Abstract
This study was designed to investigate the sensitivity and specificity of three methods for detecting the onset of cyclicity in post-partum Murrah buffaloes. The methods investigated were visual signs, transrectal ultrasonography, and serum progesterone (P4) assay. For this study, 102 post-partum Murrah buffalo cows were grouped for monitoring their ovarian activity. The first group of buffaloes was between 26 and 35 days post-partum. Thereafter, the buffalo cows that calved were grouped after every 10 days for the study sample. Thus, the study animals were adjudged between 26 and 35, 36–45, 46–55, 56–65, 66–75, 76–85, and 86–95 days post-partum with an average of 30, 40, 50, 60, 70, 80, and 90 days post-partum, respectively. Visual estrus signs were monitored twice daily, and simultaneously, ultrasound examination was carried out at 10 days interval for accessing the presence of corpus luteum (CL). Serum P4 was estimated in the animals which were adjudged cyclic by ultrasound examination, and the assay was repeated after 10 days. The buffalo cows in estrus were inseminated artificially, and pregnancy status was assessed after 30 days post-insemination. In this study, the sensitivity and specificity of visual observation were low (39.37 and 70.73%, respectively) when compared to P4 assay (98.80 and 96.47%) and ultrasound examination (single, 97.59 and 97.59%; double, 100 and 100%), respectively. Furthermore, the sensitivity and specificity of single and double ultrasound examination and P4 assay were comparable. In conclusion, this study reports that single and double ultrasound examination and P4 assay are more efficient than visual observation in detecting the onset of ovarian cyclicity in post-partum Murrah buffaloes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrus detection is a pre-requisite for efficient reproductive management in farm animals, and any failure in estrus detection affects the lifetime productivity and profitability of livestock (Rao et al. 2013). Conception rate is correlated to the proper estrus detection and timing of insemination (Verma et al. 2014). This is all more true among buffaloes as they exhibit silent heat, especially during the summers (Das and Khan 2010). Silent heat is difficult to detect, and besides, when buffaloes do exhibit signs of estrus in summer, it is likely to be in the cooler part of the day, i.e., early morning hours (Singh et al. 2000). The optimum lifetime productivity of buffaloes is achievable only if the inter-calving period is around 12–15 months. This is attainable only when the days open is optimum (60–90 days) (Perera 2011). The onset of ovarian cyclicity is correlated with the post-partum fertility which depends upon several non-genetic (estrus detection method, management, nutrition, and season) and genetic factors (breed, lactation yield, stage of lactation, and suckling) (Roy et al. 2003).
The findings of studies by Kumaresan et al. (2001) and Verma et al. (2014) indicated that in a well-managed buffalo herd, the accuracy of visual estrus detection is quite low (50%), and only 20% of buffaloes detected in estrus are inseminated at the appropriate period. Though buffaloes exhibit estrus signs similar to those of cattle, the presence of smaller ovaries and deeply embedded corpus luteum (CL) when compared to zebu and taurine cattle make the monitoring of ovarian cyclicity in buffaloes difficult using the conventional per-rectal examination (Terzano 2012; Baithalu et al. 2013).
In order to overcome the difficulties in detecting ovarian activity in buffaloes, one method is the assessment of progesterone (P4) levels either in milk or blood (Kamboj and Prakash 1993). Progesterone concentration during the luteal phase and pregnancy usually ranges from 1 to 4 ng/ml (Roy and Prakash 2009). However, the estimation of the P4 level is not routinely used for monitoring the ovarian activity due to the time-consuming analytical techniques and lack of specialized equipment (Balhara et al. 2013). In addition, transrectal ultrasonography provides an alternate method for monitoring the ovarian activity (Pierson and Ginther 1984; Baruselli et al. 1997). Use of transrectal ultrasonography to assess the follicular growth, development, and regression has significantly enhanced the understanding of ovarian activity in buffaloes (Rahman et al. 2012). The present study was designed to compare the three methods viz. visual signs of estrus, transrectal ultrasonography, and serum P4 assay for detecting the onset of ovarian cyclicity in post-partum Murrah buffaloes.
Materials and methods
Study location and experimental animals
The present study was carried out at ICAR-Central Institute for Research on Buffaloes, Hisar, India, located at 29.17 ′N latitude and 75.72° E longitude which is situated 212 m above the mean sea level. Murrah buffalo cows (primiparous, n = 21; pluriparous, n = 81) in their early stage of lactation, which calved between August 2015 and February 2016, were considered for this study. Buffaloes included for this study aged between 3 and 8 years (first to fifth lactation) with an average body weight of 547.6 ± 27.43 kg. All the animals were free from any genital diseases and had completed pregnancy to full term and normal parturition.
Management of animals
All the study animals were housed on concrete-floored sheds with asbestos roofing and sides which were partly closed during milking. After milking, the animals were moved into open paddocks adjoining each shed throughout the day. The animals were confined in the barn in the night during the winter months (November to January). The animals were provided with 6–8 kg of green fodder [Egyptian clover (Trifolium spp.) 45–55 days maturity and sorghum (Sorghum spp.) 55–60 days maturity] while dry fodder was provided as wheat straw (6–7 kg). Clean drinking water was provided ad libitum. Before each milking, the animals were individually fed concentrate supplemented with a mineral mixture. According to the quantity of milk produced, each animal was fed 3–4 kg of concentrate (0.75% of body weight). The concentrate contained 20% crude protein and 70% total digestible nutrients. The composition of the mineral mixture was as follows (kg/100 kg): di-calcium phosphate, 90.0; chalk powder, 4.0; zinc sulphate, 4.0; manganese sulphate, 1.5; copper sulphate, 0.5; potassium iodide, 0.01; and common salt. The buffaloes were hand-milked twice daily, and suckling was allowed prior to each milking for let down of milk. All experimental procedures were carried out as per guidelines of the Institutional Animal Ethics Committee (IAEC) of ICAR-Central Institute for Research on Buffaloes, Hisar, India.
Visual signs of estrus
According to Mohan et al. (2010), visual signs of estrus viz. clear mucous discharge, frequent micturition, red and swollen vulva, mounting, allowing to be mounted by other buffaloes (within the herd), chin resting, nervousness, and restlessness were observed by a single herdsman of the farm twice daily during morning (6:00 am) and evening (6:00 pm) hours. Buffalo cows showing one or more estrus signs were identified, checked per rectum for estrus confirmation, and inseminated. Pregnancy was monitored by examining the inseminated buffaloes after 30 days post-insemination using transrectal ultrasonography, and pregnant buffaloes were periodically excluded from the study.
Transrectal ultrasound scanning
All animals underwent transrectal ultrasonography by the same operator using transrectal real-time ultrasound scanner (Model 320A, Toshiba) equipped with an intraoperative 7.0-MHz micro convex transducer. Based on the period of calving, the first group of buffaloes (between 26 and 35 days post-partum) were included in the study, and thereafter, animals calved every 10 days were grouped and included in the study (Dolezel et al. 2008). Buffalo cows thus progressed and adjudged in 26–35, 36–45, 46–55, 56–65, 66–75, 76–85, and 86–95 days post-partum with an average of 30, 40, 50, 60, 70, 80, and 90 days post-partum, respectively (Choudhary et al. 2017). The day of the second examination of the first group of buffaloes overlapped with the first examination of the second group, and this followed subsequently for each period of this investigation. First ultrasound examination of post-partum animals started from 30 days (26–35 days) post-partum, and subsequent examination was done at 10 days interval till the animals were adjudged acyclic (Sharma et al. 2012; Jerome et al. 2016).
Detection of ovarian cyclicity by performing transrectal ultrasonography once or twice (at 10 days interval) was termed as single or double ultrasound examination, respectively. Animals were adjudged cyclic if they exhibited any one of the following characteristics during ultrasound examination: (a) presence of CL (luteal phase), (b) uterine tone with follicle > 12 mm (estrus), and (c) uterine tone with follicle < 7 mm (met-estrus) (Jerome et al. 2016).
Blood sampling and P4 assay
Blood sample (10 ml) was taken from the jugular vein upon detection of the CL by transrectal ultrasonography, and subsequently, a second blood sample was collected after 10 days. Acyclic buffaloes, 80 days after the first sampling, were re-sampled between 80 and 90 days for the P4 assay. Ovarian cyclicity status of the buffaloes was ascertained by CL detection ultrasonography 10 days apart which was further confirmed by P4 assay at 10 days interval.
For P4 assay, serum was collected from the sampled blood after centrifugation at 3000 rpm for 10 mins and stored at − 20 °C. Serum was thawed at 4 °C, 12 h before the assay, and P4 was estimated using a solid-phase enzyme immunoassay kit (Xema Co., Ltd., Moscow, Russia). The intra- and inter-assay variations were < 5 and < 10, respectively, with a sensitivity of 0.15 ng/ml (Ayad et al. 2014; Pandey et al. 2016).
Sensitivity and specificity
The sensitivity of estrus detection of a test was expressed as the percentage of animals identified by the test as being in estrus relative to the number truly in estrus. Specificity was defined as the proportion of non-ovulated cows in which no estrus was detected. Sensitivity and specificity were calculated according to Firk et al. (2002). Correctly detected estrus was categorized as true positive, whereas undetected estrus was considered as false negative. Non-detection of estrus during the study period was categorized as true negative, and wrongly detected estrus during the study period was termed as false positive.
Statistical analyses
Data was analyzed with SPSS (Version 16) using chi-square test to compare the efficiency of different methods in detecting the onset of cyclicity in post-partum buffaloes, and the results were considered significant at P < 0.05.
Results
Visual signs of estrus
Visual signs of estrus were not evident in 44 buffaloes (41.30%), i.e., they remained undetected; however, they were confirmed to be cyclic using ultrasonography. In this study, 19 buffaloes (18.62%) remained acyclic till 90 days post-partum. These findings (Tables 1 and 2) indicate that estrus was successfully detected visually in 29 (28%) buffaloes, and among the undetected estrus, 41.30% of the buffaloes remained acyclic after 90 days post-partum. The most common visual signs exhibited by the oestrous buffaloes were mucous discharge followed by frequent micturition (Table 1). The buffaloes were thus categorized as true positive (n = 29), false positive (n = 12), false negative (n = 44), and true negative (n = 17). The sensitivity and specificity of estrus detection by visual observation were 39.73 and 70.73%, respectively.
Transrectal ultrasound scanning
By single transrectal ultrasonography, 81 and 17 buffaloes were found to be cyclic and acyclic, respectively, till 90 days post-partum (Table 2). By using this method (single transrectal ultrasonography), there were 81, 2, 2, and 17 buffaloes who could be categorized under true positive, false positive, false negative, and true negative categories, respectively. However, when the transrectal ultrasonography was repeated (double ultrasonography), 83 and 19 buffaloes were categorized as true positive and true negative, respectively, indicating that there was no significant improvement in assessment of pregnancy status between the two transrectal ultrasonography procedures. The sensitivity of the transrectal ultrasonography procedure was significantly higher when compared to the visual method (Tables 2 and 3).
Progesterone assay
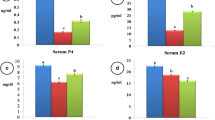
Based on the P4 assay, 83 and 19 buffaloes were adjudged to be cyclic and acyclic, respectively. The results also indicated the presence of an outlier among the group where the P4 level was < 1 ng/ml. In this study, the average P4 levels estimated were 2.34 ± 0.23 ng/ml and 0.53 ± 0.07 ng/ml in cyclic and acyclic buffaloes, respectively. True positivity of resumption of ovarian cyclicity was higher (P < 0.01) when compared to visual observation. Buffaloes under study were categorized as true positive (n = 82), false positive (n = 3), false negative (n = 1), and true negative (n = 16) using P4 assay. The sensitivity and specificity of the double P4 assay were 98.80 and 96.47%, respectively (Tables 2 and 3).
Comparison between visual observation, transrectal ultrasonography, and P4 assay
Visual observation of estrus differed significantly from single and double (P < 0.0001) transrectal ultrasonography and P4 assay (P = 0.0113). Furthermore, it was interesting to note that non-significant difference was observed between single and double transrectal ultrasonography and P4 assay in detecting the onset of ovarian cyclicity in post-partum Murrah buffaloes (Table 4).
Discussion
The findings of this study indicate that estrus detection using visual signs was less successful (39.73%) as compared to other methods (ultrasonography and P4 assay) as buffaloes, being “shy breeders,” exhibit silent heat during the summers (Suthar and Dhami 2010). Our findings agree with Kumaresan et al. (2001) who reported that estrus remains undetected in several well-managed herds, resulting in a faulty time of insemination thereby lowering the efficacy of the artificial insemination procedure. Besides, faulty detection of estrus by visual observation can be attributed to the errors/misinterpretation in addition to the poor expression of estrus signs in buffaloes (Srivastava et al. 1999; Kumaresan et al. 2001; Abdalla 2003; Dubey et al. 2015). Furthermore, estrus signs differ significantly between the buffalo heifers and cows but remained comparable across parity in buffaloes (Verma et al. 2014) and cows (Løvendahl and Chagunda 2010). Therefore, studies on the effect of parity on ovarian cyclicity onset across parity and season in buffaloes need to be investigated in the future.
The sensitivity and specificity of transrectal ultrasonography (single and double) were in accordance with Ribadu et al. (1994) in cows. In contrast, Zdunczyk et al. (2009) reported lower sensitivity (94.7%) and specificity (84%) using transrectal ultrasonography in cows. This difference could be ascribed to the type of transducers used and the experience of the examiner. Likewise, false positive and false negative results obtained in this study could be due to short oestrous cycle, misinterpretation during transrectal ultrasonography, and non-detection of early or late CL (Archbold et al. 2012; Perry and Cushman 2016).
With respect to P4 assay, the onset of ovarian cyclicity was confirmed by peripheral P4 level (> 1 ng/ml). Considering double ultrasound scanning as the gold standard for detecting the onset of post-partum cyclicity, P4 assay showed higher sensitivity (98.8%) and specificity (96.47%) than visual signs (Broes and LeBlanc 2014; Gómez-Seco et al. 2017). The study indicated that the P4 assay (98.80%) was comparable to single (97.59%) and double (100%) ultrasonography. In this study, the sensitivity and specificity of P4 assay were higher in comparison to the findings of Kaul and Prakash (1994) and Kumaresan et al. (2001) in buffaloes attributing to the difference in the assay used (Broes and LeBlanc 2014). Moreover, the erroneous (false positive and false negative) results of P4 assay can be attributed to non-detection of P4 secreted by early developing and/or regressing CL (Perry and Cushman 2016).
Higher sensitivity and specificity of ultrasound examination (single and double) and P4 assay as compared to visual observation have been reported earlier (Zdunczyk et al. 2009; Perry and Cushman 2016). Moreover, comparable specificity of ultrasound examination (single and double) and P4 assay confirms the effectiveness for monitoring the ovarian cyclicity (Stevenson et al. 2008; Gómez-Seco et al. 2017). Nonetheless, better training combined with high-resolution digital imaging (Firke 2002; Cengiz et al. 2017) can contribute to higher sensitivity and specificity of transrectal ultrasonography of the reproductive system under farm and field conditions in buffaloes.
In summary, single and double ultrasound examination and P4 assay are more efficient than visual observation in detecting the onset of ovarian cyclicity in post-partum Murrah buffaloes.
References
Abdalla, E.B., 2003. Improving the reproductive performance of Egyptian buffalo cows by changing the management system, Animal Reproduction Science, 75, 1–8.
Archbold, H., Shalloo, L., Kennedy, E., Pierce, K.M. and Buckley, F., 2012. Influence of age, body weight and body condition score before mating start date on the pubertal rate of maiden Holstein-Friesian heifers and implications for subsequent cow performance and profitability, Animal, 6, 1143–51.
Ayad, A., IguerOuada, M. and Benbarek, H., 2014. Electro-chemiluminescence immunoassay for progesterone by using a heterologous system in plasma bovine, Veterinary World, 7(8), 610–613.
Baithalu, R. K., Singh, S.K., Gupta, C., Raja, A. K., Saxena, A., Kumar, Y., Singh, R. and Agarwal, S.K., 2013. Cellular and functional characterization of buffalo (Bubalus bubalis) corpus luteum during the estrous cycle and pregnancy, Animal Reproduction Science, 140, 138–146.
Balhara, A.K., Gupta, M., Singh, S., Mohanty, A.K. and Singh, I., 2013.Early pregnancy diagnosis in bovines, current status and future directions, The Scientific World Journal, 2013, 958540. https://doi.org/10.1155/2013/958540.
Baruselli, P.S., Mucciolo, R.G., Visintin, J.A., Viana, W.G., Arruda, R.P., Madureira, E.H., Oliveira, C.A. and Molero-Filho, J. R., 1997.Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis), Theriogenology, 47, 1531–1547.
Broes, A. and LeBlanc, S.J., 2014. Comparison of commercial progesterone assays for evaluation of luteal status in dairy cows, Canadian Veterinary Journal, 55, 582–584.
Cengiz, M., Çolak, A., Hayirli, A. and Cannazik, O., 2017. Optical density changes in ultrasonographic images of the endometrium and corpus luteum in pregnant and cyclic cows, Turkish Journal of Veterinary and Animal Sciences, 41, 18–24.
Choudhary, K. K., Bharadwaj, A., Sharma, R.K., Jerome, A. and Khanna, S., 2017. Relationship of temperament with estrous behaviour, resumption of ovarian cyclicity and milk yield in postpartum Murrah buffaloes, Reproduction in Domestic Animals, 52(6), 962–968.
Das, G.K. and Khan, F. A., 2010.Summer anoestrus in buffalo—a review, Reproduction in Domestic Animals, 45(6), e483–94.
Dolezel, R., Vecera, M., Palenik, T., Cech, S. and Vyskocil, M., 2008.Systematic clinical examination of early postpartum cows and treatment of puerperal metritis did not have any beneficial effect on subsequent reproductive performance, Veterinarni Medicina, 53(2), 59–69.
Dubey, P., Singh, R.R., Choudhary, S.S. and Kharadi, V.B., 2015. Post-partum female sexual behaviour in Surti buffaloes, Livestock Research International, 3(4), 77–81.
Firk, R., Stamer, E., Junge, W. and Krieter, J., 2002. Automation of oestrus detection in dairy cows: a review, Livestock Production Science, 75(3), 219–232.
Fricke, P.M., 2002. Scanning the future-ultrasonography as a reproductive management tool for dairy cattle, Journal of Dairy Science, 85, 1918–1926.
Gómez-Seco, C., Alegre, B., González-Montaña, J. R., Martínez-Pastor, F., Alonso, M. E., Prieto, J. G. and Domínguez, J. C., 2017.Evolution of the corpus luteum volume determined ultrasonographically and its relation to the plasma progesterone concentration after artificial insemination in pregnant and non-pregnant dairy cows, Veterinary Research Communications, 41, 183–188.
Jerome, A., Srivastava, S. K. and Sharma, R. K., 2016. Study on follicular characteristics, hormonal and biochemical profile in norgestomet+PMSG treated acyclic buffaloes, Iranian Journal of Veterinary Research, 17(4), 247–252.
Kamboj, M. and Prakash, B. S., 1993. Relationship of progesterone in plasma and whole milk of buffaloes during cyclicity and early pregnancy, Tropical Animal Health and Production, 25(3), 185–192.
Kaul, V. and Prakash, B.S., 1994. Application of milk progesterone estimation for determining the incidence of false estrus detection and ovulation failure in zebu and crossbred cattle and Murrah buffaloes, The Indian Journal of Animal Sciences, 64(10), 1054–1057.
Kumaresan, A., Ansari, M.R. and Sanwal, P.C., 2001. Assessment of accuracy of oestrus detection by progesterone assay in cattle and buffalo, The Indian Journal of Animal Sciences, 71(8), 758–760.
Løvendahl, P. and Chagunda, M.G.G., 2010. On the use of physical activity monitoring for estrus detection in dairy cows, Journal of Dairy Science, 93(1), 249–259.
Mohan, K., Kumar, V., Sarkar, M. and Prakash, B.S., 2010. Temporal changes in endogenous estrogens and expression of behaviors associated with estrus during the peri-ovulatory period in Murrah buffaloes (Bubalus bubalis), Tropical Animal Health and Production, 42(1), 21–26.
Pandey, N.K.J., Gupta, H.P., Prasad, S. and Sheetal, S.K., 2016. Plasma progesterone profile and conception rate following exogenous supplementation of gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone releasing intra-vaginal device in repeat-breeder crossbred cows, Veterinary World, 9(6), 559–562.
Perera, B.M., 2011. Reproductive cycles of buffalo, Animal Reproduction Science, 124 (3–4), 194–199.
Perry, G.A. and Cushman, R.A., 2016. Use of ultrasonography to make reproductive management decisions, The Professional Animal Scientist, 32, 154–161.
Pierson, R. A. and Ginther, O. J., 1984. Ultrasonography of the bovine ovary, Theriogenology, 21, 495–504.
Rahman, M.S., Shohag, A.S., Kamal, M.M., Parveen, N. and Shamsuddin, M., 2012. Application of ultrasonography to investigate post-partum anestrus in water buffaloes, Reproduction and Developmental Biology, 36, 103–108.
Rao, T.K.S., Kumar, N., Kumar, P., Chaurasia, S. and Patel, N.B., 2013. Heat detection techniques in cattle and buffalo, Veterinary World, 6(6), 363–369.
Ribadu, A.Y., Ward, W.R. and Dobson, H., 1994. Comparative evaluation of ovarian structures in cattle by palpation per rectum, ultrasonography and plasma progesterone concentration, Veterinary Record, 135, 452–457.
Roy, B., Mehla, R. K. and Sirohi, S. K., 2003. Influence of milk yield, parity, stage of lactation and body weight on urea and protein concentration in milk of Murrah buffaloes, Asian-Australian Journal of Animal Science, 16(9), 1285–1290.
Roy, K.S. and Prakash, B.S., 2009. Plasma progesterone, oestradiol-17β and total oestrogen profiles in relation to oestrous behaviour during induced ovulation in Murrah buffalo heifers, Journal of Animal Physiology and Animal Nutrition, 93, 486–495.
Sharma, R.K., Singh, J.K., Khanna, S. and Singh, I., 2012. Ovarian response of pre-pubertal Murrah heifers to exogenous GnRH, Animal Reproduction Science, 133, 153–158.
Singh, J., Nanda, A.S. and Adams, G.P., 2000. The reproductive pattern and efficiency of female buffaloes, Animal Reproduction Science, 60, 593–604.
Srivastava, S.K., Sanwal, P.C., Umashankar, U., Varshney, V.P. and Sahni, K.L., 1999. Pregnancy rates in bovines based on plasma progesterone concentration during AI under farm and village conditions, The Indian Journal of Animal Sciences, 69(6), 396–397.
Stevenson, J.S., Tenhouse, D. E., Krisher, R. L., Lamb, G. C., Larson, J. E., Dahlen, C. R., Pursley, J. R., Bello, N. M., Fricke, P. M., Wiltbank, M. C., Brusveen, D. J., Burkhart, M., Youngquist, R. S. and Garverick, H. A., 2008. Detection of anovulation by Heat mount detectors and transrectal ultrasonography before treatment with progesterone in a timed insemination protocol, Journal of Dairy Science, 91 (7), 2901–2915.
Suthar, V.S. and Dhami, A.J., 2010. Estrus detection method in buffalo, Veterinary World, 3(2), 94–96.
Terzano, G.M., 2012. Ultrasonography and reproduction in buffalo, Journal of Buffalo Science, 1, 163–173.
Verma, K.K., Prasad, S., Mohanty, T.K., Kumaresan, A., Layek, S.S., Patbandha, T.K. and Kantwa, S.C., 2014. Behavioural signs of estrus in different parity of Murrah buffaloes (Bubalus bubalis): a comparative study, Indian Journal of Animal Research, 48(6), 620–624.
Zduńczyk, S., Janowski, T., RAŚ, A. and Barański, W., 2009. Accuracy of ultrasonography and rectal palpation in the diagnosis of silent heat in cows compared to plasma progesterone concentration, Bulletin of the Veterinary Institute, Pulawy, 53, 407–410.
Acknowledgments
The authors thank the Director, ICAR-CIRB for providing the necessary facilities for conducting this experiment. The funding of this research work under AICRP on Nutritional and Physiological approaches for enhancing reproductive performance in animals by Indian Council of Agricultural Research, New Delhi, is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Choudhary, K.K., Bharadwaj, A., Sharma, R.K. et al. Efficacy of different methods for detecting the onset of ovarian cyclicity in post-partum Murrah buffaloes. Trop Anim Health Prod 50, 1559–1564 (2018). https://doi.org/10.1007/s11250-018-1595-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1595-6