that the occurrence of sub-acute ruminal acidosis raised in early lactation cows fed with a diet containing 47% forage DM (29% NDF, DM basis). The experimental diet of our study contains 51.29% forage (DM basis) for close-up cows and 39.98% forage (DM basis) for fresh cows, and thus the concentrate levels were 48.71 and 60.02% respectively. When the levels of carbohydrate increase in diet, it is predisposed cows to sub-acute ruminal acidosis due to the accumulation of organic acids produced by microbial fermentation and the consequence is a decrease in rumen pH (Owens et al. 1998). Study by Chiquette et al. (2012, 2015) showed milk urea nitrogen (MUN) decreased in cows during sub-acute ruminal acidosis. Milk urea nitrogen (MUN) is originated from blood urea nitrogen (BUN) in ruminants (Jonker et al. 1998). A low concentration of MUN can be attributed to better utilization of NH3-N in rumen due to a suitable environment for higher stimulation of ruminal microbial protein synthesis which leads to a declined NH3-N concentration in rumen and BUN (Chiquette et al. 2015). BUN might be changed during water deprivation, diarrhea, urinary disease, pregnancy toxemia, and acidosis (Patra et al. 1996; Singh et al. 1992; Nozad et al. 2012), which were not case in experimental animals of this study. In the current study, the level of carbohydrate in the experimental diet was similar between experimental groups but BUN concentration was lower in SCW + BEN group. This result might be attributed to suitable environment for better utilization of NH3-N and better stimulation for ruminal microbial protein synthesis in the rumen. BUN level was higher in the control group that did not receive endotoxin binders, probably because of the lower number of cellulolytic ruminal bacteria that use NH3-N as source of nitrogen (Atasoglu et al. 2001). Although we did not measure rumen PH, we think that difference in the rumen pH results in different ruminal bacterial population. Khafipour et al. (2009) reported a decrease in ruminal NH3-N concentration during a sub-acute ruminal acidosis challenge. Chaucheyras-Durand et al. (2008) did a survey on the impact of yeasts on ruminal NH3-N concentration and reported that type of diet, host, and microbial-related factors has very important role. One study showed that actively dried yeast supplementation to dairy cows with sub-acute ruminal acidosis is able to improve rumen function, considering a tendency for an improved ruminal pH and greater abundance of M. elsdenii within the rumen (Malekkhani et al. 2016). We used yeast cell wall and due to a lower concentration of BUN in treatment groups, which might be because of better utilization of NH3-N: we suggest that endotoxin binders, especially combined feeding of endotoxin binders, can improve rumen function as indicated by optimizing rumen pH. Chiquette et al. (2008) demonstrated that supplementing early lactating cattle with probiotics (P. bryantii 25A) can result in reduction of lactate concentrations in the rumen which contributes to prevention of rumen acidosis. We are not certain whether supplementation of endotoxin binders can prevent sub-acute ruminal acidosis, but we suggest that it can improve rumen function in some aspect. A study of Guo et al. (2013) showed that triglyceride decreased in animals with sub-acute ruminal acidosis. A study by Van Nevel and Demeyer (1996) demonstrated that lipolysis is much more sensitive to low pH value in rumen and lipolysis and biohydrogenation decreased when the pH is low in animals fed high concentrate diet. Results of this study demonstrated that, triglyceride concentration in serum was lower in the control group, which might be due to reduction in lipolysis in the control group and also the rumen pH might have been lower in that group. Serum triglyceride concentration was high in all groups. It might be due to negative energy balance and also lipolysis was pronounced in them. Danscher et al. (2015) showed that blood-ionized calcium concentrations were lower in sub-acute ruminal acidosis group compared to animals without sub-acute ruminal acidosis. A study by Zebeli et al. (2010) in healthy lactation cows showed the negative correlations between Ca and rumen LPS and plasma serum amyloid a (SAA). Also, a study by Wenz et al. (2001) showed a decrease in the Ca concentration in lactating cows affected by coliform mastitis. The results of this study showed no treatment effect on Ca concentration in blood. An explanation for this observation might be the fact that, we did not induce acidosis in experimental animals and thus should not expect a significant decrease in Ca concentration. Although Ca concentration in serum was lower than references range in all experimental animals, especially from 1 before calving but there was not any sign of milk fever in them. Kalaitzakis et al. (2007) observed that, moderate to severe cases of fatty change of the liver had a higher level of activity of ALP compared to normal cows though ALP mostly indicating the cholestasis. Results of the current study suggested that, ALP concentration was in references range, suggesting that the experimental animals did not have cholestasis or moderate to the severe fatty liver. Due to a lower BUN concentration that could be a result of better stimulation of ruminal microbial protein synthesis in treatment groups and also due to lower triglyceride in the control group which might be associate to the lower pH in the rumen, we suggest that endotoxin binder (especially combined feeding of endotoxin binders) can modulate rumen function by optimizing rumen pH. Authors of the current study are not sure that with which mechanism endotoxin binders are able to optimize rumen pH but according to other study (Chiquette et al. 2008; Malekkhani et al. 2016). It might be because of the declined lactate concentrations in the rumen which contributes to prevention of rumen acidosis or by improved ruminal pH as a result of changing in ruminal microbial population. We suggest conducting further research to verify this theory.
Conclusion
In conclusion, BUN was lower in treatment groups. It might be due to the better stimulation of ruminal protein synthesis bacteria and better utilizing NH3-N in the rumen. Besides, pH of the rumen was suitable for cellulolytic bacteria which utilize NH3-N especially in SCW + BEN group. Furthermore, considering a lower triglyceride in control group that might have associated with lower pH in the rumen, we think that toxin binders can modulate rumen function by optimizing rumen pH. Nevertheless, further research is required to prove this claim.
References
Adams BG, Parks LW (1967) A water-soluble form of ergosterol and cholesterol for physiological studies. Biochem Biophys Res Commun 28:490–494
Ainmalamali S, Kindahl H, Fredriksson G (1992) The role of endotoxins in induced ruminal acidosis in calves. Acta Vet Scand 33:117–127
Ametaj BN, Emmanuel DGV, Zebeli Q, Dunn SM (2009) Feeding high proportions of barley grain in a total mixed ration perturbs diurnal patterns of plasma metabolites in lactating dairy cows. J Dairy Sci 92:1084–1091. https://doi.org/10.3168/jds.2008-1465
Atasoglu C, Newbold J, Wallace J (2001) Incorporation of [15N] Ammonia by the cellulolytic ruminal bacteria Fibrobacter succinogenes BL2, Ruminococcus albus SY3, and Ruminococcus flavefaciens 17. Appl Environ Microbiol:2819–2822. https://doi.org/10.1128/AEM.67.6.2819-2822.2001
Celyk K, Denly M, Savas T (2003) Reduction of toxic effects of aflatoxin by using baker yeast (Saccharomyces cerevisiae) in growing broiler chicken diets. Rev Bras Zootec 32:615–619
Chaucheyras-Durand F, Walker ND, Bach A (2008) Effects of active dry yeasts on the rumen microbial ecosystem: past, present and future. Anim Feed Sci Technol 145:5–26. https://doi.org/10.1016/j.anifeedsci.2007.04.019
Chiquette J, Allison MJ, Rasmussen MA (2008) Prevotella bryantii 25A used as a probiotic in early-lactation dairy cows: effect on ruminal fermentation characteristics, milk production, and milk composition. J Dairy Sci 91:3536–3543. https://doi.org/10.3168/jds.2007-0849
Chiquette J, Allison MJ, Rasmussen M (2012) Use of Prevotella bryantii 25A and a commercial DFM during subacute acidosis challenge in mid-lactation dairy cows. J Dairy Sci 95:5985–5995. https://doi.org/10.3168/jds.2012-5511
Chiquette J, Lagrost J, Girard CL, Talbot G, Li S, Plaizier JC, Hindrichsen IK (2015) Efficacy of the direct-fed microbial Enterococcus faecium alone or in combination with Saccharomyces cerevisiae or Lactococcus lactis during induced sub-acute ruminal acidosis. J Dairy Sci 98:190–203. https://doi.org/10.3168/jds.2014-8219
Coeuret V, Gueguen M, Vernoux JP (2004) Numbers and strains of lactobacilli in some probiotic products. Int J Food Microbiol 97:147–156. https://doi.org/10.1016/j.ijfoodmicro.2004.04.015
Constable PD, Hinchcliff KW, Done SH, Grünberg W (2017) Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goats. Veterinary Medicine, 11th edn. Elsevier Ltd., p 2217–2219
Danscher AM, Li S, Andersen PH, Khafipour E, Kristensen NB, Plaizier JC (2015) Indicators of induced sub-acute ruminal acidosis (SARA) in Danish Holstein cows. Acta Vet Scand 57:39. https://doi.org/10.1186/s13028-015-0128-9
Ditter B, Urbaschek R, Urbaschek B (1983) Ability of various adsorbents to bind endotoxins in vitro and to prevent orally induced endotoxemia in mice. Gastroenterology 84:1547–1552
Dong GZ, Liu SM, Wu YX, Lei CL, Zhou J, Zhang S (2011) Diet induced bacterial immunogens in the gastrointestinal tract of dairy cows: impacts on immunity and metabolism. Acta Vet Scand 53:1–7. https://doi.org/10.1186/1751-0147-53-48
Fairfield AM, Plaizier JC, Duffield TF, Lindinger MI, Bagg R, Dick P, McBride BW (2007) Effects of prepartum administration of a monensin controlled release capsule on rumen pH, feed intake, and milk production of transition dairy cows. J Dairy Sci 90:937–945. https://doi.org/10.3168/jds.S0022-0302(07)71577-1
Gonzalez LA, Manteca X, Calsamiglia S, Schwartzkopf-Genswein KS, Ferret A (2012) Ruminal acidosis in feedlot cattle: interplay between feed ingredients, rumen function and feeding behavior (a review). Anim Feed Sci Technol 172:66–79
Gozho GN, Plaizier JC, Krause DO, Kennedy AD, Wittenberg KM (2005) Sub-acute ruminal acidosis induces ruminal lipopolysaccharides endotoxin release and triggers an inflammatory response. J Dairy Sci 88:1399–1403. https://doi.org/10.3168/jds.S0022-0302(07)71569-2
Gozho GN, Krause DO, Plaizier JC (2006) Rumen lipopolysaccharide and inflammation during grain adaptation and sub-acute ruminal acidosis in dairy cows. J Dairy Sci 89:4404–4413. https://doi.org/10.3168/jds.S0022-0302(07)71569-2
Guo Y, Xu X, Zou Y, Yang Z, Li Y, Cao Z (2013) Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with sub-acute ruminal acidosis and its regulation with pelleted beet pulp. J Anim Sci Biotechnol 4:31. https://doi.org/10.1186/2049-1891-4-3.
Hassen A, Jamoussi F, Saidi N, Mabrouki Z, Fakhfakh E (2003) Microbial and cooper adsorption by smectitic clay – an experimental study. Environ Technol 24:1117–1127. https://doi.org/10.1080/09593330309385652
Ibrahim IK, Shareef AM, Al-Joubory KM (2000) Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Res Vet Sci 69:119–122. https://doi.org/10.1053/rvsc.2000.0390
Iranian Council of Animal Care (1995) Guide to the care and use of experimental animals vol. 1. Isfahan University of Technology, Isfahan
Jonker JS, Kohn RA, Erdman RA (1998) Using milk urea nitrogen to predict nitrogen excretion and utilization efficiency in lactating dairy cows. J Dairy Sci 81:2681–2692
Kalaitzakis M, Roubies N, Panousis N, Pourliotis K, Kaldrymidou E, Karatzias H (2007) Clinicopathologic evaluation of hepatic lipidosis in periparturient dairy cattle. J Vet Intern Med 21:835–845. https://doi.org/10.1186/1751-0147-55-48
Katsumata H, Kaneco S, Inomata K, Itoh K, Funasaka K, Masuyama K, Suzuki T, Ohta K (2003) Removal of heavy metals in rinsing wastewater from plating factory by adsorption with economical viable materials. J Environ Manag 69:187–191
Khafipour E, Krause DO, Plaizier JC (2009) A grain-based sub-acute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 92:1060–1070. https://doi.org/10.3168/jds.2008-1389
Kleen JL, Hooijer GA, Rehage J, Noordhuizen JP (2003) Subacute ruminal acidosis (SARA): a review. J Vet Med A Physiol Pathol Clin Med 50:406–414
Kleen JL, Upgang L, Rehage J (2013) Prevalence and consequences of sub-acute ruminal acidosis in German dairy herds. Acta Vet Scand 55:1–6. https://doi.org/10.1186/1751-0147-55-48
Lei CL, Dong GZ, Jin L, Zhang S, Zhou J (2013) Effects of dietary supplementation of montmorillonite and yeast cell wall on lipopolysaccharide adsorption, nutrient digestibility and growth performance in beef cattle. Livest Sci J 158:57–63. https://doi.org/10.1016/j.livsci.2013.08.019
Malekkhani M, Tahmasbi AM, Naserian AA, Danesh-mesgaran M, Kleen JL, Alzahal O, Ghafari MH (2016) Effects of supplementation of active dried yeast and malate during sub-acute ruminal acidosis on rumen fermentation, microbial population, selected blood metabolites, and milk production in dairy cows. Anim Feed Sci Technol 213:29–43. https://doi.org/10.1016/j.anifeedsci.2015.12.018
Nozad S, Gholi-Ramin A, Moghadam G, Asri-Rezaiy S, Babapour A, Ramin S (2012) Relationship between blood urea, protein, creatinine, triglycerides and macro-mineral concentrations with the quality and quantity of milk in dairy Holstein cows. Vet Res Forum 3(1):55–59
NRC (2001) Nutrient requirements of dairy cattle, 7th edn. Natl Acad Sci rev, Washington DC
Owens FN, Secrist DS, Hill WJ, Gill DR (1998) Acidosis in cattle—a review. J Anim Sci 76:275–286
Patra RC, lal SB, Swarup D (1996) Biochemical profile of rumen liquor, blood and urine in experimental acidosis in sheep. Small Rumin Res 19:177–180
Penner GB, Beauchemin KA, Mutsvangwa T (2007) Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period. J Dairy Sci 90:365–375. https://doi.org/10.3168/jds.S0022-0302(07)72638-3
Phillips TD, Lemke SL, Grant PG (2002) Characterization of clay-based enterosorbents for prevention of aflatoxicosis. Adv Exp Med Biol 504:157–171
Plaizier JC, Krause DO, Gozho GN, McBride BW (2008) Sub-acute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet J 176:21–31. https://doi.org/10.1016/j.tvjl.2007.12.016
Plaizier JC, Khafipour E, Li S, Gozho GN, Krause DO (2012) Sub-acute ruminal acidosis (SARA), endotoxins and health consequences. Anim Feed Sci Technol 172:9–21. https://doi.org/10.1016/j.als.2016.11.006
Pulsipher GD, Galyean ML, Hallford DM, Smith GS, Kiehl DE (1994) Effects of graded levels of bentonite on serum clinical profiles, metabolic hormones, and serum swainsonine concentrations in lamb fed locoweed (Oxytropis sericea). J Anim Sci 72:1561–1569
Singh SK, Prasad MC, Nem S (1992) Clinico-biochemical studies on induced pregnancy toxemia in sheep. Ind J Vet Pathol 16:85–90
Steele MA, Croom J, Kahler M, AlZahal O, Hook SE, Plaizier K, McBride BW (2011) Bovine rumen epithelium undergoes rapid structural adaptations during grain induced subacute ruminal acidosis. Am J Phys Regul Integr Comp Phys 300:R1515–R1523. https://doi.org/10.1152/ajpregu.00120.2010
Stone WC (2004) Nutritional approaches to minimize sub-acute ruminal acidosis and laminitis in dairy cattle. J Dairy Sci 87:E13–E26
Tajik J, Nazifi S (2011) Diagnosis of sub-acute ruminal acidosis: a review. Asian J Anim Sci 5:80–90. https://doi.org/10.3923/ajas.2011.80.90
Tajik J, Nadalian MG, Raoofi A, Mohammadi GR, Bahonar AR (2009) Prevalence of sub-acute ruminal acidosis in some dairy herds of Khorasan Razavi province, northeast of Iran. J Vet Res 10:28–32
Thompson ED, Knights BA, Parks LW (1973) Identification and properties of a sterol-binding polysaccharide isolated from Saccharomyces cerevisiae. Biochem Biophys Acta 304:132–141
Trckova M, Matlova L, Dvorska L, Pavlic I (2004) Kaolin, bentonite, and zeolites as feed supplements for animals: health advantages and risks. Vet Med – Czech 49(10):389–399
Van Nevel CJ, Demeyer DI (1996) Influence of pH on lipolysis and biohydrogenation of soybean oil by rumen contents in vitro. Reprod Nutr Dev 36:53–63
Wenz JR, Barrington GM, Garry FB, McSweeney KD, Dinsmore RP, Goodell G, Callan RJ (2001) Bacteremia associated with naturally occurring acute coliform mastitis in dairy cows. J Am Vet Med Assoc 219:976–981
Zebeli Q, Ametaj BN (2009) Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. J Dairy Sci 92:3800–3809. https://doi.org/10.2527/jas.2009-2203.
Zebeli Q, Dunn SM, Ametaj BN (2010) Strong associations among rumen endotoxin and acute phase proteins with plasma minerals in lactating cows fed graded amounts of concentrate. J Anim Sci 88:1545–1553. https://doi.org/10.2527/jas.2009-2203.
Funding
This study was financed by PhD student project grant by School of Veterinary Medicine, Shiraz University, Shiraz, Iran. The authors would like to thank the Moghufat Malek industry for provision of cows especially Mr. Miri, Mr. Naghavi, and Mr.Ershadi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal right
All animals were treated in accordance with the regulations on the guidelines of the Iranian Council of Animal Care (1995), and the experiment was approved by the Institutional Animal Care Committee for Animals Used in Research.
Rights and permissions
About this article
Cite this article
Razavi, S.A., Pourjafar, M., Hajimohammadi, A. et al. Effects of dietary supplementation of bentonite and yeast cell wall on serum blood urea nitrogen, triglyceride, alkaline phosphatase, and calcium in high-producing dairy cattle during the transition period. Comp Clin Pathol 28, 419–425 (2019). https://doi.org/10.1007/s00580-018-2849-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2849-4