Abstract
Citrus bacterial canker, caused by Xanthomonas citri subsp. citri (Xcc), is a major disease of citrus plants, causing a significant loss in the citrus industry. The pthA is a bacterial effector protein mediates protein–protein and protein-DNA interactions and modulates host transcription. Injection of pthA effector protein into the host cell induces the expression of the susceptibility gene CsLOB1 which is required for citrus canker disease development. In this study, we described in planta expression of a specific anti-pthA single-chain variable fragment (scFv) recombinant antibody, scFvG8, and assessed its function using molecular docking, immunoblotting, and indirect enzyme-linked immunosorbent assay (ELISA). Based on the results, homology-based molecular docking suggested that at least eight intermolecular hydrogen bonds are involved in pthA-scFvG8 interactions. Immunoblotting and indirect ELISA results reconfirmed specific binding of scFvG8 to pthA protein. Moreover, gene fragment encoding scFvG8 was cloned into plant expression vector and transiently expressed in leaves of Nicotiana tabacum cv. Samson by agroinfiltration method. Transient expression of scFvG8 (at the expected size of 35 kDa) in N. tabacum leaves was confirmed by western blotting. Also, immunoblotting and indirect ELISA showed that the plant-derived scFvG8 had similar activity to purified scFvG8 antibody in detecting pthA. Additionally, in scFvG8-expressing tobacco leaves challenged with Xcc, a reduction (for up to 70%) of hypersensitive response (HR) possibly via direct interaction with pthA, was observed in the necrotic leaf area compared to control plants infected with empty vector. The results obtained in this study confirm that scFvG8 can suppress the function of pthA effector protein within plant cells, thus the induction of stable expression of scFvG8 in lime trees can be considered as an appropriate approach to confer resistance to Xcc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus bacterial canker caused by Xanthomonas citri subsp. citri (Xcc) causes severe damage to many citrus species, resulting in quality loss and yield reduction by forming yellow chlorotic rings on fruits and other parts of the plant (Gurlebeck et al. 2006). The pathogenic effector protein pthA plays a substantial role in the interaction between Xcc and citrus. In fact, pthA is the major determinant of Xcc pathogenicity that contributes to the hypersensitive response (HR) in resistant hosts and pathogenicity in susceptible genotypes and non-host plants (Mysore and Ryu, 2004; Gurlebeck et al. 2006; Roeschlin et al. 2017). The pthA protein is encoded by the avrBs3/pthA gene family and it is translocated into the plant by a type III secretion system of the bacterium and transferred into the host cell nucleus by a nuclear localization signal (NLS) (Kay et al. 2005; Boch and Bonas, 2010). The protein translocation via T3SS depends on ring structures that enclose an inner transport channel; these structures are associated with HrpE, an extracellular filamentous appendage, in Xcc and they act as a channel through which, effector proteins are transferred into the host cell cytosol (Ghosh, 2004; Gottig et al. 2009). The entry of pthA into the cell affects mRNA processing and translation (De Souza et al. 2012), since pthA-NLS mimics host transcription factors as it inhibits peptidyl-prolyl cis–trans isomerase activity of cyclophilin (CYP) and targets the promoters of genes such as lateral organ boundary 1 (LOB1), 2-oxo-glutarate/FE(II)-dependent dioxygenase (DIOX), and cyclin B genes (CCNBS) (Pereira et al. 2014). Additionally, some studies have discussed that importins (α and β) help in the transport of pthA into the nucleus where they interfere with transcription (Domingues et al. 2010; Boch and Bonas, 2010; Gochez et al. 2018). Interestingly, mutations of pthA can lead to a loss of hypertrophy (cell enlargement) but do not affect bacterial growth in host leaf tissue (Hu et al. 2014), confirming the role of pthA in the disease development in the host.
Recently, as an alternative approach for plant disease control, recombinant antibodies have been employed to confer resistance in plants (Safarnejad et al. 2011; Peschen et al. 2016). Due to the essential role of pthA in the canker development, scFvG8 expression in citrus seedlings can lead to the formation of anti-pthA antibody pthA complex that may impair pthA activity, interrupt the pathogenesis, confer resistance towards Xcc and eventually, effectively control the disease (Peschen et al. 2016). Since plants do not have an antibody-producing immune system to identify and inactivate pathogens, transformation of anti-pthA antibody gene and induction of its expression into the plant cell can be as a suitable approach to produce transgenic plants resistant to Xcc.
In this context, recombinant antibodies are applied to develop so-called plantibodies that produce resistance to pathogens; this approach involves the introduction of a gene cassette encoding a single-chain variable fragment (scFv) antibody against virulence factor(s) of a pathogen in plants (Schillberg et al. 2001; Cervera et al. 2010; Hemmer et al. 2018; Diamos et al. 2020). An scFv is comprised of light- and heavy-chain variable domains (VL and VH, respectively) with a glycine-serine flexible linker located between them to improve scFv folding, flexibility, and stability. These fragment antibodies are about one-fifth that of full-length immunoglobulin G (IgG) antibodies (Holliger and Hudson 2005) and have a molecular mass of about 30 kDa. An scFv contains six complementarity-determining regions (CDRs) namely, CDR L1-L3 and CDR H1-H3, that may interact with the antigen (Monnier et al. 2013). Notably, scFv antibodies have been preferred over full-length antibodies for the production of transgenic plants because they can be easily engineered and expressed in the cytosol, and they are effective even at low accumulation levels (Schillberg et al. 2001). The use of scFv antibodies for producing transgenic plants was established in the early 1990s and for the first time, antibody-mediated resistance in plants was reported by Tavladoraki et al. (1993) who induced the expression of a specific scFv against the viral coat protein of Artichoke mottle crinkle virus (AMCV) in bacteria and then in transgenic Nicotiana benthamiana and reported resistance to AMCV (Tavladoraki et al. 1993). Subsequently, Le Gall et al. (1998) showed that antibody-based resistance based on scFv fragments is also useful against bacterial pathogens. This study investigated the expression of specific scFv for major membrane proteins of Candidatus Phytoplasma solani; since Ca. P. solani is limited to phloem tissues, anti-phytoplasma scFv antibodies should be directed through the secretory pathway. This study showed that grafting of transgenic tobacco shoots expressing anti-phytoplasma scFv antibodies onto tobacco plants infected with phytoplasma, can greatly reduce disease severity (Le Gall et al.1998). In another work, Chen and Chen (1998) investigated the cytosolic expression of scFv against corn stunt spiroplasma (CSS) and reported that scFv could be detected in transgenic plants; however, no effect on CSS disease progression in challenged plants was observed. Since CSS is restricted to sieve tubes, the use of antibodies directed to the secretory pathway in phloem cells should be considered in future studies. Later, the production of transgenic plants that express scFv fragments against various pathogens including Potato virus Y (PVY) (Gargouri-Bouzid et al. 2006), Tomato yellow leaf curl virus (TYLCV) (Safarnejad et al. 2009), Broad bean mottle virus (BBMV) (Ghannam et al. 2015) and Grapevine fanleaf virus (GFLV) (Hemmer et al. 2018) was reported.
In our earlier work, we described a pthA-specific scFv antibody derived from phage display library screening (Raeisi et al. 2019a, b). In the present study, transient expression of the corresponding cDNA in Nicotiana tabacum cv. Samson was studied to examine the efficacy of the anti-pthA scFv antibody against Xcc. Our results showed that the application of anti-pthA scFv antibody fragment expressed in plants, increases resistance to Xcc in a tobacco plant model. The present findings provide a basis for further research on the development of disease-resistant cultivars by introducing selected antibody-encoding genes in citrus. To the best of our knowledge, this is the first report on the functionality of a specific scFv against pthA and Xcc in plants.
Materials and methods
In silico analysis of scFvG8 antibody affinity for pthA
Homology modeling and molecular docking were performed to assess interactions between scFvG8 and pthA. Three dimensional (3D) structures of recombinant pthA and scFvFG8 were modeled by I-TASSER server service (https://zhanglab.ccmb.med.umich.edu/I-TASSER/). The 3D models were generated by collecting the highest scoring of template models based on the target model amino acid sequence. ModRefiner server service (http://zhanglab.ccmb.med.umich.edu/ModRefiner/) was used to refine I-TASSER-predicted 3D structural models. Finally, ProSA server service (https://prosa.services.came.sbg.ac.at/prosa.php) was used to evaluate quality, accuracy, and reliability of the generated homology model (Wiederstein and Sippl, 2007). Further, Haddock server service (https://haddock.science.uu.nl/services/HADDOCK2.2/) was used to investigate the mode of interactions between pthA and scFvG8 refined models. The final model was then selected based on the largest cluster size and minimal local energy. Possible interactions of the structural models were analyzed using Pymol software version 1.5.0.1 (http://pymol.findmysoft.com) and visualized by ligplot plus software version 4.5.3 (Laskowski and Swindells 2011).
Bacterial expression and purification of pthA and scFvG8 recombinant proteins
The pET28a-pthA construct previously prepared by Raeisi et al (2019b) was used for production of pthA protein (supplementary Fig. 1a). This construct harbor c-terminal of pthA gene (606 bp) isolated from an Iranian A* isolate. The expression of foreign gene was induced in the Rosetta (DE3) strain of Escherichia coli by 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 16 h at 30 °C, and then, it was purified under native conditions by immobilized metal ion affinity chromatography (IMAC) (Qiagen, Hilden, Germany) using the attached His-tag as described earlier (Safarnejad et al. 2008).
As previously described (Raeisi et al. 2019a), the scFv antibody fragment was isolated by screening Tomlinson I naïve scFv phage display library against the pthA recombinant protein. A highly specific scFvG8 antibody was selected and sequenced to confirm the existence of full-length VH and VL domains. The pHEN primers were used for Sanger DNA sequencing (Pishgam Biotech Co. Tehran, Iran), and sequence alignments were performed using IMGT/V-QUEST alignment tool (http://www.imgt.org). The E. coli HB2151 was used to express scFvG8. The protein expression was induced by treatment with 1 mM IPTG for 16 h at 30 °C. The His-tagged scFvG8 was purified by IMAC for subsequent analyses (Raeisi et al. 2019a).
Preparation of Xanthomonas citri subsp. citri (Xcc)-infected samples
The natural Xcc-infected Mexican lime (Citrus aurantifolia) were collected from Jiroft area located in southern regions of Iran. The samples with symptoms of chlorotic, necrotic, and canker lesions on leaves, fruit, and stems were used in serological assays. The extraction of plant protein was conducted in 1:3 (m/v) extraction buffer (1X PBS buffer (5.85 g/L NaCl, 4.72 g/L Na2HPO4, 2.64 g/L NaH2PO4.2H2O, pH 7.2), 5 mM EDTA, 5 mM β-mercaptoethanol or 2% (v/v) polyvinylpyrrolidone, pH 7.5).
Cloning into plant transformation vector
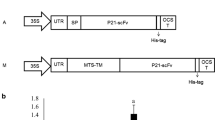
The entire gene encoding scFvG8 protein was sub-cloned to pCAMBIA1300 plant expression vector under the control of 35S cauliflower mosaic virus (CaMV) promoter via SalI/NotI restriction digestion. Since no leader peptide or tag has been added before or after scFv gene, these construct were assigned for cytosolic expression of scFv fragment in plant cells. The novel construct, pCAMBIA-scFvG8, was used to express scFvG8 in Nicotiana tabacum cv. Samson leaves by agroinfiltration. The competent cells of Agrobacterium radiobacter (formerly known as Agrobacterium tumefaciens) GV3101 strain resistant to gentamicin, kanamycin, and rifampicin (Gmr, Kmr, and Rifr) were prepared (Hofgen and Willmitzer, 1988) and transformed using pCAMBIA-scFvG8 via heat shock method (Weigel and Glazebrook, 2006). Recombinant agrobacteria were identified by polymerase chain reaction (PCR) using specific primers (forward: 5′-ATCCTTCGCAAGACCCTTCCTCT-3′ and reverse: 5′-AGAGAGAGATAGATTTGTAGAGA-3′). PCR was initiated at 94 °C (for 10 min) and followed by 35 cycles of 94 °C (for 30 s), 57 °C (for 30 s), and 72 °C (for 2 min) and a final extension at 72 °C (for 10 min).
Transformed agrobacterium was cultured in Luria broth (LB) medium containing 50 mg/L of kanamycin and 10 mg/L rifampicin at 28 °C under agitation at 160 rpm. The bacterial cells at OD600 of 1 were collected, and transferred to 10 mL MMA (0.43% (w/v) Murashige & Skoog (MS) salts, 10 mM 2-(N-morpholino) ethanesulfonic acid (MES), 2% (w/v) sucrose, and 200 mM Acetosyringone (pH5.6) and incubated at 22˚C for 3 h before infiltration (Shahryari et al. 2013).
Transient expression of anti-pthA scFvG8 in tobacco leaves
The six-week-old plants were grown in a greenhouse at 22 °C, with 80% relative humidity, under 16:8 h light:dark photoperiod. Six biological replicates with at least six leaves per replicate were considered for each treatment. The lower epidermis of N. tabacum cv. Samson intact leaves were agroinfiltrated by pCAMBIA-scFvG8 using a syringe. PBS buffer and agrobacterium containing empty pCAMBIA plasmid were injected as negative controls and suspensions of Xcc (108 CFU/mL of NIGEB-088 prepared in PBS) were used as positive controls. After three days, total soluble proteins were isolated from the injected leaves using 2 mL of extraction buffer per gram of leaf. Cell debris was removed by centrifugation at 16,000×g, at 4 °C for 30 min. Then, scFvG8 expression was assessed by immunoassays. Western blotting was done for detecting scFvG8 antibody in leaf total proteins as previously described.
Western blotting, Dot immunobinding assay (DIBA), and indirect enzyme-linked immunosorbent assay (ELISA)
The binding activity of purified scFvG8 against recombinant pthA and native pthA presented in extract of infected lime was evaluated via western blotting, dot immunobinding assay (DIBA), and indirect enzyme-linked immunosorbent assay (ELISA) (Raeisi et al. 2020). Moreover, to estimate the efficiency of the plant-produced scFvG8 in pthA detection, total soluble proteins extracted from transformed tobacco leaves expressing scFvG8 were used as a primary antibody in immunoassays.
Western blot was conducted using purified pthA (10 μg/mL) and bovine serum albumin (BSA, 100 μg/mL). The proteins (10 μL) were separated on 12% SDS-PAGE gel and transferred to Millipore polyvinylidene difluoride (PVDF) membrane (Sigma-Aldrich, Germany) according to the manufacturer's instructions. The membrane was blocked with 5% (w/v) skimmed milk powder (Fluka, Germany) in 1X PBS buffer and incubated with purified scFvG8 protein (10 μg/mL) or plant-produced scFvG8 at 4 °C for 16 h. The scFvG8 protein was detected by anti- c-myc monoclonal antibody (1:5000) (Abcam, USA), and then, by anti-mouse IgG conjugated to alkaline phosphatase (1:7000) (Sigma-Aldrich, USA). The substrate 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT) (Sigma-Aldrich, Germany) were used to detect the proteins.
DIBA was performed using purified pthA protein (10 μg/mL) and protein extracts of healthy and infected plant samples. Protein extracts (5 μL) were spotted on a nitrocellulose membrane. After blocking by 5% (w/v) skimmed milk powder, the membrane was washed three times with 1X PBS buffer containing 0.1% Tween 20 (PBST). The purified scFvG8 antibody (10 μg/mL) or plant-produced scFvG8 was added and incubated for 2 h at 37 °C and the membrane was washed with 0.1% PBST. Anti-c-myc antibody was added that followed by goat anti-mouse IgG conjugated alkaline phosphatase for 2 h at 37 °C. The substrate BCIP/NBT was used to make the target proteins visible.
In indirect ELISA, healthy and infected plant extracts were applied to coat microplate wells (Thermo Fisher Scientific, USA), and purified recombinant pthA protein (10 µg/mL) was considered as a positive control. To determine the specificity of scFvG8, recombinant protein HrpE of Xcc (previously reported by Raeisi et al. 2018a, b) and BSA were used as negative controls. The plate was stored at 4 °C for 16 h. After blocking with 3% (w/v) skimmed milk for 2 h at 37 °C, the plate was washed three times with 0.1% (w/v) PBS. The purified scFvG8 antibody (10 μg/mL) or plant-produced scFvG8 was added and incubated for 2 h at 37 °C. The reaction between the antibody and antigen was detected by adding anti-c-myc antibody and then, goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) (Abcam, USA). The reaction was detected by adding 2,2-azino-di-3-ethylbenz-thiazoline sulfonate (ABST) (Fermentase, Vilnius, Lithuania) as substrate. The absorbance was recorded at 405 nm by an ELISA reader (Tecan, Switzerland). Reactions were considered positive when the corresponding ELISA readings were at least twice those of the controls.
Characterization of plant-produced scFvG8 and assessment of transgenic plant resistance against Xanthomonas citri subsp. citri (Xcc)
To confirm the ability of scFvG8-expressing tobacco to reduce Xcc symptoms, agroinfiltration of pCAMBIA-scFvG8 was performed in leaves of wild type N. tabacum cv. Samson plants. Three days after infiltration, suspension of Xcc (108 CFU/mL) was infiltrated in the same area. The ability to elicit HR by Xcc on the N. tabacum cv. Samson was investigated by measuring the size of the necrotic lesions developed by Xcc. Plant responses were examined for 24–72 h post-inoculation.
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software, CA, USA) was used for statistical analysis. Results are presented as mean ± standard deviation (SD) for triplicate ELISA reading. Results of the inoculation experiments (area of necrosis) were analyzed using one-way ANOVA followed by Duncan’s test (P < 0.05).
Results
scFvG8 sequence analysis
The isolation of specific DNA sequences encoding scFvG8 antibody (accession No. MT997070) from Tomlinson I naïve scFv phage library (MRC, Cambridge, England, ReIn_0017) was previously reported by screening with recombinant pthA (Raeisi et al. 2019a, b). To confirm the existence of full-length VH and VL domains, the sequence was translated and aligned with other available sequences in IMGT database (http://www.imgt.org). The CDRs of scFvG8 are shown in Table 1 and supplementary Fig. 1b. CDRI, II, and III of VH domain were respectively found at positions 28–35, 53–60, and 99–107 in the primary protein sequence. CDRI, II, and III of VL were respectively located at positions 160–166, 183–187, and 223–231.
Homology modelling and molecular docking between pthA and ScFvG8
I-TASSER server service was used to generate 3D models of recombinant pthA structure and scFvG8. For the next steps, the structure with the least energy was chosen among the 5 models proposed by the server. Based on Ramachandran plots, 91.7% of the residues were placed into the combination of favored and allowed categories (Supplementary Fig. 2a and b). Thus, the structure with the least energy was chosen for both pthA (Supplementary Fig. 3a) and scFvG8 (Supplementary Fig. 3b). The total model quality calculated by ProSA Z-score was − 7.15 for pthA and − 6.06 for scFvG8, implicating the high quality of the models (supplementary Fig. 3c and d). Based on the results of ProSA web tool, considering the bold black circle, the predicted scFvG8 and pthA models were in the range of experimentally solved structures and they were suitable for the molecular docking study.
Haddock was used to visually evaluate the interaction between scFvG8 and pthA. As a negative control, the BSA (PDB: 4f5s) 3D model was docked onto scFvG8 model (data not shown). In the case of scFvG8, the model with the lowest energy docking (among 40 models) was selected (Fig. 1a). Docking results of the scFvG8 antibody and pthA showed a binding energy of − 738 kcal/mol which was approximately twice that of the interaction between scFvG8 and BSA (− 389 kcal/mol). Furthermore, hydrogen bond interaction analyses showed that the complex was stabilized by eight intermolecular hydrogen bonds that interact with pthA [i.e. CDRI (Thr: 30, Ser: 33, and Tyr: 34) and CDRII (Ala: 55 and Thr: 60) of VH domain, and CDRII (Asp: 184 and Asp: 187) and CDRIII (Lys: 228) of VL domain (Fig. 1b)]. Among these residues, CDRIII of VH and CDRI of VL were not involved in intermolecular hydrogen bonding. Taken together, these results indicate that four CDRs are involved in scFvG8-pthA interaction (Table 2).
In vitro pthA binding activity of purified scFvG8
Both scFvG8 and pthA were expressed in E. coli strains and the purified proteins were used for subsequent analyses. The serological reactivity of scFvG8 was investigated using western blot analysis, DIBA, and indirect ELISA against recombinant pthA, Xcc-infected plants, and healthy samples.
Western blotting showed that scFvG8 could detect recombinant pthA protein and native pthA in the infected plant, as it revealed a protein band at the expected size of 25 kDa for recombinant pthA and 70 kDa for native pthA while no reaction with BSA was observed (Fig. 2a). The protein band revealed for two Xcc-infected plant samples was at the expected size but the detected band was much stronger in infected sample 1 than infected sample 2, indicating differences in pthA protein levels between the samples. DIBA confirmed the results of western blotting (Fig. 2b) and showed that the scFvG8 antibody can react with pthA in infected samples and with recombinant pthA.
The characterization of in vitro binding activity of scFvG8. a Western blot analysis. M: pre-stained protein ladder (Fermentas, Lithuania); b DIBA; c Indirect ELISA. scFv antibody-pthA binding was determined at 405 nm. BSA was used as a negative control. Data are presented as mean ± SD (n = 3). Different letters indicate significant differences (p < 0.01) among different samples
To confirm the ability of scFvG8 to detect pthA protein in infected plants, indirect ELISA was performed using several Xcc-infected samples. In ELISA, recombinant pthA protein as a positive control at a concentration of 10 μg/mL, showed the highest absorption rate, followed by samples one and four of Xcc-infected plants; scFvG8 showed 50% less absorption compared to the positive control. Moreover, the rate of adsorption for scFvG8 in other Xcc-infected samples was about 36–47% while in samples of healthy plants, the binding ranged between 10 and 15%. Negative control samples BSA and HrpE had similar absorption rates to healthy samples, which was about 13% compared to the positive control. These results verified the specificity of scFvG8 for detecting both recombinant pthA and pthA secreted by Xcc in infected plants and confirmed the absence of cross-reactivity with other proteins (Fig. 2c).
In planta expression of scFvG8
To study the expression of scFvG8 antibody in the cytosol of Nicotiana tabacum cv. Samson leaves, an expression cassette bearing genes encoding scFvG8 antibody, named pCAMBIA-scFvG8, was constructed (Fig. 3a). To ensure the presence of scFvG8 cDNA in the pCAMBIA plasmid, PCR was done using scFv-specific primers (supplementary Fig. 4).
a Schematic presentation of the pCAMBIA constructs used for scFvG8 expression in plants as well as the restriction sites; and b Western blot analysis of scFvG8 transiently produced in plant (N. tabacum cv. Samson). M: pre-stained protein ladder (Fermentas, Lithuania), Lane 1: BSA; lane 2: scFvG8 expression in tobacco leaves; and Lane 3: untransformed tobacco leaves (as a negative control)
The construct was introduced into N. tabacum leaves by agroinfiltration and then, transiently expressed in transformed tissues. Three days later, the transformed tobacco leaves were analyzed by western blotting to confirm scFvG8 expression. The result showed a ~ 35 kDa band for scFvG8 (Fig. 3b), confirming the correct expression of scFvG8 in plant cells.
Function of scFvG8 expressed in tobacco leaves
The pthA binding activity of plant-produced scFvG8 was evaluated using the total protein extracted from the transformed plant as a primary antibody in western blot, DIBA, and indirect ELISA.
The results of western blotting showed that plant-derived scFvG8 antibody functioned similarly to purified scFvG8 and could detect pthA protein at the expected size of 25 and 70 kDa for recombinant and native forms, respectively (Fig. 4a). These results were also confirmed by DIBA (Fig. 4b).
a The ability of scFvG8 expressed in leaves of N. tabacum to detect recombinant pthA and native pthA in infected sample. The total extracted proteins of tobacco leaves were used as a primary antibody to detect pthA. Lanes 1–4: pthA and Lane 5: BSA; and b DIBA was used to detect Xcc-infected samples and purified pthA protein, using scFvG8 produced by the plants; c Indirect ELISA done using plant-derived scFvG8 to detect Xcc-infected samples and purified pthA protein. Data are presented as mean ± SD (n = 3). Different letters indicate significant differences (p < 0.01) among different samples
The ability of scFvG8 expressed in tobacco leaves to detect various Xcc-infected plants was assessed by indirect ELISA (Fig. 4c). The results showed that plant-derived scFvG8 could detect Xcc-infected plants so that compared to the positive control, scFvG8 showed about 35–54% absorption in Xcc-infected plant samples, but near 14% in healthy and negative control samples. These results showed that plant-produced scFvG8 binds strongly to pthA and is functional in the cytosol of transformed plant tissues.
To study the specificity of the antibody, pCAMBIA-scFvG8 was initially infiltrated into fully expanded leaves of N. tabacum cv. Samson, followed by infiltrating a suspension of Xcc (108 CFU/mL) in the same area of the leaves. The results showed that plants infiltrated only with Xcc developed a distinct necrotic local lesion in two days, while the controls, i.e. PBS and vector control, were asymptomatic (Fig. 5a). A comparison of the size of the necrotic lesions developed by Xcc, PBS, and vector control, showed that Xcc could well induce HR and necrotic lesions of about 1.5 cm2 in the tobacco leaves, while necrotic lesions were not considerable in PBS, and vector control (Fig. 5b; Table 3a).
a Appearance of untransformed N. tabacum leaves inoculated with Xanthomonas citri subsp. citri (Xcc). The leaves infiltrated with vector control and PBS were used as negative controls. b Comparison of necrosis area in terms of mean lesion size (2 dpi). c Appearance of transformed N. tabacum leaves by pCAMBIA-scFvG8, PBS, or vector control that after three days were inoculated with Xcc and scored 2 dpi. d Comparison of the necrosis area in terms of mean lesion size. Data presents the average lesion size of three independent experiments with six plants in each replicate. Different letters indicate significant differences (p < 0.01) among different samples
Interestingly, when the leaves were injected with scFvG8 first and then with Xcc, HR within the infiltrated area was suppressed and the tissue lesions became smaller (Fig. 5c). The resulting necrotic area on PBS and vector control leaves was significantly greater compared to that of the scFvG8-expressing leaves. Necrotic lesions arising from Xcc were larger than 2 cm2 on PBS and vector control leaves, while they were very limited on scFvG8-expressing leaves (Table 3b). These results showed that the antibody decreased the necrotic area on the leaves for up to 70% compared to control plants, indicating the prevention of Xcc pathogenicity by blocking pthA activity.
Discussion
The antibody-mediated resistance-inducing strategies attempt to limit/suppress pathogenicity and confer resistance toward pathogens (Peschen et al. 2016). In these strategies, the key products of a pathogen, especially its virulence factor, are targeted as antigens by specific antibodies produced in plants to develop resistance in them. Here, due to the key role of pthA in bacterial pathogenesis, we investigated the transient expression of a recombinant anti-pthA antibody, scFvG8, in N. tabacum cv. Samson leaf cytosol. For this purpose, scFvG8 was first selected from naïve phage display libraries by exploiting their high affinity for recombinant pthA. Assessment of scFvG8 encoding sequence confirmed the presence of six CDRs (three in each chain) and their position was characterized using the IMGT database. Docking results of the scFvG8 antibody and pthA showed a binding energy of − 738 kcal/mol which was approximately twice that of scFvG8-BSA interaction, indicating specific and strong binding of the antibody to the antigen. As mentioned in other studies, the estimated binding energy in an antibody-antigen interaction is usually higher than − 600 kcal/mol and in most cases, it is about twice that of antibody-BSA interaction (Pashaki et al. 2017; Shahmirzaie et al. 2020; Raeisi et al 2020; Raeisi et al 2021). Furthermore, our results showed that the binding domain was mainly formed by eight intermolecular hydrogen bonds that propose the involvement of four CDRs (CDRH1, CDRH2, CDRL2, and CDRL3) in scFvG8-pthA interaction. Some studies showed that each of the six CDRs can be responsible for antigen binding and various numbers of CDRs were reported to be involved in antibody-antigen interaction; however, antibody-antigen interaction is often reported to occur mainly at the CDR3 (Kunik et al. 2012; Monnier et al. 2013; D'Angelo et al. 2018). Notably, most human antibody libraries are designed based on randomizing the CDR3 regions, indicating their key role in antigen–antibody interaction (D'Angelo et al. 2018).
In this study, scFvG8 was successfully expressed in bacterial cells and purified under native conditions by IMAC. scFvG8 protein concentration was about 500 μg/mL, and we used it at 10 μg/mL in western blotting, DIBA, and indirect ELISA. Results from immunoassay tests were indicative of high sensitivity and specificity of scFvG8 antibody in pthA detection. As shown by previous studies, the ability of a concentration of 10 μg/mL scFv antibody to detect antigen implicates its high sensitivity (Tohidkia et al. 2017; Raeisi et al. 2021). Thus, in silico and in vitro results imply scFvG8 antibody specificity for pthA detection.
Transient expression scFvG8 in Nicotiana. tabacum cv. Samson leaves revealed antibody accumulation at detectable levels within the tobacco leaves three days post-infiltration. In western blotting done using anti-scFv antibodies to verify the presence of scFv fragments in transgenic plants, a protein band at the expected size of 35 kDa for scFvG8 was observed. In some studies, the inability of western blotting in detecting scFv expressed in the cytosol of plants was explained to be due to extremely low levels of antibody in the cytosol, however, even low levels of plant-produced antibody significantly enhanced resistance to pathogens compared to wild type controls (Zimmermann et al. 1998). The ability to detect scFv expressed in the cytosol of plants by western blot confirms the presence of high levels of the antibody in plant cells that can confer resistance to pathogens (safarnejad et al. 2009; Cervera et al. 2010; Shahryari et al. 2013). Moreover, our immunoassays findings suggest that scFvG8 acquired a correct protein folding and was detectable in plant cells. The accuracy of scFv folding and preservation of its properties in plant cells were reported previously (Lombardi et al. 2005; safarnejad et al. 2009; Shahryari et al. 2013). In our study, the ability of scFvG8 produced in plants to recognize and bind pthA in serological assays, supported its correct protein folding and indicated that it has a high binding activity for its cognate antigen so that comparing ELISA data for purified scFvG8 and plant-produced scFvG8 showed comparable ability in detecting infected plants for the two antibodies except in one case (infected sample 3). Since the concentration of purified protein used in ELISA is specific, scFvG8 was expressed at an acceptable amount in the plant cells, consistent with previous studies (Safarnejad et al. 2009; Shahryari et al. 2013). The limited HR observed in scFvG8-expressing leaves was indicative of the functionality of this antibody in plant cells. Thus, the expression of scFvG8 in plant reduced pthA-induced necrotic lesions development. As a result, there was a significant difference in the diameter of the lesions between antibody-expressing (2.0 cm) and control (0.5 cm) plants, indicating a reduction of about 70% in the severity of Xcc-produced symptoms. Some previous studies were remarkably successful in this field; in this regard, a 70% reduction in local lesion numbers was achieved by expressing a full-size IgG against Tobacco mosaic virus coat protein in tobacco (Voss et al. 1995) and a reduction of 70–90% in local lesion size was yielded by expressing scFv targeting the same protein (Zimmermann et al. 1998). Our data were in accordance with the available literature concerning the functional equivalence of recombinant proteins produced using different expression systems (Villani et al. 2005; Cervera et al. 2010; Kopertekh et al. 2019). Hence, scFvG8 expression in citrus seedlings can be effective in disease management. Furthermore, the functionality and stability of scFvG8 during transient expression in plants hold promise for Xcc suppression. The only earlier study on the use of a recombinant antibody generated against Xcc employed a hybridoma technology (Li et al. 2010); therefore, to the best of our knowledge, the present study is the first investigation of the isolation of a specific scFv from phage display libraries and its application for controlling citrus canker bacterial infection in citrus culture.
Taken together, our findings are the first step toward developing stable expression of scFvG8 antibody in citrus plants to produce resistance to Xcc.
References
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48:419–436. https://doi.org/10.1146/annurev-phyto-080508-081936
Cervera M, Esteban O, Gil M, Gorris MT, Martinez MC, Peoa L (2010) Transgenic expression in citrus of single chain antibody fragments specific to Citrus tristeza virus confers virus resistance. Transgenic Res 19(6):1001–1015. https://doi.org/10.1007/s11248-010-9378-5
Chen YD, Chen TA (1998) Expression of engineered antibodies in plants: a possible tool for spiroplasma and phytoplasma disease control. Phytopathology 88:1367–1371. https://doi.org/10.1094/PHYTO.1998.88.12.1367
D’Angelo S, Ferrara F, Naranjo L, Erasmus MF, Hraber P, Bradbury A (2018) Many routes to an antibody heavy-chain CDR3: necessary, yet insufficient, for specific binding. Front Immunol 9:395. https://doi.org/10.3389/fimmu.2018.00395
De Souza TA, Soprano AS, de Lira NP, Quaresma AJ, Pauletti BA, Paes Leme AF, Benedetti CE (2012) The TAL effector pthA4 interacts with nuclear factors involved in RNA-dependent processes including a HMG protein that selectively binds poly (U) RNA. PLoS ONE 7:e32305. https://doi.org/10.1371/journal.pone.0032305
Diamos AG, Hunter J, Pardhe MD, Rosenthal SH, Sun H, Foster BC, Dipalma MP, Chen Q, Mason HS (2020) High level production of monoclonal antibodies using an optimized plant expression system. Front Bioeng Biotechnol 7:472. https://doi.org/10.3389/fbioe.2019.00472
Domingues MN, De Souza TA, Cernadas RA, De Oliveira ML, Docena C, Farah CS (2010) The Xanthomonas citri effector protein PthA interacts with citrus proteins involved in nuclear transport, protein folding and ubiquitination associated with DNA repair. Mol Plant Pathol 11:663–675. https://doi.org/10.1111/j.1364-3703.2010.00636.x
Gargouri-Bouzid R, Jaoua L, Rouis S, Saïdi MN, Bouaziz D, Ellouz R (2006) PVY-resistant transgenic potato plants expressing an anti-NIa protein scFv antibody. Mol Biotechnol 33(2):133–140. https://doi.org/10.1385/MB:33:2:133 (PMID: 16757800)
Ghannam A, Kumari S, Muyldermans S (2015) Camelid nanobodies with high affinity for broad bean mottle virus: a possible promising tool to immunomodulate plant resistance against viruses. Plant Mol Biol 87:355–369. https://doi.org/10.1007/s11103-015-0282-5
Gochez AM, Huguet-Tapia JC, Minsavage GV, Shantaraj D, Jalan N, Strauß A, Lahaye T, Wang N, Canteros BI, Jones JB, Potnis N (2018) Pacbio sequencing of copper-tolerant Xanthomonas citri reveals presence of a chimeric plasmid structure and provides insights into reassortment and shuffling of transcription activator-like effectors among X. citri strains. BMC Genom 19:16. https://doi.org/10.1186/s12864-017-4408-9
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J (2009) A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS ONE 4:e4358. https://doi.org/10.1371/journal.pone.0004358
Gurlebeck D, Thieme F, Bonas U (2006) Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J Plant Physiol 163(3):233–255. https://doi.org/10.1016/j.jplph.2005.11.011
Hemmer C, Djennane S, Ackerer L, Hleibieh K, Marmonier A, Gersch S, Garcia S, Vigne E, Komar V, Perrin M, Gertz C, Belval L, Berthold F, Monsion B, Schmitt-Keichinger C, Lemaire O, Lorber B, Gutiérrez C, Muyldermans S, Demangeat G, Ritzenthaler C (2018) Nanobody-mediated resistance to Grapevine fanleaf virus in plants. Plant Biotechnol J 16(2):660–671. https://doi.org/10.1111/pbi.12819
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23(9):1126–1136. https://doi.org/10.1038/nbt1142
Hu Y, Zhang HuY, Sosso J, Jia H, Frommer D, Li T, Yang WB, White B, Wang FF, Jones JB (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. PNAS 111:521–529. https://doi.org/10.1073/pnas.1313271111
Kay S, Boch J, BonasU, (2005) Characterization of AvrBs3-like effectors from a Brassicaceae pathogen reveals virulence and avirulence activities and a protein with a novel repeat architecture. MPMI 18:838–848. https://doi.org/10.1094/MPMI-18-0838
Kopertekh L, Meyer T, Freyer C, Hust M (2019) Transient plant production of Salmonella typhimurium diagnostic antibodies. Biotechnol Rep 21:e00314. https://doi.org/10.1016/j.btre.2019.e00314
Kunik V, Peters B, Ofran Y (2012) Structural consensus among antibodies defines the antigen binding site. PLoS Comput Biol 8(2):e1002388. https://doi.org/10.1371/journal.pcbi.1002388
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51(10):2778–2786. https://doi.org/10.1021/ci200227u
Le Gall F, Bove JM, Garnier M (1998) Engineering of a single-chain variable-fragment (scFv) antibody specific for the stolbur phytoplasma (Mollicute) and its expression in Escherichia coli and tobacco plants. Appl Environ Microbiol 64:4566–4572. https://doi.org/10.1128/AEM.64.11.4566-4572
Li N, Hu CH, Long GY, Long GY, Dai SM, Xie Y, Deng ZN (2010) Preparation of monoclonal antibody against PthA-NLS and cloning of the relative ScFv Gene. ASC 9(1):101–105. https://doi.org/10.1016/S1671-2927(09)60071-7
Lombardi A, CantaleC S, Giacomini P, Galeffi P (2005) Functional expression of a single-chain antibody specific for the HER2 human oncogene in a bacterial reducing environment. Protein Expr Purif 44:10–15. https://doi.org/10.1016/j.pep.2005.05.013
Monnier PP, Vigouroux RJ, Tassew NG (2013) In Vivo applications of single chain Fv (variable domain) (scFv) fragments. Antibodies 2:193–208. https://doi.org/10.3390/antib2020193
Mysore KS, Ryu CM (2004) Nonhost resistance: how much do we know? Trends Plant Sci 9:97–104. https://doi.org/10.1016/j.tplants.2003.12.005
Pashaki AS, Safarnejad MR, Asgari Safdar AH, Safarpour H, Tabatabaie M (2017) Production of a phage-displayed single chain variable fragment antibody against infectious bursal disease virus. Trop J Pharm Res 16:2801–2809. https://doi.org/10.4314/tjpr.v16i12.3
Pereira AL, Carazzolle MF, Abe VY, De Oliveira ML, Domingues MN, Silva JC (2014) Identification of putative TAL effector targets of the citrus canker pathogens shows functional convergence underlying disease development and defense response. BMC Genom 15:157. https://doi.org/10.1186/1471-2164-15-157
Peschen D, Schillberg S, Fischer R (2016) Antibody-mediated pathogen resistance In Plants. Methods Mol Biol 1385:273–291. https://doi.org/10.1007/978-1-4939-3289-4_19
Raeisi H, Safarnejad MR, Alavi SM, Elahinia SA, Farrokhi N (2018a) Production of polyclonal phages harbouring antibody fragment genes against Xanthomonas citri subsp. citri using phage display technology. J Appl Entomol 85(2):265–276. https://doi.org/10.22092/jaep.2017.115980.1194
Raeisi H, Safarnejad MR, Alavi SM, Elahinia SA, Farrokhi N (2018b) Gene cloning and expression of Xanthomonas citri subsp. citri pilin. ABJ 10:35–48. https://doi.org/10.22103/jab.2018.2066
Raeisi H, Safarnejad MR, Alavi SM, Farrokhi N, Elahinia SA, Safarpour H, Sharifian F (2019a) Development and molecular analyses of Xanthomonas pthA specific scFv recombinant monoclonal antibodies. J Crop Prot 8(4):417–429
Raeisi H, Safarnejad MR, Alavi SM, Elahinia SA, Farrokhi N (2019b) Applying of pthA effector protein of Xanthomonas citri subsp. citri for production of specific antibodies and its application for detection of infected plants. Plant Pathol J. https://doi.org/10.1007/s42161-019-00385-5
Raeisi H, Safarnejad MR, Moeini P, Safarpour H, Sokhansanj Y (2020) Isolation of single-chain variable fragment (scFv) antibodies for detection of Chickpea chlorotic dwarf virus (CpCDV) by phage display. Arch Virol 165:2789–2798. https://doi.org/10.1007/s00705-020-04813-1
Raeisi H, Safarnejad MR, Sadeghkhani F (2021) A new single-chain variable fragment (scFv) antibody provides sensitive and specific detection of citrus tristeza virus. J Virol Methods 300:114412. https://doi.org/10.1016/j.jviromet.2021.114412
Roeschlin RA, Favaro MA, Chiesa MA, Alemano S, Vojnov AA, Castagnaro AP, Filippone MP, Gmitter FGJ, Gadea J, Marano MR (2017) Resistance to citrus canker induced by a variant of Xanthomonas citri ssp. citri is associated with a hypersensitive cell death response involving autophagy-associated vacuolar processes. Mol Plant Pathol 18(9):1267–1281. https://doi.org/10.1111/mpp.12489
Safarnejad MR, Fischer R, Commandeur U (2008) Generation and characterization of functional recombinant antibody fragments against tomato yellow leaf curl virus replication-associated protein. Commun Agric Appl Biol Sci 73(2):311–321
Safarnejad MR, Fischer R, Commandeur U (2009) Recombinant-antibody-mediated resistance against Tomato yellow leaf curl virus in Nicotiana benthamiana. Arch Virol 154(3):457–467. https://doi.org/10.1007/s00705-009-0330-z
Safarnejad MR, Jouzani GS, Tabatabaei M, Twyman RM, Schillberg S (2011) Antibody-mediated resistance against plant pathogens. Biotechnol Adv 29(6):961–971. https://doi.org/10.1016/j.biotechadv.2011.08.011.2011
Schillberg S, Zimmermann S, Zhang MY, Fischer R (2001) Antibody-based resistance to plant pathogens. Transgenic Res 10(1):1–12. https://doi.org/10.1023/A:1008945126359
Shahmirzaie M, Safarnejad MR, Rakhshandehroo F, Safarpour H, Shirazi FH, Zamanizadeh HR, Elbeaino T (2020) Generation and molecular docking analysis of specific single-chain variable fragments selected by phage display against the recombinant nucleocapsid protein of fig mosaic virus. J Virol Methods 276:113796. https://doi.org/10.1016/j.jviromet.2019.113796
Shahryari F, Safarnejad MR, Shams-Bakhsh M, Schillberg S, Nölke G (2013) Generation and expression in plants of a single-chain variable fragment antibody against the immunodominant membrane protein of Candidatus phytoplasma aurantifolia. J Microbiol Biotechnol 23(8):1047–1054. https://doi.org/10.4014/jmb.1301.01054 (PMID: 23727814)
Tavladoraki P, Benvenuto E, Trinca S, De Martinis D, Cattaneo A, Galeffi P (1993) Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 366(6454):469–472. https://doi.org/10.1038/366469a0
Tohidkia MR, Sepehri M, Khajeh S, Bara J, Omidi Y (2017) Improved soluble ScFv ELISA screening approach for antibody discovery using phage display technology. SLAS Discov 22(8):1026–1034. https://doi.org/10.1177/2472555217701059
Villani ME, Roggero P, Bitti O, Benvenuto E, Franconi R (2005) Immunomodulation of cucumber mosaic virus infection by intrabodies selected in vitro from a stable single frame work phage display library. Plant Mol Biol 58:305–316. https://doi.org/10.1007/s11103-005-4091-0
Voss A, Niersbach M, Hain R (1995) Reduced virus infectivity in N. tabacum secreting a TMV-specific full-size antibody. Mol Breed 1:39–50. https://doi.org/10.1007/BF01682088
Weigel D, Glazebrook J (2006) Transformation of agrobacterium using the freeze-thaw method. Cold Spring Harb Protoc 1(7):4666. https://doi.org/10.1101/pdb.prot4666
Wiederstein M, Sippl MJ (2007) ProSA-wb: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35(suppl_2):W407–W10
Zimmermann SD, Schillberg S, Liao Y, Fisher R (1998) Intracellular expression of TMV-specific single-chain Fv fragments leads to improved virus resistance in shape Nicotiana tabacum. Mol Breed 4:369–379
Acknowledgements
The authors would like to thank Iranian Research Institute of Plant Protection for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All authors critically assessed the manuscript and approved it for publication. This article does not contain any studies on human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raeisi, H., Safarnejad, M.R., Alavi, S.M. et al. Transient expression of an scFvG8 antibody in plants and characterization of its effects on the virulence factor pthA of Xanthomonas citri subsp. citri. Transgenic Res 31, 269–283 (2022). https://doi.org/10.1007/s11248-022-00301-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-022-00301-1