Abstract
Soy-based fatty acid methyl ester disulfide (FAME-S2) was synthesized in good yield by oxidation of polymercaptanized soybean oil fatty acid methyl ester (PM-FAME). The chemical structure of FAME-S2 is of interest because of its potential as a biobased multi-functional additive in lubricant formulations. Neat FAME-S2 and their blends (1–10% w/w) in polyalphaolefin (PAO-6) and high oleic sunflower oil (HOSuO) were characterized for its chemical, physical and tribological properties. Blends of FAME-S2 in HOSuO relative to similar blends of PM-FAME displayed higher oxidation onset temperature (> 15 °C) that remained constant with increasing concentration. Evaluation of FAME-S2 as an extreme pressure (EP) additive on a 4-ball tribometer showed increasing weld point (WP) with increasing concentration to a maximum of 220 and 180 kgf in HOSuO and PAO-6, respectively, at 10% w/w concentration. The results indicate that FAME-S2 has both anti-oxidant and EP properties and can be applied as a multi-functional biobased additive in lubricant formulation. This work demonstrates an encouraging progress towards the development of effective biobased lubricant additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There has been a steady progress in the development and commercialization of biobased lubricants. However, biobased lubricants still comprise a meager 1% of the global lubricant market [1]. Thus, more work is needed to develop biobased lubricants that are commercially competitive in performance and cost. This will require progress in both biobased base oil and biobased additive development.
There has been some progress in the commercial use of soybean and other vegetable oils and their derivatives for biobased base oil and additives applications [2]. As a result, formulators of biobased lubricants are not totally dependent on commercial petroleum based additives for their lubricant development. In addition, there are sustained efforts at developing new biobased additives based on soybean and other vegetable oils. One example is the biobased extreme pressure (EP) additive obtained from the transesterification reaction between high oleic sunflower oil (HOSuO) and lipoic acid. Lipoate ester products did display good EP properties but were not as effective as the commercial EP additive di-t-dodecyl pentasulfide (TPS-32, from Arkema) [3, 4]. A second example involves a series of biobased phosphonate anti-wear (AW) additives synthesized by the reaction of dialkyl phosphites with the double bond of limonene, vegetable oils (soybean oil, high oleic sunflower oil), or their derivatives (methyl oleate, methyl linoleate) [5,6,7,8]. In all cases, these phosphonate biobased AW additives displayed wear scar diameters in a 4-ball AW test that were comparable or lower than those from similar concentrations of zinc dialkyl dithiophosphate (ZDDP) [5,6,7,8], which is a common commercial AW additive used in engine and gear oils, hydraulic and metalworking fluids, etc.
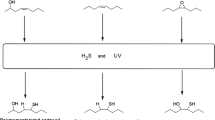
In the work described here, we discuss the synthesis and investigation of disulfides of soybean oil fatty acid methyl esters (FAME-S2) as biobased additives for EP and other properties. FAME-S2 is synthesized by the oxidation of fatty acid methyl ester of polymercaptanized soybean oil (PM-FAME). Polymercaptanized soybean oil (PM-SOY) is a commercial product obtained by the reaction of soybean oil with hydrogen sulfide using a patented procedure [9]. PM-SOY has been investigated for its tribological properties [10] and as a precursor for the synthesis of a wide range of products [11, 12].
Oxidation of thiols to disulfides is an important and well-known reaction [13,14,15,16,17,18,19], and a subject of active research [20,21,22,23,24,25]. The oxidation of thiols to disulfides using air as an oxidant without catalyst has been reported [21, 22]. Dimethyl sulfoxide has been reported to be a good oxidant of thiols to disulfides [15], but the reaction releases highly odorous and volatile dimethyl sulfide. A promising method [25] involves Cs2CO3-catalyzed oxidation of alkyl thiols in ambient air, acetonitrile solvent and 60 °C. In the current work, we used a modified procedure of Kirihara et al. [17] to oxidize thiols to disulfides at room temperature In this method, the oxidizing reagent is H2O2, the catalyst is sodium iodide and the solvent is ethyl acetate. In the modified procedure, which was also reported by He et al. [19], tetrabutylammonium iodide was used as the catalyst instead of sodium iodide, and ethanol as the co-solvent. The modified procedure resulted in improved yield of secondary thiols.
Polysulfides are known to provide excellent lubricant additive properties, both as antioxidants and as EP additives [26,27,28]. It has been demonstrated that biobased disulfides based on lipoic acid and garlic oil display good EP properties [3, 4, 29]. Fatty acid methyl esters of polymercaptanized soybean oil (PM-FAME) did display some EP properties, which was attributed to the presence of some polysulfides in PM-SOY [30]. Since PM-FAME is derived from polymercaptanized soybean oil (PM-SOY), it is of interest to increase its polysulfide content using the oxidation reaction discussed above and depicted in Scheme 1. The product from the oxidation of PM-FAME according to Scheme 1 is FAME-S2. In the pages to follow, FAME-S2 synthesis, identification, properties and evaluation as lubricant additives is discussed.
2 Experimental
2.1 Materials
Polymercaptanized soybean oil (PM-SOY) was a free sample from Chevron-Phillips Chemical Company (Woodlands, TX, USA). High oleic sunflower oil (HOSuO) was obtained from Columbus Foods (Des Plaines, IL, USA). Polyalphaolefin (PAO-6), with a kinematic viscosity of 6 cSt at 100 °C, was a free sample from Ineos Oligomers (League City, TX, USA). Methanol, HPLC grade, and ethyl acetate, HPLC grade, were from EMD Millipore (Billerica, MA, USA). Hexanes, HPLC grade, tetrahydrofuran (THF), certified, toluene, certified ACS, Na2SO4, anhydrous, certified ACS, hydrogen peroxide, 30%, certified ACS, and Na2HPO4, certified ACS, and NaHCO3, certified ACS, were from Fisher Chemicals (Fair Lawn, NJ, USA). NaOMe, 25 wt. % in methanol, butylated hydroxytoluene (BHT), and tetrabutylammonium iodide, 98%, reagent grade, were from Sigma-Aldrich (St. Louis, MO, USA). Ethanol was from AAPER Alcohol and Chemical Company (Shelbyville, KY, USA).
2.2 Synthesis
2.2.1 Synthesis of Polymercaptanized Soybean Oil Fatty Acid Methyl Ester (PM-FAME)
To 1 L (972 g) Polymercaptanized Soybean Oil (PM-SOY), were added 300 mL methanol and 60 mL 25% sodium methoxide. The mixture was boiled at ~ 65 °C for 1 h, allowed to cool, the catalyst neutralized with 60 mL acetic acid, and the glycerol layer separated. The crude PM-FAME was dissolved in hexanes, washed three times with aq. sodium bicarbonate solution, dried overnight over anhydrous sodium phosphate, filtered over Whatman #4 filter and the hexanes removed by rotary evaporation at low pressure (30 mbars), 70 °C during 5 h. The solvent-free product was weighed to give 878 g of purified PM-FAME product or 90% yield.
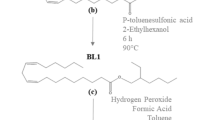
2.2.2 Synthesis of Fatty Acid Methyl Ester Disulfide (FAME-S2)
Ethyl acetate (1440 mL) and PM-FAME (405 mL) were mixed in a 5 L three-neck round-bottom flask equipped with a magnetic stirrer, condenser and temperature probe. While stirring, 30% hydrogen peroxide (144 mL) and a solution of 2.3 g tetrabutylammonium iodide in ethanol (720 mL) were added to the mixture. The homogeneous mixture was stirred for 8 h, after which the reaction was stopped, and 1 L deionized water and 1 L hexanes added to the mixture. The lower water phase was then removed and the top phase washed twice with disodium phosphate solution. The organic phase was dried over anhydrous sodium sulfate for 3.5 h, filtered over a Whatman #2v filter. The hexane solvent was then removed by two consecutive rotary evaporations at 43 mbar, 60 °C for 3 h; and at 2 mbar, 100 °C for 2 h.
2.3 Analysis and Identification
Fourier transform infrared (FTIR) spectra were obtained on a Varian 3100 FTIR spectrometer and 64 scans were averaged. The scans were collected at 5 kHz at a spectral resolution of 4 cm−1 from the neat sample on a multi-pass horizontal attenuated total reflectance accessory with Ge crystal.
Gas chromatography–mass spectroscopy (GC–MS) was used to monitor the oxidation reaction. Approximately 50 µL sample was diluted to 1 mL with ethyl acetate, and 0.2 µL were injected in a GC–MS for analysis. The GC–MS instrument was equipped with a model 6890 N GC oven from Agilent Technologies (Santa Clara, CA, USA) and a SPB-1 column (30 m × 0.25 mm × 0.25 μm) from Supelco Inc. (Bellefonte, PA, USA). The inlet temperature was 250 °C. The oven program was held for 3 min at 130 °C, followed by a ramp of 5 °C/min from 130 to 290 °C, with a H2 flow rate of 1 mL/min. A model 5973 Network MSD detector in electron impact mode from Agilent Technologies (Santa Clara, CA, USA) was used. The conditions were: mass range 34–750 amu, 22 sampling rate, electron multiplier 0 V relative (1059 V). The areas of the total ion counts were scaled to the combined areas of the methyl palmitate and methyl stearate peaks.
Nuclear magnetic resonance (NMR) spectra were obtained in CDCl3 on a Bruker Avance 500 NMR spectrometer (Bruker Corporation, Billerica, MA, USA) using a 5-mm BBO probe operating at 500 MHz for 1H NMR and 126 MHz for 13C NMR. Chemical shifts are reported in parts per million (ppm) from tetramethylsilane calculated from the lock signal. 1H, 13C, 13C-Dept 135, HSQC, HMBC, COSY and HSQC-TOCSY were acquired and analyzed.
Gel permeation chromatography (GPC) was conducted on unfiltered samples. The GPC consisted of a precolumn PLgel 3 μm Guard (50 × 7.5 mm, Part 1110–1320), two PLgel 3 μm mixed E columns (300 × 7.5 mm) from Agilent Technologies (Santa Clara, CA, USA) connected in a series, a Wyatt Optilab REX differential refractive index (dRI) detector from Wyatt Technology (Santa Barbara, CA, USA), a Waters 717 autosampler, and a Waters 1515 isocratic pump from Waters Corporation (Milford, MA, USA). About 40 mg of each sample was dissolved in 1 mL of THF with 0.1% BHT (stabilizer) and 0.5% toluene (internal standard for the flow rate). Calibration curve was prepared with EasiVial PS-L low-MW polystyrene standards from Agilent Technologies (Santa Clara, CA, USA). The standards contained 12 polystyrene samples with peak molecular weight from 162 to 45,120 Da. The log of molecular weight vs. elution volume was fitted to a cubic calibration curve.
2.4 Chemical and Physical Properties
Total acid number (TAN) was measured on 751 GPD Titrino (Metrohm Ltd., Herisau, Switzerland), according to the official AOCS Method Te 2a-64 [31].
Refractive index (RI) was measured as a function of temperature, in the range 30–80 °C, on a model Mark II Plus Abbe refractometer (Reichert Inc., Depew, NY). The instrument provides data to four-digit precision. RI at 100 °C was obtained by extrapolation of measured data.
Density, dynamic and kinematic viscosity as a function of temperature were obtained from measurements on a Stabinger SVM3000/G2 viscometer (Anton Paar GmbH, Graz, Austria) according to ASTM D 7042 [32].
Viscosity index (VI) was calculated from measured kinematic viscosity values at 40 and 100 °C, in accordance with ASTM D 2270-93 [33].
Onset (OT) and peak (PT) oxidation temperatures were measured on a Q20P pressurized differential scanning calorimeter (PDSC, TA Instruments—Waters LLC, New Castle, DE, USA) in accordance with ASTM D 6186 [34]. Tests were conducted using pressurized cells (500 ± 25 psig of pure oxygen) with positive oxygen flow rate of 100 ± 10 mL/min, as described before [3].
Pour point (PP) was measured according to ASTM D 97 on a Lawler Model DR-4-20L automated pour point analyzer (Lawler Manufacturing Corp., Edison, NJ, USA) [35].
Cloud point (CP) was measured according to ASTM D 2500 on a Lawler Model DR-4-10L automated cloud point analyzer (Lawler Manufacturing Corp., Edison, NJ, USA) [36]
2.5 Tribological Properties
Four-ball extreme pressure (EP) tests were conducted on a model KTR-30L and K93100 four-ball tribometers equipped with TriboDATA software (Koehler Instrument Company, Bohemia, NY, USA). At least two measurements per test lubricant were conducted, and average values are reported. EP tests were conducted according to ASTM D 2783 [37], which involves a series of 10 s tests with increasing load until welding of the four balls occurs. The load at which welding is observed is recorded as the weld point (WP) of the test lubricant.
Data analysis was conducted using IgorPro Version 5.0.3.0 software (WaveMetrics, Inc., Lake Oswego, OR, USA).
3 Results and Discussion
3.1 Synthesis and Characterization
Oxidation of PM-FAME in air without catalyst in ethanol solvent [22] did not produce disulfides. We followed published procedures [17, 19] using 30% H2O2 as oxidant, a small amount (~ 0.5 mol%) of tetrabutylammonium iodide salt as a catalyst, and ethanol-ethyl acetate co-solvents to ensure miscibility of the H2O2 with thiols.
The progress of the reaction was monitored by GC–MS. Several PM-FAME peaks could be identified: MW = 270 (methyl palmitate), MW = 298 (methyl stearate), MW = 296 (methyl oleate), MW = 294 (methyl linoleate), MW = 330 (monothiol, formed by addition of hydrogen sulfide to methyl oleate), MW = 328 (monothiols or cyclic sulfides, each formed by addition of one molecule hydrogen sulfide to methyl linoleate), MW = 362 (dithiol, formed by addition of two molecules of hydrogen sulfide to methyl linoleate), and MW = 360 (cyclic disulfides, formed by oxidation of dithiol). We used methyl palmitate and methyl stearate (which do not participate in the oxidation reactions) as internal standards to monitor the reaction. We observed a decrease of monothiols, a disappearance of dithiols, an increase in cyclic disulfides, and no change of cyclic sulfides as the reaction progressed (see Fig. 1).
One disadvantage of the GC–MS was that it did not detect the oligomeric fatty acids, formed due to sulfide or disulfide bridges between two or more fatty acid chains. These were analyzed using GPC, which clearly showed peaks corresponding to PM-FAME monomers, dimers, and higher oligomers (see Fig. 2 and Table 1). The increase of oligomeric structures in FAME-S2 is well-pronounced and indicates that many of the new bonds, formed in the FAME-S2, were intra-molecular (see Fig. 2).
GPC calibration using polystyrene is known to yield higher than the actual molecular weights of lipids and lipid-derived oligomers [5, 38]. The discrepancy is due to the differences in the limiting viscosity numbers of the two dissimilar compounds. A more accurate determination of molecular weight by GPC requires use of a universal calibration curve [39] or static light scattering method [40]. The light scattering method is not sensitive to low-molecular weight compounds and did not generate a useful signal for our oligomer samples. The universal calibration assumes that polymers with the same molecular weight and limiting viscosity number elute at the same volume, if the same column and conditions are applied. A universal calibration thus requires knowledge of the limiting viscosity numbers of the oligomers or an elaborate calculation procedure for its estimation [41, 42]. Since we did not apply a universal calibration method, the reported molecular weights from GPC are apparent molecular weights and expected to be higher than the actual values.
After the dithiols have been completely reacted, water and hexane were added to the reaction mixture to separate the hydrogen peroxide phase from the organic phase. GC–MS showed that the disappearance of dithiols was accompanied by the appearance of peaks with molecular weight of 360 Da, which corresponds to cyclic disulfides (see structures 2, 3, 4 in Scheme 1). No other GC–MS peaks were observed that correlate with the disappearance of the monothiols. The data from the GPC (see below) suggest that the monothiols were oxidized to dimeric disulfides (molecular weight > 650 Da), which probably were too big to pass through the GC–MS column.
In preliminary experiments, prolonged exposure of PM-FAME to H2O2/tetrabutylammonium iodide led to the formation of semisolid and solid products. A sample from such a prolonged exposure experiment was subjected to elemental analysis and showed approximate composition corresponding to C18.8H35.5O2.73S1.28Na1.09. This suggests that a significant amount of the thiols were oxidized to sulfenic acid (R–S–O–H) or its thiosulfinate precursor (R–S(O)–S–R) which, when washed with aqueous sodium bicarbonate, converted to sodium sulfenates (R–S–O–Na). Thus, in order to avoid such overoxidation, we shortened the oxidation time, which might have caused some unreacted monothiols in the final product.
FTIR did not show big differences between the spectra of PM-FAME and FAME-S2 (Fig. 3). This is due to the C–S, S–H and S–S bond stretches giving very weak IR signals [43]. There was a weak 2570 cm−1 signal due to the S–H stretches in the PM-FAME spectrum (see the inset of Fig. 3) that disappeared in the FAME-S2 spectrum. In the FAME-S2 spectrum, there were no new peaks at 1070–1030 cm−1 (sulfoxides S = O stretch), 1350–1300 or 1160–1120 cm−1 (sulfones and sulfonic acids S = O stretches). Such peaks should be very strong if present in the reaction mixture [44]. The FTIR thus indicates that the thiols had reacted but did not over-oxidize.
The 1H NMR showed that most regions of the molecule remained unchanged after oxidation of PM-FAME to FAME-S2 (See Fig. 4a). The signal associated with S–H groups (~ 1.45 ppm) disappeared, while the peak associated with HCS groups shifted from ~ 2.75 ppm (HCSH) to ~ 2.60 ppm (HCSS). There was no broad peak in the region 10.0–13.5 ppm to indicate the presence of a carboxylic acid [44].
a1H NMR spectra of PM-FAME (dotted line) and FAME-S2 (solid line); b partial 13C NMR spectra of PM-FAME (bottom blue line) and FAME-S2 (top red line). The numbers refer to the structures in Scheme 1 with the corresponding assigned peaks (Color figure online)
In 13C NMR (see Fig. 4b), the CSH signals at 41.1–40.8 ppm disappeared after oxidation while new signals appeared for CSS at 52.6–52.1 ppm. We attribute these new signals to 1,2-dithiane (six-membered cycles with 2 sulfur atoms, 3), 1,2-dithiepane (7-membered cycles with 2 sulfur atoms, 4) and to oligomer disulfides (see structure numbers in Schematic I). Signals appearing at 3.6 ppm (1H) and 55.8 ppm (13C) were attributed to the CH group in 1,2-dithiolane (five-membered ring with two S atoms, 2).
Odor and color are very important properties of sulfurized compounds, and are taken into account when deciding on whether or not to apply them in lubricant formulations. PM-SOY had a faint hydrogen sulfide odor which we attribute to some contamination or insufficient purging of the hydrogen sulfide used in its manufacture. PM-FAME obtained from PM-SOY had a weak odor of hydrogen sulfide, which disappeared after oxidation to FAME-S2. We attribute the low odor of PM-FAME and the absence of odor of FAME-S2 to their relatively high molecular weight and corresponding low volatility. PM-SOY has a light yellow color, which didn’t change much after conversion to PM-FAME. On the other hand, FAME-S2 had a darker yellow or amber color.
3.2 Neat Oil Properties
In this section, we compare the properties of PM-FAME, and FAME-S2 to each other and to biobased (high oleic sunflower oil, HOSuO) and petroleum (polyalphaolefin, PAO-6) base oils. HOSuO and PAO-6 were selected as representatives of their corresponding base oil groups because they have similar viscosity at 40 and 100 °C but differ in chemical structure. While PAO-6 is a non-polar pure hydrocarbon, HOSuO comprises tri-ester functional groups and is polar. As a result, HOSuO and vegetable oils in general adsorb on friction surfaces and lower coefficient of friction (COF) whereas PAO-6 and petroleum base oils do not [45]. HOSuO and vegetable oils also substantially enhance the performance additives much higher than that attainable with PAO-6 or mineral oil base oils [28, 46]. The two base oils also differ substantially in many other properties, including: viscosity index (VI), oxidation stability, cold flow, hydrolytic stability, bioresitance, etc.[47, 48]. In the following sections, we will examine how blending FAME-S2 to these important classes of base oils affects their properties. In this section, we will explore how the properties of FAME-S2 compare with these two base oils.
The solubility of PM-FAME and FAME-S2 in HOSuO and PAO-6 is shown in Table 2. The two materials display similar solubility in HOSuO at room temperature (RT). However, FAME-S2 was substantially less soluble in PAO-6 at RT which improved with heating. This could be the increased polarity of FAME-S2 due to the disulfide structure making it less compatible with the non-polar PAO-6.
The density, kinematic viscosity and VI of PM-FAME and FAME-S2 are also compared in Table 2. Both PM-FAME and FAME-S2 are denser than the two base oils, which is attributable to the presence of multiple sulfur groups in their structures. The higher amount of sulfur per molecule in FAME-S2 (see "Experimental") is also responsible for its higher density and viscosity relative to PM-FAME. As expected, HOSuO displayed the highest VI whereas FAME-S2 and PM-FAME displayed VI above that of PAO-6 (Table 2).
The refractive index (RI) as a function of temperature, and total acid number (TAN) of PM-FAME and FAME-S2 are compared with those of PAO-6 and HOSuO in Table 3. RI values from 30 to 80 °C were measured, and RI value at 100 °C was obtained by extrapolation of measured data. As expected, RI values for all four materials decreased with increasing temperature, as was the case with the density of these materials discussed above (Table 4). The RI values also increased in the order PAO-6 < HOSuO < PM-FAME < FAME-S2, which was also the order of increasing density (Table 2), which confirms the well-known direct correlation between density and RI [49]
Measured total acid number (TAN) increased in the order PAO-6 < HOSuO < PM-FAME < FAME-S2 (Table 3). The low TAN values for PAO-6 and HOSuO were expected since these are highly purified commercial products. The TAN value for FAME-S2 was more than fivefold higher than that for PM-FAME and is attributed to the presence of residual sulfenic (R-SOH), sulfinic (R-SO2H) or sulfonic (R-SO3H) acids in the product mixture as a result of overoxidation of FAME during conversion to FAME-S2 as discussed before (please see "Synthesis and Characterization"). The fact that the sulfenic, sulfinic and sulfonic acids products were not detected by IR or NMR indicates that they are present in concentrations below the detection limits of these analytical methods. The FTIR absorption in the range 3000–3600 cm−1 indicates that the high TAN value was not due to the presence of carboxylic acids (R-COOH) from hydrolysis FAME-S2.
The pour point and cloud point of PM-FAME and FAME-S2 are shown in Table 4 along with the values for the base oils. As expected, PAO-6 showed excellent pour point value whereas PM-FAME and FAME-S2 displayed very poor pour point and cloud point values. The pour point and cloud point values of PM-FAME and FAME-S2 increased with increasing sulfur content, which can be rationalized to be a function of their molecular weight, which increases with increasing sulfur content.
The onset (OT) and peak (PT) oxidation temperatures of PM-FAME and FAME-S2 are compared to each other and to the base oils also in Table 4. As expected, PAO-6 displayed superior oxidation stability relative to HOSuO. However, PM-FAME and FAME-S2 displayed values that surpassed those of PAO-6. This indicates that two different mechanisms are involved with the suppression of oxidation in PM-FAME and FAME-S2. The first mechanism is the elimination of double bonds in the hydrocarbon chain which also eliminates the allylic and bis-allylic protons responsible for oxidation. This mechanism should bring the oxidation stability to the level of PAO-6, which is also free of allylic and bis-allylic protons. The second mechanism, which is responsible for the additional oxidation stability, can be attributed to the anti-oxidant property of sulfur atoms in the FAME and FAME-S2 molecules [27, 50].
3.3 Properties of Blends in Polyalphaoefin (PAO-6)
The effect of blending 0–10 (% w/w) PM-FAME and FAME-S2 on properties of PAO-6 were investigated. Figure 5 shows the effect on density of PAO-6 at 40 and 100 °C. Increasing the concentration of both PM-FAME and FAME-S2 resulted in increasing blend density that is almost linear with concentration. The blend density appears to be a simple proportional combination of the density of PAO-6 with the higher densities of PM-FAME and FAME-S2. This result is consistent with expectations from blending a higher density component.
The effect of PM-FAME and FAME-S2 concentration (0–10% w/w) on PAO-6 blend kinematic viscosity at 40 and 100 °C is illustrated in Fig. 6. As was discussed before (Table 2), both PM-FAME and FAME-S2 are denser and more viscous than PAO-6. Thus, it is reasonable to expect a monotonous increase of viscosity with increasing concentration, as was observed with density above. However, the opposite was observed in that the kinematic viscosity decreased with increasing PM-FAME and FAME-S2 concentration. Even though the decrease was much sharper for blends with the relatively lower viscosity PM-FAME than those with FAME-S2, both blends indicate the presence of a strong antagonistic effect. The reason for the antagonistic effect is not clear and could be due to the vast difference in the polarity between the hydrocarbon PAO-6 and the polar PM-FAME and FAME-S2 with multiple sulfur in their structures.
The viscosity index (VI) of PM-FAME and FAME-S2 blends (0–10% w/w) in PAO-6 displayed a linear increase with increasing concentration (data not included). This result may not be due to the higher VI of the neat additives (Table 2) positively influencing the VI of the blend, as was observed with density (Fig. 5). As discussed above, the higher viscosity of these additives did not produce a positive influence on blend viscosity (Fig. 6). An alternative explanation could be that PM-FAME and FAME-S2 are acting as viscosity index improvers or VIIs.
3.4 Properties of Blends in HOSuO
The effect of PM-FAME and FAME-S2 concentration (0–10%, w/w) on the pour point and cloud point of HOSuO blends is illustrated in Fig. 7. For both additives, pour point increased with increasing concentration above 1% (w/w). Pour point values were larger for PM-FAME blends below 5% (w/w) concentration, whereas FAME-S2 blends were larger above 5% (w/w) concentration. The two additives displayed similar cloud point values which increased with increasing concentration up to 3% (w/w) but remained constant with further increase of concentration to 10% (w/w).
Figure 8 compares onset (OT) and peak (PT) oxidation temperatures as a function of concentration (0–10%, w/w) of PM-FAME and FAME-S2 blends in HOSuO. No change in OT was observed for FAME-S2 blends with increasing concentration or for PM-FAME blends above 1% concentration. The OT data show that FAME-S2 is more effective at maintaining a higher oxidation resistance by the blends than PM-FAME. The PT data show decreasing oxidation resistance with increasing concentration of both additives. However, as with the OT results, FAME-S2 was more effective at maintaining a higher peak oxidation temperature (∆T ≈ 20 °C) than PM-FAME.
3.5 Tribological Properties of Blends of FAME-S2 in PAO-6 and HOSuO
Additives containing di- or polysulfides in their structures are known to display extreme pressure (EP) properties [51]. Therefore, it was of interest to see if FAME-S2 have such properties. Figure 9 shows the effect of FAME-S2 concentration (0–10%, w/w) on 4-ball EP weld point. Without EP additives, most hydrocarbons such as PAOs display WP values in the range 100–120 kgf. As shown in Fig. 9, blending 1% w/w of FAME-S2 in PAO-6 increased the WP to 160 kgf, which remained unchanged with a further increase of FAME-S2 to 5% (w/w). At 8% w/w of FAME-S2 in PAO-6, the WP increased to 180 kgf and remained unchanged with a further increase of FAME-S2 concentration to 10% w/w. The results in Fig. 9 indicates that FAME-S2 contains considerably more disulfides in its structure than PM-FAME as demonstrated by its higher WP (180 kgf) relative to the value for PM-FAME (160 kgf—data not shown).
Figure 9 also shows the effect of FAME-S2 concentration on the 4-ball EP weld point of HOSuO blends. Weld point values increased to 200 kgf with increasing concentration of FAME-S2 up to 3% w/w. Weld point was unchanged with further increase of FAME-S2 to 5% w/w but increased to 220 kgf at 10% w/w of FAME-S2.
Comparison of the effect of FAME-S2 concentration on WP of PAO-6 vs. HOSuO blends indicates that, generally, FAME-S2 provides about 40 kgf higher WP in HOSuO compared to those in PAO-6. Such enhancement of additive EP properties by biobased base oils such as HOSuO relative to petroleum base oils such as PAO-6 has been reported before [28, 46]. The data provide further confirmation of one of the most important benefits of biobased base oils, enhancing the performance of EP additives [28, 46]. This, along with high VI and low traction coefficient, are considered major strengths of biobased base oils over petroleum base oils.
4 Summary/Conclusion
Soy-based fatty acid methyl ester disulfide (FAME-S2) was synthesized by oxidation of the fatty acid methyl ester of polymercaptanized soybean oil (PM-FAME). The FAME-S2 product was obtained in good yield and thoroughly authenticated using a wide range of analytical techniques. In general, FAME-S2 comprised more than 60% oligomers and less than 40% monomers, compared to less than 20% oligomers and more than 80% monomers for the PM-FAME used in the synthesis.
Neat FAME-S2 and its blends (1–10% w/w) in polyalphaolefin (PAO-6) and high oleic sunflower oil (HOSuO) were further characterized for its chemical, physical and tribological properties relative to PM-FAME. Among the findings were:
Blends of FAME-S2 in HOSuO displayed higher oxidation onset temperature (> 15 °C), that remained constant with increasing concentration, than similar blends of PM-FAME. This indicates that FAME-S2 has anti-oxidant additive properties
Evaluation of blends of FAME-S2 as an extreme pressure (EP) additive in HOSuO and PAO-6 base oils on a 4-ball tribometer showed that, FAME-S2 provides higher weld point (WP) than the neat base oils. In addition, the WP increased with increasing concentration (1–10% w/w) of FAME-S2 in both base oils. FAME-S2 also displayed higher weld point (ΔWP = 40 kgf) in HOSuO than in PAO-6 base oil; which is an indication of better compatibility between the biobased base oil and biobased additive.
The results indicate that FAME-S2 has both EP and anti-oxidant properties in PAO-6 as well as HOSuO base oils, and is a good candidate for use as a multi-functional biobased additive in lubricant formulation.
This work demonstrates that effective biobased additives can be synthesized from thoughtful chemical modification of vegetable oils and their derivatives.
References
Swedberg, S., Sullivan, T.: Biobased, biodegradable and food grade lubes. Lubes ‘N’ Greases 25(2), 28–33 (2019)
Sharma, B.K., Biresaw, G. (eds.): Environmentally Friendly and Biobased Lubricants. CRC Press Taylor & Francis Group, Boca Raton (2016)
Biresaw, G., Laszlo, J.A., Evans, K.O., Compton, D.L., Bantchev, G.B.: Synthesis and tribological investigation of lipoyl glycerides. J. Agric. Food Chem. 62, 2233–2243 (2014). https://doi.org/10.1021/jf404289r
Biresaw, G., Compton, D., Evans, K., Bantchev, G.: Lipoate ester multi-functional lubricant additives. Ind. Eng. Chem. Res. 55, 373–383 (2016). https://doi.org/10.1021/acs.iecr.5b03697)
Bantchev, G.B., Moser, B.R., Murray, R.E., Biresaw, G., Hughes, S.R.: Synthesis and characterization of phosphonates from methyl linoleate and vegetable oils. J. Am. Oil Chem. Soc. 93, 1671–1682 (2016)
Biresaw, G., Bantchev, G.B.: Tribological properties of biobased ester phosphonates. J. Am. Oil Chem. Soc. 90, 891–902 (2013)
Biresaw, G., Bantchev, G.: Tribological properties of limonene bisphosphonates. Tribol. Lett. 60(1), 11–25 (2015)
Biresaw, G., Bantchev, G.B., Harry-O’kuru, R.E.: Biobased poly-phosphonate additives from methyl linoleates. Tribol. Trans. 62(3), 428–442 (2019)
Matson, M.S., Refvik, M.D., Netemeyer, E.J., Cameron, C., Marrion, A.R., Wright, A.C.: Methods of mercaptanizing olefinic hydrocarbons and compositions produced therefrom. US Patent 8,461,293 (Assignee: Chevron-Phillips Chemical Company LP The Woodlands, Texas) (2013)
Biresaw, G., Lansing, J., Bantchev, G., Murray, R., Harry-O’kuru, R.: (2017) Tribological Investigation of Polymercaptanized Soybean Oil. Tribol. Lett. 65, 87 (2017). https://doi.org/10.1007/s11249-017-0866-0)
Ionescu, M., Radojcic, D., Wana, X., Petrovic, Z.S., Upshaw, T.A.: Functionalized vegetable oils as precursors for polymers by thiol-ene reaction. Eur. Polym. J. 67, 439–448 (2015)
Javni, I., Bilic, O., Ljubic, D., Wan, X., Petrovic, Z.S., Upshaw, T.A.: Polymercaptan-based polyurethane foams. J. Cell. Plast. 52(6), 643–656 (2016)
Liu, K.T., Tong, Y.C.: A facile conversion of thiols to disulfides. Synthesis 1978, 669–670 (1978)
Evans, B.J., Doi, J.T., Musker, W.K.: 19F NMR Study of the Reaction of p -Fluorobenzenethiol and Disulfide with Periodate and Other Selected Oxidizing Agents. J. Org. Chem. 55, 2337–2344 (1990)
Tam, J.P., Wu, C.-R., Liu, W., Zhang, J.-W.: Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 113, 6657–6662 (1991)
Shi, T., Rabenstein, D.L.: Discovery of a highly selective and efficient reagent for formation of intramolecular disulfide bonds in peptides. J. Am. Chem. Soc. 122(29), 6809–6815 (2000)
Kirihara, M., Asai, Y., Ogawa, S., Noguchi, T., Hatano, A., Hirai, Y.: A mild and environmentally benign oxidation of thiols to disulfides. Synthesis 21, 3286–3289 (2007)
Harutyunyan, R., Rezvani, M.A., Heravi, M.M.: H5[PMo10V2O40] as a green, reusable, and highly efficient catalyst for the oxidation of dithiols in intermolecular reactions using permanganate as an oxidizing reagent. Synth. React. Inorg. Met. Org., Nano-Met. Chem. 41(1), 94–99 (2011)
He, Y., Hang, D., Lu, M.: A simple and practical method for the oxidation of thiols to disulfides at mild conditions without solvents. Phosphorus Sulfur Silicon Relat. Elem. 187(9), 1118–1124 (2012)
Bayraq, S.S., Nikseresht, A., Khosravi, I.: (NH4)6Mo7O24•4H2O as an efficient, selective, and reusable catalyst for the oxidation of thiols to disulfides using potassium bromate. Phosphorus Sulfur Silicon Relat. Elem. 188(9), 1236–1243 (2013)
Lui, Y., Wang, H., Wang, C., Wan, J.-P., Wen, C.: Bio-based green solvent mediated disulfide synthesis via thiol couplings free of catalyst and additive. RSC Adv. 3, 21369–21372 (2013)
Wang, H., Huang, G., Sun, Y., Liu, Y.: Simple conversion of thiols to disulfides in EtOH under ambient aerobic conditions without using any catalyst or additive. J. Chem. Res. 38, 96–97 (2014)
Yuan, J., Ma, X., Yi, H., Liu, C., Lei, A.: I2-catalyzed oxidative C(sp3)–H/S–H coupling: utilizing alkanes and mercaptans as the nucleophiles. Chem. Commun. 50, 14386–14389 (2014)
Shah, S.S., Karthik, S., Singh, N.D.P.: Vis/NIR light driven mild and clean synthesis of disulfides in the presence of Cu2(OH)PO4 under aerobic conditions. RSC Adv. 5(56), 45416–45419 (2015)
Qiu, X., Yang, X., Zhang, Y., Song, S., Jiao, N.: Efficient and practical synthesis of unsymmetrical disulfides via base-catalyzed aerobic oxidative dehydrogenative coupling of thiols. Org. Chem. Front. 6(13), 2220–2225 (2019)
Rossrucker, T., Fessenbecker, A.: Sulfur carriers. In: Rudnick, L.R. (ed.) Lubricant Additives. Chemistry and Applications, pp. 259–291. CRC Press, Boca Raton (2003)
Bantchev, G.B., Biresaw, G., Mohamed, A., Moser, J.: Temperature dependence of the oxidative stability of corn oil and polyalphaolefin in the presence of sulfides. Thermochim. Acta 513(1–2), 94–99 (2011)
Biresaw, G., Asadauskas, S.J., McClure, T.G.: Polysulfide and biobased extreme pressure additive performance in vegetable vs paraffinic base oils. Ind. Eng. Chem. Res. 51(1), 262–273 (2012)
Li, W., Jiang, C., Chao, M., Wang, X.: Natural garlic oil as a high-performance, environmentally friendly, extreme pressure additive in lubricating oils. ACS Sustain. Chem. Eng. 2(4), 798–803 (2014)
Biresaw, G., Lansing, J.C., Bantchev, G.B., Murray, R.E., Harry-O’Kuru, R.E.: Chemical, physical and tribological investigation of polymercaptanized soybean oil. Tribol. Lett. 65(87), 1–16 (2017)
A.O.C.S. Official: Method Te 2a-64, Acid Value, p. 1 (1997)
ASTM D 7042-11a: “Standard test method for dynamic viscosity and density of liquids by Stabinger viscometer (and the Calculation of Kinematic Viscosity),” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 186–193 (2012)
ASTM D 2270-93: “Standard practice for calculating viscosity index from kinematic viscosity at 40 and 100 °C,” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 849–854 (2012)
ASTM D 6186-08: “Standard test method for oxidation induction time of lubricating oils by pressure differential scanning calorimetry (PDSC),” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 52–56 (2012)
ASTM D 97: “Standard test method for pour point of petroleum products,” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 100–104 (2012)
ASTM D 2500: “Standard test method for cloud point of petroleum products,” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 949–953 (2012)
ASTM D 2783–88: “Standard test method for measurement of extreme-pressure properties of lubricating fluids (Four-Ball Method),” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 130–137 (2012)
Noor, M.A.M., Sendijarevic, V., Hoong, S.S., Sendijarevic, I., Ismail, T.N.M.T., Hanzah, N.A., Noor, N.M., Palam, K.D.P., Ghazali, R., Hassan, H.A.: Molecular weight determination of palm olein polyols by gel permeation chromatography using polyether polyols calibration. J. Am. Oil Chem. Soc. 93, 721–730 (2016). https://doi.org/10.1007/s11746-016-2812-y
Grubisic, Z., Rempp, P., Benoit, H.: A universal calibration for gel permeation chromatography. Polym. Lett. 5, 753–759 (1967). https://doi.org/10.1002/pol.1967.110050903. Re-published. In: (1996) Journal of Polymer Science, Part B: Polymer Physics, 34:1707-1713. DOI: 10.1002/polb.1996.922
Wyatt, P.J.: Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272, 1–40 (1993)
Braun, J.L., Kadla, J.F.J.: A relatively simple method for calculating Mark-Houwink parameters using basic definitions. Appl. Polym. Sci. 114, 3303–3309 (2009)
Bantchev, G.B., Cermak, S.C., Durham, A.L., Price, N.P.J.: Estolide molecular weight distribution via gel permeation chromatography. J. Am. Oil Chem. Soc. 96, 365–380 (2019)
Mayo, D.W., Miller, F.A., Hannah, R.W.: Course Notes on the interpretation of infrared and Raman Spectra. Wiley, Hoboken (2004)
Silverstein, R.M., Webster, F.X., Kiemle, D.J.: Spectroscopic Identification of Organic Compounds, 7th edn. Wiley, Hoboken (2005)
Biresaw, G., Adharyu, A., Erhan, S.Z., Carriere, C.J.: Friction and adsorption properties of normal and high oleic soybean oils. J. Am. Oil Chem. Soc. 79(1), 53–58 (2002)
Asadauskas, S.J., Biresaw, G., McClure, T.G.: Effects of chlorinated paraffin and ZDDP concentrations on boundary lubrication properties of mineral and soybean oils. Tribol. Lett. 37(2), 111–121 (2010)
Hope, K.: Balancing lubricant properties with vegetable oil and PAO blends. Proceedings USB Lube TAP (2006)
Biresaw, G.: Environmentally friendly lubricant-development programs at USDA. In: Sik, R. (ed.) Environmentally Considerate Lubricants, STP 1575, pp. 1–23. ASTM International, West Conshohocken (2014)
Galiatsatos, V., Neaffer, R.O., Sen, S., Sherman, B.J.: Refractive index, stress-optical coefficient, and optical configuration arameters of polymers. In: Mark, J.E. (ed.) Physical properties of Polymers Handbook, pp. 535–543. AIP Press, Woodbury (1996)
Bala, V., Hartley, R.J., Hughes, L.J.: The influence of chemical structure on the oxidative stability of organic sulfides. Lubr. Eng. 52, 868–873 (1996)
Schey, J.A.: Tribology in Metalworking Friction, Lubrication and Wear. American Society of Metals, Metals Park (1983)
Acknowledgements
The authors are very grateful to Ms. Linda Cao, Ms. Linda Manthey, Mr. Dan Knetzer and Dr. Karl Vermillion for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Biresaw, G., Bantchev, G.B., Lansing, J. et al. Sulfurized Methyl Esters of Soya Fatty Acids: Synthesis and Characterization. Tribol Lett 68, 61 (2020). https://doi.org/10.1007/s11249-020-01292-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-020-01292-y