Abstract
Coccidian organisms of poultry have proved very hard to control due to their abilities to resist most anticoccidial drugs. Pleurotus ostreatus (Fr.) Jacq. ex (Pleurotaceae), a medicinal mushroom, was investigated in vivo against Eimeria spp. Ninety-six broilers (day-old) naturally infected with Eimeria spp. were divided into eight groups (12 birds per group). Group A was infected untreated (negative control) and group B was treated with toltrazuril (positive control) while groups C–H were gavaged with graded doses of P. ostreatus extract at 100, 200, 300, 400, 500, and 600 mg/kg, respectively. The phytochemical analysis of the aqueous extract of P. ostreatus which revealed saponins, flavonoids, anthraquinones, and alkaloids was evaluated for anticoccidial activity by assessing the inhibition of oocyst output, lesion score, faecal score, weight differences, haematological parameters, and leucocyte differential counts. The acute toxicity study showed extract of P. ostreatus to be non-toxic at 600 mg/kg. The weight of the groups treated with the extracts and toltrazuril increased significantly (P < 0.05) compared with the untreated control. Treated groups significantly (P < 0.05) reduced oocyst output except groups C and D. The therapeutic best-fit ED50 value for the extract was 448 mg/kg. The post-treatment mean packed cell volume and red blood cell count were significantly higher (P < 0.05) than the untreated group, while the WBC count was significantly higher (P < 0.05) in the untreated group. Pleurotus ostreatus therefore could be a potential source of new anticoccidial medicine which could find application in the control of avian coccidiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Commercial poultry production is expanding rapidly providing cheaper sources of balanced diets for consumers worldwide (Ghafoor et al. 2010). However, poultry farming is still faced with various enteric illnesses like coccidiosis that limits the progress (Hafez 2011). The coccidial parasites grow and replicate in the epithelia tissue of the intestine destroying the villi leading to epithelial damage, haemorrhages, loss of appetite, inadequate nutrient absorption, dehydration, poor growth, and death (Seifert 2006). Anticoccidial drugs and feed additives are administered for chemoprophylactic and therapeutic reasons but Eimeria strains resistant to drugs and accumulation of drug metabolites in chicken products have aggravated the problem (Abbas et al. 2011). Another effective control method of coccidiosis is the use of live Eimeria oocysts as vaccine (Shirley and Lillehoj 2012); however, the use of live vaccine under poor production management system in broiler chickens may cause negative adverse effects resulting in reduced flock performances (Chapman 2000). Moreover, researchers have been working on cheaper, non-toxic, and effective treatment options for chicken coccidiosis of which herbs and herb extracts are important (Zaman et al. 2011). As a result of these, more investigations are tailored toward plants and their extracts for prophylaxis and control of intestinal diseases caused by parasites (Jung et al. 2011).
Mushrooms have been used because of their medicinal properties for five millennia (Borchers et al. 1999). Glucans and proteoglycans found in mushrooms are potent sources of gastrointestinal, biologically active immune stimulants which concurrently produce enhancing effect on all important parts of immune system (Selegean et al. 2009). Pleurotus species are called ‘oyster’ mushrooms. Pleurotus ostreatus proximate analysis showed the presence of steroidal glycosides, carbohydrates, terpenoids, and tannin in small amounts, whereas cyanogenic glycosides, alkaloids, and flavonoids were absent (Iwalokun et al. 2007). P. ostreatus extracts were demonstrated to have antifungal and antibacterial activities against 89.8% Saccharomyces cerevisiae (10.5–10.8 mm), Bacillus subtilis (7.6–7.8 mm), and Escherichia coli (7.6–8.2 mm), producing greatest effects against fungi and gram-positive and gram-negative organisms in agar well diffusion (Iwalokun et al. 2007). Phenolic content of P. ostreatus extract was reported high (24.012 mg AE/g) when methanol was used as solvent but low in aqueous extract (16.5468 mg GAE/g) with potent antioxidant attributes demonstrating high free superoxide free radicals removing property (Sanjay et al. 2015). In a study, Allen et al. (1998) discovered that compounds with antioxidant decreased the undesirable effect of E. tenella by improving the level of oxidative stress in the intestine. More recently, Ademola and Odeniran (2016) reported trypanocidal effect of Pleurotus sajo-caju aqueous extract in mice infected with Trypanosoma congolense. It has been reported that host animals treated with mushroom polysaccharides possess strong immunity against micro-organisms by promoting lysosomal enzyme operation, phagocytosis and interleukin-1 stimulation (Estrada et al. 1997). This study investigated the anticoccidial efficacy of Pleurotus ostreatus aqueous mushroom extract against Eimeria spp. in broiler chickens.
Materials and methods
Ethical approval
The study was conducted with the permission of the University of Ibadan Animal Ethics Committee (UI-ACUREC/App/2015/056) and in line with the guidelines of the committee.
Extraction of mushroom
Fresh cultivated Pleurotus ostreatus mushrooms were obtained from the Forest Research Institute of Nigeria (FRIN). The mushrooms were air-dried at 45 °C in a drier. The dried mushroom was ground to a fine powder by a milling machine. Extract was prepared by maceration with intermittent shaking in distilled water with a 10:1 solvent:dry weight ratio (Eloff 1998). The extract was filtered using a Whatman No. 1 filter paper and was administered to birds immediately. The mixture was filtered using a previously weighed filter paper. A brown filtrate was obtained. The residue was dried and weighed again and the difference in weight gave the amount of powdered mushroom dissolved in water (w/v). Appropriate aliquots of the aqueous extract were taken and diluted to prepare graded doses of the extract for the test.
Phytochemical analysis
Qualitative and quantitative preliminary phytochemical screening on ethanol and aqueous crude extract of P. ostreatus was evaluated using Wagner’s test for alkaloids, 10% ferric chloride test for tannins, 20% sodium hydroxide with sulphuric acid and lead(II) acetate test for flavonoids, frothing test for saponins, Salkowski test for terpenoids and steroids, and Keller-Killiani test for cardiac glycosides, while anthraquinones was evaluated using Borntrager’s test (10% HCl + chloroform + 10% ammonia solution) (Wandati et al. 2013). Qualitative and quantitative assessment was determined by colour intensity or relative rate of colour formation with reagent addition, but for alkaloids, it is a function of quantity of precipitation upon reagent addition and is signified by the number of plus.
Toxicity test
Preliminary acute toxicity study was conducted by using 24 1-day-old broiler chickens that were divided into six groups of four chickens each. Each bird in groups A–F was individually gavaged orally with the graded doses (100, 200, 300, 400, 500, and 600 mg/kg body weight) of aqueous mushroom extract of Pleurotus ostreatus. The chickens were observed for 24 h for any signs of toxicity (eye blinking, panting, lethargy, salivation, and incoordination), including change in behaviour or death.
Parasite
Birds were naturally infected with mixed field Eimeria species. The field isolates contained the following species (differentiated on the basis of oocyst morphology, site of colonisation, pathology, and clinical signs): Eimeria tenella, Eimeria acervulina, Eimeria necatrix, Eimeria maxima, and Eimeria brunetti.
Maintenance of experimental animals
Broiler chicks were purchased from Farm support® limited, Ibadan, Nigeria, and fed ad libitum on an anticoccidial free proprietary broiler ration from Premier Feed limited (Top feeds), Ibadan, Nigeria. Water was given ad libitum. The chicks were raised on used deep litter system from a day old at the Teaching and Research Farm, University of Ibadan, Nigeria. The chickens were routinely vaccinated against Newcastle and Gumboro diseases. No antibiotic was administered throughout the study.
Experimental design
A total of 96 1-day-old Arbor Acres breed of broiler chickens of both sexes were reared as a single group and were adequately infected with field strains of Emeria spp. by the 28th day of age. The birds were randomly assigned to eight treatment groups (A–H) according to the oocyst level in faeces which ranged from 26,650 to 30,950 and faecal samples were assessed in triplicate for the number of oocyst per gramme (OPG) of faeces prior to treatment. Group A was the untreated infected group (negative control), group B (positive control) was treated with toltrazuril (Keprocox® 2.5% ORAL, Batch No. 13G919) at a dose rate (25 mg/kg) prescribed by the manufacturer, and groups C–H were gavaged with graded doses of P. ostreatus aqueous extract (100, 200, 300, 400, 500, and 600 mg/kg) from day 29 to day 33 (5-day treatment duration). Koinarski et al. (2005) estimated the period of oxidant insult induced by the coccidian parasites to be 5 days; hence, the birds were treated for 5 days.
Measurement
Birds from each treatment group were weighed individually on days 28/0 (pre-treatment), 32/4 (mid-treatment), and 35/7 (post-treatment) to determine the mean body weight. Pooled daily oocyst counts per group were undertaken. Individual oocyst counts were not feasible because the birds were housed in groups. A total of 10 g of faeces was collected daily beginning from day 28/0 to 35/7 post-treatment. The modified McMaster technique as described by Vassilev (2002) was used to estimate oocyst per gramme of faeces. This was repeated three times and the mean value calculated. The method described by Youn and Noh (2001) was used to determine the extent of bloody diarrhoea daily from days 28/0 to 35/7 of age/treatment by assigning it to one of five levels, from 0(-) to 4(++++). Zero was the normal status, while 1, 2, 3, and 4 represented less than 25, 26–50, 51–75, and over 76% bloody faeces, respectively.
Two broilers per group were randomly euthanised on day 28/0 (pre-treatment), day 32/4 (mid-treatment), and day 35/7 (post-treatment) and subjected to necropsy. Any coccidial lesions found in the intestine were scored from 0 to 4. Zero was no lesion status, while 1, 2, 3, and 4 represented small scattered petechial haemorrhages, numerous petechial haemorrhages, extensive haemorrhages, and extensive haemorrhage that gives a dark colour to the intestine respectively, as described by Johnson and Reid (1970). A score was assigned to each category and a mean calculated from the individual scores to give a global lesion score.
Blood samples (2 ml) were collected from the jugular veins of three chickens in each group on days 28/0, 32/4, and 35/7 post-treatment. The packed cell volume (PCV), red blood cells (RBC), total white blood cell (TWBC), mean corpuscular volume (MCV), and haemoglobin (Hb) were determined using Auto-haematology analyzer (Minray BC-2800). Differential leucocyte counts were determined by microscopic examination of Giemsa-stained blood smear (Jain 1986).
Statistical analysis
The bioactivity of various doses of the extract and the positive and negative controls were assessed by comparing the oocyst reduction values by one-way ANOVA and Tukey’s multiple comparison test. Weight gains and haematological parameters for each group for the different time points were also compared using one-way ANOVA and Tukey’s multiple comparison tests to ascertain if weights differed significantly over the period. All data analyses were performed using GraphPad Prism version 5.0 for Windows (San Diego, CA). Sigmoidal dose-response curve was used to determine the ED50 of P. ostreatus aqueous extract using this formula:

Results
Yields of extract
The aqueous mushroom extract of Pleurotus ostreatus gave a yield of 14.77 g (11.36% w/w) from an initial dried mushroom weight of 130 kg.
Phytochemical results
Phytochemical assessment was done using spectra intensity. The phytochemicals present in the ethanol extract were saponins (++), flavonoids (+), alkaloids (+), terpenoids (++), anthraquinones (++), and steroids (++), while tannins and cardiac glycoside were absent. The aqueous extract contained saponins (+++), flavonoids (+), anthraquinones (+), and alkaloids (+), while tannins, cardiac glycoside, terpenoids, and steroids were absent.
Acute toxicity study and effect of aqueous mushroom extract on weight
In vivo preliminary acute toxicity study showed that extract of P. ostreatus was non-toxic (no eye blinking, panting, lethargy, salivation, incoordination, or death in any of the treated groups of broiler chickens) including the group treated with the highest dose (600 mg/kg body weight).
Anticoccidial effect of Pleurotus ostreatus extract on oocyst output and ED50
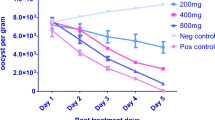
Administration of P. ostreatus extract to groups E (300 mg/kg), F (400 mg/kg), G (500 mg/kg), and H (600 mg/kg) and toltrazuril (25 mg/kg) to group B produced significant Eimeria oocyst reduction (P < 0.05) in broilers infected with mixed species of Eimeria when compared with group A untreated. The Eimeria oocyst output level in untreated group A remains very high. Oocyst reduction in group B treated with toltrazuril was significant and parasite clearance occurred 48 h post-treatment. There was clearance of parasites in groups G and H on day 4 and day 5 post-treatment, respectively. Anticoccidial effect of the extract on groups C–F was dose-dependent (Fig. 1a). The statistical parameter of the curve fitting analysis and the best-fit ED50 values for the aqueous extract were 448 mg/kg (Fig. 2).
Oocyst counts and average weight of broilers treated with Pleurotus ostreatus aqueous extract and toltrazuril. The asterisks (*) indicate significant difference (P < 0.05) when compared with the untreated group. Panel a shows significant difference in oocyst counts of treated groups B, E, F, G, and H when compared with the untreated group. Panel b shows significant difference in weight of groups B, E, F, and G when compared with the untreated group. Values are mean ± SEM (12 mice per group)
Weight gain
Changes in the body weight of broiler chickens infected with species of Eimeria pre- and post-treatment are shown in Fig. 1b. The weight in all the groups treated with the extracts and toltrazuril increased compared with the untreated control. There was weight loss of 3.9% in the untreated control group on the 32nd day with further weight loss to 12.5% by the 35th day. The group treated with toltrazuril had 36.8% weight gain, while the group treated with aqueous extract of P. ostreatus of 100, 200, 300, 400, 500, and 600 mg/kg had weight increase of 24.8, 33.3, 37.5, 44.7, 39.7, and 35.7%, respectively, post-treatment. Generally, the weight shows significant increase (P = 0.026) when compared to the untreated group. Tukey’s multiple comparison tests showed the progression of weight gain between treated groups which varied significantly (P < 0.05) between groups B, C, F, and G when compared with the untreated group. The weight of broilers treated with 400 mg/kg of P. ostreatus was slightly higher than that of the other groups (Fig. 1b). However, there was no significant difference (P < 0.05) between all the treated groups.
Faecal score
Brown or bloody diarrhoea (51–75%) was observed in all treatment groups (A–H) on day 28 pre-treatment. Group A which was untreated passed out bloody diarrhoea consistently throughout the study. The faeces in groups B (toltrazuril 25 mg/kg), G (500 mg/kg extract), and H (600 mg/kg extract) were observed to be normal on days 3, 5, and 4 post-treatment, respectively (Table 1).
Lesion score
The lesion scores showed numerous petechial haemorrhages to extensive haemorrhages in the intestines in all the groups (A–H). Necropsy findings showed that the broilers were infected with Eimeria tenella (caecal lesions), Eimeria acervulina, E. maxima, and Eimeria praecox (duodenal and jejunal lesions). The untreated group (A) showed numerous petechiae haemorrhages in the duodenal and caecal surfaces (both serosal and mucosal) in pre-treatment, mid-treatment, and post-treatment. Groups B treated with 25 mg/kg body weight toltrazuril and H treated with 600 mg/kg extract of P. ostreatus reduced lesions remarkably (Table 2).
Haematology
The mean packed cell volume was significantly higher in the treated groups (P = 0.0094, r2 = 0.64, F = 4.08) when compared with the untreated group. The PCV values obtained in the untreated group ranged 27.3–33.2%, while the treated group ranged 31.1–40%. Hence, the packed cell volume (PCV) in all treated groups was slightly higher on day 4 post-treatment (30–37%) and further increased (31.3–40.0%) on day 7 post-treatment (Fig. 3a). Haemoglobin (Hb) concentration was higher in treated broilers but not significantly (P > 0.05) when compared with the untreated group (Fig. 3b). The Hb values ranged from 7.7 to 11.9 g/dl. The white blood cells (WBC) in the treated group reveals significant decrease (P = 0.0368) when compared with the untreated group (Fig. 3c). The red blood cells showed significant increase in the treated groups (P = 0.04, r2 = 0.538, F = 2.66) when compared with the untreated group (Fig. 3d). The red blood cell values range from 1.5 to 3.4 × 1012/l. Mean corpuscular volume of the group treated with toltrazuril and groups treated with 100 and 500 mg/kg showed significant (P < 0.05) decrease compared with the untreated group (Fig. 4a). Those treated with 200, 300, 400, and 600 mg/kg of P. ostreatus have their MCV decreased but not significantly when compared with the untreated group. The mean corpuscular haemoglobin (MCH) of all the treated groups decreases significantly (P < 0.05) when compared with the untreated control group (Fig. 4b). However, the mean corpuscular haemoglobin concentration (MCHC) varied among the treated groups with no significant (P > 0.05) difference when compared with the untreated group (Fig. 4c). Differential counts (heterophils, lymphocytes, eosinophils, monocytes, basophils, and thrombocytes) of broiler chickens naturally infected with mixed Eimeria species revealed heterocytosis, eosinophilia, lymphopaenia, and thrombopaenia in the untreated group on day 35 (Fig. 5a–f). All the treated groups maintained constant heterophil counts throughout the experiment (Fig. 5a). On day 4 post-treatment, treated groups showed lymphopaenia although not significantly (P > 0.05) from the untreated groups. However, an increase was observed on day 7 post-treatment, but did not rise to the pre-treatment threshold counts (Fig. 5c). The eosinophil counts observed in broilers treated with 400 and 500 mg/kg of P. ostreatus showed marked eosinopaenia on day 7 post-treatment when compared with the untreated group (Fig. 5b). An initial monocytosis was observed on day 4 post-treatment before it reverted to basal level on day 7 post-treatment in all the groups except group E (Fig. 5d). Basophils were only observed in group B at before treatment commenced and none was observed throughout the course of experiment (Fig. 5e). Thrombopaenia was observed in untreated control group on day 35; however, no significant changes were observed in all the treated groups (Fig. 5f).
a–c Blood differential counts of experimental broilers pre-treatment, mid-treatment, and post-treatment with Pleurotus ostreatus aqueous extract. The asterisks (*) indicate significant difference (P < 0.05) when compared with the untreated group. d–f Blood differential counts of experimental broilers pre-treatment, mid-treatment, and post-treatment with Pleurotus ostreatus aqueous extract. The asterisks (*) indicate significant difference (P < 0.05) when compared with the untreated group. Each column represents the mean ± SEM. ANOVA shows significant effect of groups E and F (b) of treated broilers compared with the negative control group
Discussion
For many decades, conventional coccidiosis control methods have relied solely on anticoccidial chemical compounds and live vaccines; however, the development of drug-resistant strains of Eimeria and an increasing concern over the presence of drug residues in animal products led researches into alternatives in the form of plant extracts containing many compounds which are safe, effective, and cheaper (Abbas et al. 2012). This study was designed to investigate anticoccidial effect of P. ostreatus extract against field isolates of Eimeria species of broiler birds.
Acute toxicity study of P. ostreatus extract proved to be safe and without any side effect at the highest dose of 600 mg/kg which could be the reason it is consumed by people of different races since ancient time. The aqueous extract of P. ostreatus demonstrated an ED50 value of 448 mg/kg on day 3 of post-treatment. The therapeutic potency of 600 mg/kg extract in broilers infected with field strains of Eimeria species was 100% on the day 4 post-treatment and birds remained Eimeria-free throughout the experiment, while the group treated with toltrazuril demonstrated oocyst clearance 48 h post-treatment. While the crude extracts phytochemical components of plants have been well-investigated in several studies, only few have been on medicinal mushrooms (Wandati et al. 2013). The significant (P < 0.05) oocyst reduction in group H when compared with the untreated group could be due to P. ostreatus strong antioxidant components. Aqueous and methanolic extracts of P. ostreatus possess phenolic compounds with high antioxidant properties (González-Palma et al. 2016). The difference in oocyst reduction time between toltrazuril and extract of P. ostreatus could probably be due to the components with anticoccidial properties that have not been separated and purified compared to synthetic drug where chemical compounds are purified.
The effectiveness of anticoccidia can further be evaluated from weight measurements of the chickens. Birds treated with the extract of P ostreatus, on average, had improved body weight at comparable levels to pre-treatment values which could be due to its anticoccidial, anti-stress, appetite-stimulating, and immune-enhancing abilities even though the difference is not significant (P > 0.05), while those in the untreated infected group demonstrated progressive reduction in body weights throughout the study. The reason might be due to the damaging effect of coccidial parasites in the intestine leading to loss of appetite and poor absorption of nutrients.
During necropsy, haemorrhagic lesions reduced in all treated groups especially at high dose of P. ostreatus extract. This might be as a result of reduction in the number of Eimeria sporozoites causing the lesions. The untreated group was observed to retain numerous petechial haemorrhages throughout this study which may be due to the damaging effect of the coccidial parasites. The lesions observed during necropsy showed that broilers were infected with Eimeria acervulina, Eimeria maxima, Eimeria praecox (affecting the upper intestine), and Eimeria tenella (caeca). The reduction in the number of sporozoites causing damages to the intestinal epithelium was responsible for the decrease in bloody diarrhoea in groups treated with extract. The haematological parameters of broiler chickens naturally infected with Eimeria species were not significantly (P > 0.05) different in all treated groups but there was slight increase in packed cell volume. This may be due to reduction in the number of sporozoites causing blood loss from the epithelial lining of the intestine and could also be due to strong amino acid constituents of P. ostreatus mushroom extract. The absence of anaemia in the treated birds could be due to the compensatory increase in absorption of nutrients in greater parts of jejunum and ileum that were not infected coupled with the immunomodulatory effect of the extract administered. The control group had significantly high MCV and MCH values which indicated macrocytosis coupled with anaemia. This could be due to constant electrolyte and blood losses from intestinal and caeca bleeding. There was a significant (P < 0.05) reduction in eosinophil count of infected broilers treated with the extract. The reason could be because P. ostreatus possesses immune-stimulating ability. Following bloody faeces, after which the broilers have shown heavy signs of infection, a progressive leucocytosis was observed in the untreated group which proved to be significantly different when compared with the treated group. Lymphocytosis was observed before the commencement of the treatment and the groups treated with higher doses of the extract showed lowered oocyst output which seemed to prevent lymphocytosis, suggesting that this phenomenon is a result of parasite load. However, the lymphopaenia in the control group could be due to affected lymphatic organs. In conclusion, the aqueous extract of Pleurotus ostreatus mushroom demonstrated anticoccidial activity as observed in the inhibition of Eimeria oocyst output. Therefore, the use of the extract may be an effective alternative medicine for the treatment of coccidiosis in poultry production. Further studies should be carried out to determine the mechanism of action of Pleurotus ostreatus extract. Bioactivity-guided fractionation could also give a better understanding of the active component(s) responsible for anticoccidial and immune-enhancing activity.
References
Abbas, R.Z., Iqbal, Z., Blake, D., Khan, M.N. & Saleemi, M.K. (2011). Anticoccidial drug resistance in fowl coccidia: the state of play revisited. World’s Poultry Science Journal, 67, 337–350.
Abbas, R.Z., Colwell, D.D. & Gilleard, J. (2012). Botanicals: an alternative approach for the control of avian coccidiosis. World's Poultry Science Journal, 68, 203–215.
Ademola, I.O. & Odeniran, P.O. (2016). Novel trypanocide from an extract of Pleurotus sajor-caju against Trypanosoma congolense. Pharmaceutical Biology, 55(1), 132–138.
Allen, P.C., Danforth, H.D. & Augustine, P.C. (1998). Dietary modulation of avian coccidiosis. International Journal of Parasitology, 28, 1131–1140.
Borchers, A.T., Stern, J.S., Hackman, R.M., Keen, C.L. & Gershwin, M.E. (1999). Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biological Medicine, 221, 281–293.
Chapman, H.D. (2000). Practical use of vaccines for the control of coccidiosis in the chicken. World's Poultry Science Journal, 56, 7–20.
Eloff, J.N. (1998). Which extract should be used for the screening and isolation of antimicrobial components from plants. Journal of Ethnopharmacology, 60, 1–8.
Estrada, A., Yun, C.H., Andrew, V.K., Bing, L., Shirley, H. & Bernard, L. (1997). Immunomodulatory Activities of Oat β-glucan In vitro and In vivo. Microbiology and Immunology, 41, 991–998.
Ghafoor, A., Badar, H., Hussain, N. & Tariq, N. (2010). An empirical estimation of the factors affecting demand and supply of poultry meat. Pakistan Veterinary Journal, 30, 172–174.
González-Palma, I., Escalona-Buendía, H.B., Ponce-Alquicira, E., Téllez-Téllez, M., Gupta, V. K., Díaz-Godínez, G. & Soriano-Santos, J. (2016). Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Frontiers in Microbiology, 12, 1–9.
Hafez, H.M. (2011). Enteric Diseases of Poultry with Special Attention to Clostridium perfringens. Pakistan Veterinary Journal, 31, 175–184.
Iwalokun, B.A., Usen, U.A., Otunba, A.A. & Olukoya, D.K. (2007). Comparative phytochemical evaluation, antimicrobial and antioxidant properties of Pleurotus ostreatus. African Journal of Biotechology, 6(15), 1732–1739.
Jain, N.C. (1986). Schalm’s veterinary hematology. 4th ed. Philadelphia (PA): Lea and Febiger. p. 610–612.
Johnson, J. & Reid, W.M. (1970). Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28, 30–36.
Jung, W., Chun-Nam, C., Yeo-Eun, L., Chang-Yeol, Y., Eun-Kee, P., Suk, K. & Hu-Jang, L. (2011). Anti-diarrheal effects of a combination of Korean traditional herbal extracts and dioctahedralsmectite on piglet diarrhea caused by Escherichia coli and Salmonella typhimurium. Pakistan Veterinary Journal, 31, 336–340.
Koinarski, V., Georgieva, N., Gadjeva, V. & Petkov, P. (2005). Antioxidant status of broiler chickens infected with Eimeria acervulina. Revue de Medecine Veterinaire 156, 498–502.
Sanjay, P., Kartik, D.V., Pithawala, E.A., Shukla, M.D., Lahiri, S.K., Jain, N.K. & Modi, H.A. (2015). Phytochemical screening, total phenolic content, antimicrobial and antioxidant activity of wild edible mushroom Pleurotus ostreatus. International Research Journal of Pharmacy, 6(1), 65–69.
Seifert, H. (2006). Tropical Animal Health. Kluwee academic Publishers, Boston. 57.
Selegean, M., Putz, M.V. and Rugea, T. (2009). Effect of polysaccharide extract from the edible mushroom Pleurotus ostreatus against infections bursal disease virus. International Journal Molecular Science, 10, 3616–3634.
Shirley, M.W. & Lillehoj, H.S. (2012). The long view: a selective review of 40 years of coccidiosis research. Avian Pathology, 41, 111–121.
Vassilev, D.G. (2002). Special modification of McMaster. In: B. Cumming (ed). Manual of veterinary parasitological techniques (Central Veterinary Laboratory, London).
Wandati, T.W., Kenji, G.M. & Onguso, J.M. (2013). Phtochemicals in edible wild mushrooms from selected areas in Kenya. Journal of Food Research, 2(3), 137–144.
Youn, H.J. & Noh, J.W. (2001). Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Veterinary Parasitology, 96, 257–263.
Zaman, M.A., Iqbal, Z., Abbas, R.Z. & Khan, M.N. (2011). Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology, 139, 237–243.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ademola, I.O., Ojo, P.O. & Odeniran, P.O. Pleurotus ostreatus extract inhibits Eimeria species development in naturally infected broiler chickens. Trop Anim Health Prod 51, 109–117 (2019). https://doi.org/10.1007/s11250-018-1665-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1665-9